Abstract
Reduced synaptic inhibition due to dysfunction of ionotropic GABAA receptors has been proposed as one factor in cerebral ischaemia-induced excitotoxic cell death. However, the participation of the inhibitory metabotropic GABAB receptors in these pathological processes has not been extensively investigated. We used oxygen–glucose deprivation (OGD) and NMDA-induced excitotoxicity as models to investigate whether ischaemia-like challenges alter the protein levels of GABAB1 and GABAB2 receptor subunits in rat organotypic hippocampal slice cultures. Twenty-four hours after the insult both OGD and NMDA produced a marked decrease in the total levels of GABAB2 (~75%), while there was no significant change in the levels of GABAB1 after OGD, but an increase after NMDA treatment (~100%). The GABAB receptor agonist baclofen (100 μM) was neuroprotective following OGD or NMDA treatment if added before or during the insult. GABAB receptors comprise heterodimers of GABAB1 and GABAB2 subunits and our results suggest that the separate subunits are independently regulated in response to extreme neuronal stress. However, because GABAB2 is required for functional surface expression, down-regulation of this subunit removes an important inhibitory feedback mechanism under pathological conditions.
Keywords: Cerebral ischaemia, GABAB receptors, Baclofen, Organotypic hippocampal slice cultures, Oxygen–glucose deprivation, NMDA-induced excitotoxicity
1. Introduction
Cerebral ischaemia/stroke is a major cause of death and severe long-term disability due to excessive neuronal excitation and consequent cell death (Choi, 1992). The involvement of excitatory glutamate receptors in ischaemic cell death has been extensively investigated but the contributions of inhibitory gamma-aminobutyric acid (GABA) receptors are less well established (Schwartz-Bloom and Sah, 2001). GABA acts at three main classes of receptors in the mammalian brain, the GABAA, GABAB and GABAC receptors. Both GABAA and GABAC receptors are ligand gated Cl−-permeable ion channels, while GABAB receptors are G-protein-coupled receptors that exert much longer lasting synaptic inhibition. GABAB receptors require two subunits, GABAB1 and GABAB2, to form a functional receptor and they are present at both post- and presynaptic compartments. GABAB receptors act presynaptically by inhibiting Ca+ channels, and postsynaptically by activating inwardly rectifying K+ channels (GIRKs). Changes in the number, activity and/or localisation of GABAB receptors can dramatically alter the level of synaptic inhibition (Bettler and Tiao, 2006). Thus, GABAB receptors seem likely candidates to play a role in balancing excessive glutamatergic excitation that occurs during ischaemia. For example, activation of presynaptic GABAB receptors that can down-regulate glutamate release might provide a mechanism to counteract excitotoxic neuronal cell death.
The GABAB receptor agonist baclofen inhibits glutamate release (Huston et al., 1995) and has been reported to be neuroprotective in vivo (Babcock et al., 2002; Jackson-Friedman et al., 1997; Lal et al., 1995; Ouyang et al., 2007) and in organotypic hippocampal slice cultures during oxygen–glucose deprivation (OGD) (Dave et al., 2005). It has also been reported that both GABAA and GABAB receptor agonists can protect neurons against death induced by ischaemia/reperfusion in vivo via a mechanism involving inhibition of NMDA receptor-mediated nitric oxide (NO) production by neuronal NO synthase (nNOS) (Zhou et al., 2008). Interestingly, GABAB receptors are stable at the plasma membrane showing little basal down-regulation or baclofen induced internalisation making them an attractive therapeutic target (Fairfax et al., 2004). Indeed, baclofen has been used for many years primarily as a muscle relaxant to treat spasticity (Bowery, 2006).
To gain insight into the potential roles of GABAB receptors in ischaemia we investigated changes in GABAB1 and GABAB2 protein levels in organotypic hippocampal slice cultures exposed to OGD or NMDA-induced excitotoxicity, two models widely used to elicit neuronal cell death. In addition, we tested the neuroprotective effects of baclofen in these models. Our data suggest that the individual subunits are differentially regulated following ischaemic insult. The total levels of GABAB2 are dramatically reduced whereas GABAB1 levels remain unchanged. Surprisingly, we also found that although baclofen was neuroprotective it did not prevent the ischaemia-induced reduction in GABAB2.
2. Methods
Animal care and all experimental procedures were conducted in accordance with British animal protection legislation and the experimental protocols were approved by the British National Committee for Ethics in Animal Research.
2.1. Organotypic slice cultures of rat hippocampus
Organotypic hippocampal slice cultures were prepared using the method of Stoppini and colleagues (Stoppini et al., 1991). Transverse hippocampal slices (400 μm) from 7-day-old male Wistar rats were cut using a McIlwain tissue chopper and transferred to Hank’s balanced salt solution (HBSS). Six slices were placed on each Millicell culture insert in 6-well culture plates together with 1 ml of culture medium per well. Culture medium (pH 7.3) consisted of minimum essential medium 50%, horse serum 25% and HBSS 25% supplemented with glucose 36 mM, HEPES 25 mM, NaHCO3 4 mM, and penicillin/streptomycin 1%. The cultures were incubated at 37 °C in an atmosphere of 5% CO2 for 14 days in vitro (DIV) prior to use. Medium was changed every 3 days.
2.2. OGD and NMDA treatment
On DIV 14, the cultures were exposed to OGD (Cimarosti et al., 2001). Each insert was washed twice with OGD medium (pH 7.2) composed of CaCl2 1.26 mM, KCl 5.36 mM, NaCl 136.89 mM, KH2 PO4 0.44 mM, Na2HPO4 0.34 mM, MgCl2 0.49 mM, MgSO4 0.44 mM, HEPES 25 mM, NaHCO3 4 mM and penicillin/streptomycin 1%. To deplete glucose from intracellular stores and extracellular space, the inserts were incubated in 1 ml of OGD medium for 10 min. Following this period, the medium was exchanged for OGD medium previously bubbled with N2/CO2 (95%/5%) for 10 min. The slice cultures were then transferred to an anaerobic chamber at 37 °C with N2-enriched atmosphere where they were maintained for 45 min. After OGD, the slices were removed from the chamber, washed twice with HBSS, returned to culture medium, and incubated for a further 24 h. Where appropriate baclofen or AP5 was incorporated in culture medium and in OGD medium during the periods indicated.
For assessments of NMDA-induced cell death, on DIV 14 the cultures were exposed to NMDA 50 μM for 45 min (in the same culture medium; 37 °C atmosphere air with 5% CO2). After NMDA exposure, the inserts were washed twice with HBSS and returned to culture medium for 24 h before being assessed for cell death. Baclofen or AP5 was incorporated in culture medium with or without NMDA during the periods indicated.
Slices in the control groups were treated in parallel to slices in the OGD or NMDA groups, and incubated for 45 min, but were washed and maintained in culture medium and exposed to 37 °C atmosphere air with 5% CO2.
2.3. Assessment of cell death due to OGD or NMDA excitotoxicity
Cellular damage was assessed by fluorescent image analysis of propidium iodide (PI) uptake (Cimarosti et al., 2001). PI is excluded from healthy cells, but following loss of membrane integrity it enters cells, binds to DNA and becomes highly fluorescent. Twenty-four hours after OGD or NMDA-induced excitotoxicity, PI 7.5 μM was added to cultures and incubated for 1 h. Cultures were observed with an inverted microscope (Nikon Eclipse TE 300) fitted with a standard rhodamine filter set. Images were captured and analysed using Scion Image software. The intensity of PI fluorescence in the selected region of interest, (CA1, CA2 + CA3 or DG (dentate gyrus)), was used as an index of cell death. The pixel intensity and area in which PI fluorescence was detectable above background level was determined using the ‘density slice’ function of the software. The mean percentage values of fluorescence in the slices treated with test compounds (baclofen or AP5) were calculated and compared to standard damage (Cimarosti et al., 2001). Standard damage was obtained as the mean of the intensity of PI fluorescence in the organotypic slices subjected to OGD or NMDA with no added drug.
2.4. Western blots and densitometry
For Western blot analysis, slices were added to Tris–HCl (pH 7.4) 50 mM, NaCl 150 mM, EDTA 1 mM, SDS 0.1%, Triton X-100 1% and mammalian protease inhibitor 1%, and homogenized on ice. The homogenates were sonicated for 10–15 s at 4 °C and the protein concentration determined. Samples were heated for 5 min at 37 °C with β-mercaptoethanol 5%, and subjected to SDS-PAGE (8%) loaded at 15 μg protein/lane. Proteins were blotted onto Immobilon-P membrane (Millipore Corporation, Bedford, MA, USA) and probed with appropriate primary antibodies after blocking with non-fat dry milk. The primary antibodies used were: rabbit polyclonal anti-GABAB1a,b antibody (1 μg/ml, Santa Cruz Biotechnology), guinea pig polyclonal anti-GABAB2 (1:1000, Chemicon), and mouse monoclonal anti-β-actin (1:5000, Sigma Chemicals). The membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (1:10 000, Sigma Chemicals) for 1 h followed by substrate incubation with BM Chemiluminescence Blotting Substrate (POD, Roche Molecular Biochemicals) or SuperSignal West Femto (Pierce). The chemiluminescence signal was detected on Hyperfilm HP (Amersham Biosciences). The band intensity was quantified by densitometry using ImageJ (NIH). OGD and NMDA groups were compared to control groups, which were designated as 100%, on the same Western blot to avoid any differences in signal intensity due to exposure times. All the blots were then re-probed with β-actin antibody as an internal control to ensure equal protein loading in all lanes.
2.5. Statistical analysis
Data are presented as mean ± SEM (standard error of the mean) of the indicated number of independent experiments. One-way analysis of variance (ANOVA), followed by Duncan’s multiple-range method, was applied to the means to determine significant differences between experimental groups. p Values < 0.05 were considered statistically significant.
3. Results
3.1. Dose–response effect of baclofen present during oxygen–glucose deprivation (OGD)
Organotypic hippocampal slice cultures were subjected to OGD for 45 min in the absence or presence of baclofen. In the CA1 region of OGD slices, baclofen at concentrations of 5, 50, 100 and 200 μM decreased cell death assessed by the incorporation of propidium iodide (PI) by 37 ± 7%, 36 ± 10%, 34 ± 7% and 49 ± 10%, respectively, compared to the CA1 region of untreated OGD slices (Fig. 1B). Similar neuroprotection was observed in the CA2 + CA3 region, where baclofen at the concentrations of 5, 50 and 100 μM decreased PI uptake by 30 ± 10%, 27 ± 10% and 38 ± 8%, respectively whereas the concentration of 200 μM conferred higher levels of neuroprotection (71 ± 11%, Fig. 1C). In the DG baclofen decreased the PI uptake by 32 ± 7% at 100 μM and by 33 ± 15% at 200 μM (Fig. 1D). To validate the OGD protocol as a model to assess neuroprotection, we tested the NMDA receptor antagonist AP5, an established neuroprotective agent (Fatokun et al., 2008). AP5 (50 μM) added during exposure to OGD decreased PI incorporation by ~95% in all regions analysed (see Fig. 3) indicating that nearly all OGD-invoked cell death is mediated by NMDA receptor activation.
Fig. 1.
Effects of different doses of baclofen on cellular damage induced by oxygen–glucose deprivation (OGD) for 45 min in organotypic slice cultures of rat hippocampus. (A) Representative images of cultures showing propidium iodide (PI) uptake 24 h after OGD. (B)–(D) Quantitative analysis of incorporation of PI in CA1, CA2 + CA3 and DG of control and baclofen-treated OGD slices compared with the corresponding region of untreated OGD slices (standard damage). The results are presented as percentage of standard damage ± SEM (n = 9). (*) indicates significant difference from standard damage and (#) indicates significant difference from all groups, p < 0.05 (one-way ANOVA and Duncan’s test).
Fig. 3.
Effects of baclofen (100 μM) added for 45 min before, during or after oxygen–glucose deprivation (OGD) on cellular damage induced by OGD for 45 min in organotypic slice cultures of rat hippocampus. (A) Representative images of cultures showing propidium iodide (PI) uptake 24 h after OGD. (B)–(D) Quantitative analysis of incorporation of PI in CA1, CA2 + CA3 and DG of baclofen-treated OGD slices compared with the corresponding region of untreated OGD slices (standard damage). The results are presented as percentage of standard damage ± SEM (n = 9). (*) indicates significant difference from standard damage and (#) indicates significant difference from all groups, p < 0.05 (one-way ANOVA and Duncan’s test).
3.2. GABAB receptors levels after OGD are not affected by baclofen treatment
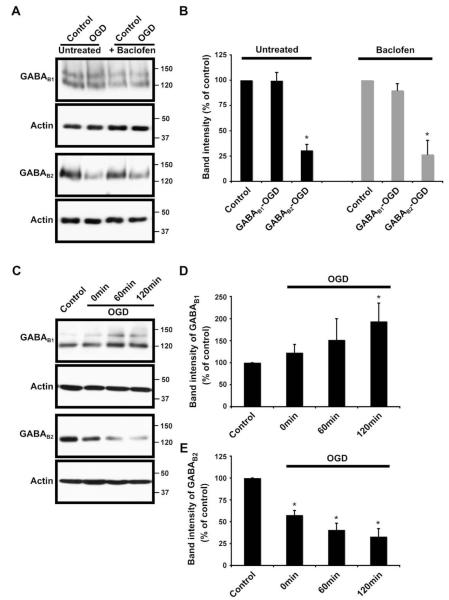
We next investigated the effects of OGD on GABAB receptor levels in control and baclofen-treated slices. Slices analysed 24 h after OGD showed dramatically decreased levels of GABAB2. Levels of GABAB1, however, remained unchanged by OGD (Fig. 2A and B). Interestingly, inclusion of baclofen (100 μM) during the 45 min OGD did not alter the profiles of GABAB receptor subunits (Fig. 2A and B, see Fig. 4). To determine whether the OGD effect on GABAB receptor levels was an immediate or delayed response to cellular stress, we analysed slices collected immediately after (0 min), 60 and 120 min after OGD. The levels of GABAB2 were decreased at 0 min and decreased further at 60 min and 120 min time points (Fig. 2C and E). In contrast, GABAB1 did not decrease rather there was a trend towards increased levels following OGD, and the changes were statistically significant at 120 min (Fig. 2C and D).
Fig. 2.
Effects of oxygen–glucose deprivation (OGD) for 45 min on the protein levels of GABAB receptors in organotypic slice cultures of rat hippocampus. (A) and (B) Untreated and baclofen-treated (100 μM) organotypic slice cultures of rat hippocampus were subjected to 45 min OGD and analysed 24 h after the lesion induction. Baclofen was present during the 45 min of OGD. (A) Representative pattern of GABAB1 and GABAB2 immunoreactivity detected using Santa Cruz anti-GABAB1 antibody and Chemicon anti-GABAB2 antibody. (B) Cumulative GABAB1 and GABAB2 results showing quantified data from separate immunoblots using slices from three different experiments. (C)–(E) Protein levels of GABAB receptors in organotypic slice cultures of rat hippocampus exposed to 45 min OGD and analysed immediately (0 min), 60 and 120 min after OGD. (C) Representative blots showing the pattern of GABAB1 and GABAB2 immunoreactivity detected using Santa Cruz anti-GABAB1 antibody and Chemicon anti-GABAB2 antibody. (D) and (E) Cumulative GABAB1 and GABAB2 results showing quantified data from separate immunoblots using slices from five different experiments. The results are presented as percentage of control ± SEM. (*) indicates significant difference p < 0.05 (one-way ANOVA and Duncan’s test).
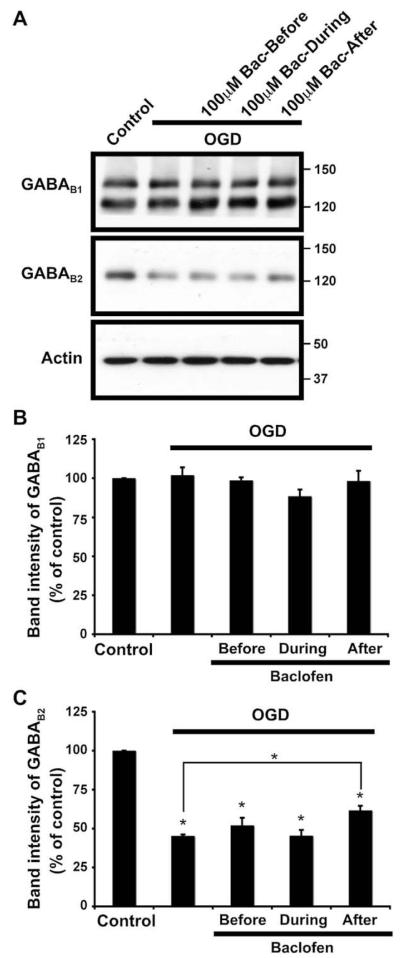
Fig. 4.
Effects of oxygen–glucose deprivation (OGD) for 45 min on the protein levels of GABAB receptors in untreated and baclofen-treated (100 μM) organotypic slice cultures of rat hippocampus. Baclofen was added for 45 min before, during or after OGD. (A) Representative pattern of GABAB1 and GABAB2 immunoreactivity detected using Santa Cruz anti-GABAB1 antibody and Chemicon anti-GABAB2 antibody. (B) and (C) Cumulative GABAB1 and GABAB2 results showing quantified data from separate immunoblots using slices from three different experiments. The results are presented as percentage of control ± SEM. (*) indicates significant difference p < 0.05 (one-way ANOVA and Duncan’s test).
We next tested the time window for neuroprotection by baclofen. Baclofen (100 μM) was added either 45 min before, during or immediately after OGD (Fig. 3). While not as effective as AP5, significant levels of protection were observed with baclofen in all the analysed regions. In the CA1 region the PI incorporation was reduced by 28 ± 7%, 42 ± 11% and 56 ± 9%, when baclofen was added before, during or after OGD, respectively (Fig. 3B). Similar neuroprotection was conferred in the CA2 + CA3 region (38 ± 11%, 63 ± 9% and 50 ± 10%, respectively, Fig. 3C) and in the DG (40 ± 11%, 48 ± 12% and 50 ± 9%, respectively, Fig. 3D).
Baclofen either before or during OGD did not affect the OGD-induced changes in the levels of GABAB2 (Fig. 4). However, when baclofen was added after OGD, a slight but significant increase in the levels of GABAB2 compared to baclofen-untreated OGD slices was observed (Fig. 4), indicating that baclofen stimulation may provide some level of protection against OGD-induced GABAB2 degradation.
3.3. Effects of baclofen on NMDA-induced excitotoxicity
In parallel experiments we investigated the fate of GABAB receptor subunits and the effects of baclofen on slice cultures exposed to NMDA (50 μM) for 45 min. Baclofen (100 μM) was added for 45 min before, during or after NMDA-induced excitotoxicity. When added before or during NMDA exposure, baclofen decreased the incorporation of PI by ~50% in the CA1 region, ~40% in the CA2 + CA3 region and ~35% in the DG (Fig. 5). However, when baclofen was added after NMDA treatment, it did not exert a significant neuroprotective effect (Fig. 5). Surprisingly, the presence of baclofen before, during or after NMDA treatment caused a significant increase in the levels of GABAB1 compared to control exposed slices whereas GABAB2 levels were decreased in all NMDA-treated slices both with and without baclofen (Fig. 6).
Fig. 5.
Effects of baclofen (100 μM) added for 45 min before, during or after NMDA exposure on cellular damage induced by NMDA (50 μM) added for 45 min in organotypic slice cultures of rat hippocampus. (A) Representative images of cultures showing propidium iodide (PI) uptake 24 h after OGD. (B)–(D) Quantitative analysis of incorporation of PI in CA1, CA2 + CA3 and DG of baclofen-treated NMDA-exposed slices compared with the corresponding region of untreated NMDA-exposed slices (standard damage). The results are presented as percentage of standard damage ± SEM (n = 6). (*) indicates significant difference from standard damage and (#) indicates significant difference from all groups, p < 0.05 (one-way ANOVA and Duncan’s test).
Fig. 6.
Effects of NMDA (50 μM) exposure for 45 min on the protein levels of GABAB receptors in untreated and baclofen-treated (100 μM) organotypic slice cultures of rat hippocampus. Baclofen was added for 45 min before, during or after NMDA exposure. (A) Representative pattern of GABAB1 and GABAB2 immunoreactivity detected using Santa Cruz anti-GABAB1 antibody and Chemicon anti-GABAB2 antibody. (B) and (C) Cumulative GABAB1 and GABAB2 results showing quantified data from separate immunoblots using slices from three different experiments. The results are presented as percentage of control ± SEM. (*) indicates significant difference p < 0.05 (one-way ANOVA and Duncan’s test).
4. Discussion
We used rat organotypic hippocampal slice cultures to investigate the effects of in vitro models of ischaemia on the total levels of GABAB receptor subunits and to assess the neuroprotective effect of baclofen. Unlike dispersed neuronal cell cultures, organotypic slice cultures retain some structure and neuronal pathways (Cimarosti and Henley, 2008). This makes them well suited to study the balance between excitatory and inhibitory neurotransmission regulated by neuronal network connections involving distinct types of GABAergic interneurons and glial cells (DeFazio et al., 2009; Maccaferri et al., 2000).
The roles of GABA in brain damage after energy deprivation have been investigated by using several experimental approaches (Green et al., 2000; Schwartz-Bloom and Sah, 2001) however there is some discrepancy in the literature. Enhanced GABAergic transmission has been reported to be neuroprotective (Calabresi et al., 2003; Chen Xu et al., 2000; Galeffi et al., 2000; Schwartz-Bloom et al., 2000). Conversely, GABA receptor agonists have been reported to exacerbate cerebral damage caused by energy deprivation (Erdo et al., 1991; Muir et al., 1996; Stokes et al., 2001). In our in vitro ischaemic models baclofen was strongly neuroprotective but this effect did not appear to be related to changes in the total levels of GABAB receptor subunits. Ischaemic preconditioning (IPC) in vivo in rats promotes a robust release of GABA after lethal ischaemia compared with control rats and IPC also increases the activity of glutamate decarboxylase, the major GABA synthesis pathway in the brain (Dave et al., 2005). In rat organotypic hippocampal slices, IPC and preconditioning by activation of the epsilon-isoform of protein kinase C confer neuroprotection that depends on functional modifications of GABA synapses (DeFazio et al., 2009). In addition, baclofen also provided a significant decrease in cellular death in organotypic hippocampal slices exposed to OGD suggesting that activation of GABAB contributes significantly to neuroprotection against ischaemia (Dave et al., 2005). Similarly, baclofen administration in vivo (50 mg/kg) prevented both the loss of hippocampal CA1 pyramidal cells and the reduction in hippocampal CaM kinase immunoreactivity observed in control animals following ischaemic insult although it did not prevent ischaemia-induced working memory deficits in behavioral tests (Babcock et al., 2002).
A major factor on neuronal death following OGD is cytotoxic calcium entry through NMDA receptors (Papadia and Hardingham, 2007). Consistent with this, we show that inclusion of AP5, an NMDA receptor antagonist, during OGD resulted in very high levels of neuroprotection. Nonetheless, OGD is an extreme insult that likely also affects other signalling pathways. We therefore propose that the increase in GABAB1 following NMDA-induced excitotoxicity but not OGD may be due to subtle differences in the signalling cascades activated. Baclofen was most neuroprotective in CA1, moderately neuroprotective in CA3 regions of hippocampal slices but least effective in DG. These results are consistent with previously published expression studies of distributions of the GABAB1 subunit which is strongly expressed in the CA1, less strongly expressed in CA3 regions and weakly expressed in DG (López-Bendito et al., 2004). Similarly NMDA receptors are weakly expressed in the CA3 region and highly expressed in DG (Coultrap et al., 2005). Indeed, consistent with this we found that in NMDA-induced excitotoxicity, AP5 was more neuroprotective in DG compared to other regions.
We have shown previously that ischaemia in vivo results in marked decreases in both AMPAR and KAR levels (Cimarosti et al., 2008). Further, ischaemia has been shown to dramatically down-regulate expression of the AMPA receptor subunit GluR2 and thereby increase the Ca2+ permeability of AMPARs (reviewed by Liu and Zukin, 2007; Soundarapandian et al., 2005). Here we demonstrate a decrease in GABAB2 protein levels 24 h after OGD or NMDA-induced excitotoxicity in organotypic hippocampal slice cultures, both in the absence or presence of baclofen. Interestingly, there was large reduction of GABAB2 protein levels immediately following OGD suggesting that these subunits were degraded during OGD protocol. Although unexpected, similar rapid rates of receptor turnover (~50% loss within 45 min) have been reported previously. For example we, and others, have shown similar extents and rates of degradation for EGF receptor (Kantamneni et al., 2009; Lu et al., 2003). In contrast, the levels of the GABAB1 subunit were largely unchanged following our in vitro ischaemia models. This observation of differential GABAB subunit regulation argues against a general loss of surface receptor proteins in response to excitotoxic stress, and supports a model whereby GABAB2 is being specifically degraded.
The data presented here are broadly consistent with analysis of GABAB subunit mRNAs using in situ hybridization following transient global ischaemia in the gerbil hippocampus. Both GABAB1 and GABAB2 mRNAs decreased and then disappeared in the CA1 region of the hippocampus in conjunction with neuronal death (Vollenweider et al., 2006). GABAB2, which is less abundant, was more affected than the GABAB1. Two days after ischaemia there was a pronounced decrease in GABAB2 while GABAB1 was only slightly altered. However, four days after ischaemia when there was widespread cell death, expression of both subunits was nearly absent in CA1. In organotypic hippocampal slice cultures, ischaemic models induce neuronal death within 24 h in the CA1 region, and the damage extends to the CA3 region during the following 72 h (Cho et al., 2004). The analysis at different time points after ischaemia could explain the different findings between our in vitro study in organotypic cultures and the previous in vivo study in gerbils (Vollenweider et al., 2006). Furthermore, it is difficult to directly correlate mRNA levels with protein levels. This may be especially true in the case of GABAB − levels of GABAB2 in GABAB1−/− mice are almost undetectable, despite GABAB2 mRNA levels and distribution in the brain being unchanged (Schuler et al., 2001).
The reasons and mechanisms for the differential regulation of GABAB receptor subunits following ischaemic insult observed here are unclear. Although it has been published previously that plasma membrane expression of GABAB receptors is stable with little net endocytosis even in the presence of baclofen (Fairfax et al., 2004), there is general theme emerging in the field that GABAB receptors undergo constitutive cycling. This constitutive cycling is increased upon baclofen application (Wilkins et al., 2008; Laffray et al., 2007). It is possible this may be a way of keeping the receptors at the surface active during prolonged stimulation by exchanging desensitized surface receptors for newly exocytosed non-desensitized receptors. This would provide an explanation, at least in part, for the apparent lack of GABAB desensitization in some systems (Cruz et al., 2004; Wetherington and Lambert, 2002).
Despite the ~50% loss of GABAB2 during the 45 min OGD baclofen is still neuroprotective It therefore remains possible that during excitotoxic/ischaemic conditions there is a compensatory increase in GABAB receptor signalling sensitivity. Interestingly, there is some evidence to support this. GABAB receptors are regulated by 5′ AMP-dependent protein kinase (AMPK) (Kuramoto et al., 2007). AMPK regulates cellular energy levels by stimulating catabolic metabolism and simultaneously inhibiting anabolic pathways and is rapidly activated following ischaemia (Kahn et al., 2005; Ramamurthy and Ronnett, 2006). AMPK binds GABAB1 and directly phosphorylates S783 in GABAB2 to enhance GABAB receptor activation of GIRKs. Phosphorylation of S783 was dramatically increased following ischaemic injury leading to the suggestion that it may play a role in neuroprotection after ischaemia by increasing GABAB receptor function and reducing excitotoxicity (Kuramoto et al., 2007). In that study, no change in GABAB2 staining was detected following middle cerebral artery occlusion (MCAO) in vivo or 80 min after transient anoxic insult (10 mM deoxyglucose and 10 mM azide) in dispersed neuronal cultures. However, because of the different model systems, ischaemia paradigms and sampling times used it is difficult to compare directly the reduced levels of GABAB2 after ischaemic insult here and not in the AMPK study.
It is feasible that GABAB1 is constitutively synthesized and that GABAB2 is the determining, and therefore most stringently regulated, factor in the number of functional GABAB receptors. In that case, down-regulation of GABAB2 will result in decreased inhibitory neurotransmission and remove a counter balance of excitatory glutamate release and enhance the neurotoxicity. While further research work is required to resolve these questions, GABAB receptor agonists that can access the brain could provide a new potential avenue for the development of neuroprotective therapies in the treatment of ischaemia.
Acknowledgements
We would like to thank Kevin Wilkinson for critical reading of the manuscript. H.C. is a Marie Curie Research Fellow. We are grateful to the MRC, the Wellcome Trust and the EU (GRIPPANT) for funding.
References
- Babcock AM, Everingham A, Paden CM, Kimura M. Baclofen is neuroprotective and prevents loss of calcium/calmodulin-dependent protein kinase II immunoreactivity in the ischemic gerbil hippocampus. J. Neurosci. Res. 2002;67:804–811. doi: 10.1002/jnr.10169. [DOI] [PubMed] [Google Scholar]
- Bettler B, Tiao JY. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol. Ther. 2006;110:533–543. doi: 10.1016/j.pharmthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor: a site of therapeutic benefit. Curr. Opin. Pharmacol. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy in brain ischemia. Ann. Neurol. 2003;53:693–702. doi: 10.1002/ana.10603. [DOI] [PubMed] [Google Scholar]
- Chen Xu W, Yi Y, Qiu L, Shuaib A. Neuroprotective activity of tiagabine in a focal embolic model of cerebral ischemia. Brain Res. 2000;874:75–77. doi: 10.1016/s0006-8993(00)02554-3. [DOI] [PubMed] [Google Scholar]
- Cho S, Liu D, Fairman D, Li P, Jenkins L, McGonigle P, Wood A. Spatiotemporal evidence of apoptosis-mediated ischemic injury in organotypic hippocampal slice cultures. Neurochem. Int. 2004;45:117–127. doi: 10.1016/j.neuint.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Rodnight R, Tavares A, Paiva R, Valentim L, Rocha E, Salbego C. An investigation of the neuroprotective effect of lithium in organotypic slice cultures of rat hippocampus exposed to oxygen and glucose deprivation. Neurosci. Lett. 2001;315:33–36. doi: 10.1016/s0304-3940(01)02310-2. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54:280–289. doi: 10.1016/j.neuropharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Henley JM. Investigating the mechanisms underlying neuronal death in ischemia using in vitro oxygen-glucose deprivation: potential involvement of protein SUMOylation. Neuroscientist. 2008;14:626–636. doi: 10.1177/1073858408322677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Nixon KM, Alvestad RM, Valenzuela CF, Browning MD. Differential expression of NMDA receptor subunits and splice variants among the CA1, CA3 and dentate gyrus of the adult rat. Brain Res. Mol. Brain Res. 2005;135:104–111. doi: 10.1016/j.molbrainres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat. Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Dave KR, Lange-Asschenfeldt C, Raval AP, Prado R, Busto R, Saul I, Perez-Pinzon MA. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J. Neurosci. Res. 2005;82:665–673. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Raval AP, Lin HW, Dave KR, Della-Morte D, Perez-Pinzon MA. GABA synapses mediate neuroprotection after ischemic and epsilonPKC preconditioning in rat hippocampal slice cultures. J. Cereb. Blood Flow Metab. 2009;29:375–384. doi: 10.1038/jcbfm.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdo S, Michler A, Wolff JR. GABA accelerates excitotoxic cell death in cortical cultures: protection by blockers of GABA-gated chloride channels. Brain Res. 1991;542:254–258. doi: 10.1016/0006-8993(91)91575-l. [DOI] [PubMed] [Google Scholar]
- Fairfax BP, Pitcher JA, Scott MG, Calver AR, Pangalos MN, Moss SJ, Couve A. Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J. Biol. Chem. 2004;279:12565–12573. doi: 10.1074/jbc.M311389200. [DOI] [PubMed] [Google Scholar]
- Fatokun AA, Stone TW, Smith RA. Adenosine receptor ligands protect against a combination of apoptotic and necrotic cell death in cerebellar granule neurons. Exp. Brain Res. 2008;186:151–160. doi: 10.1007/s00221-007-1218-3. [DOI] [PubMed] [Google Scholar]
- Galeffi F, Sinnar S, Schwartz-Bloom RD. Diazepam promotes ATP recovery and prevents cytochrome c release in hippocampal slices after in vitro ischemia. J. Neurochem. 2000;75:1242–1249. doi: 10.1046/j.1471-4159.2000.0751242.x. [DOI] [PubMed] [Google Scholar]
- Green AR, Hainsworth AH, Jackson DM. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacology. 2000;39:1483–1494. doi: 10.1016/s0028-3908(99)00233-6. [DOI] [PubMed] [Google Scholar]
- Huston E, Cullen GP, Burley JR, Dolphin AC. The involvement of multiple calcium channel sub-types in glutamate release from cerebellar granule cells and its modulation by GABAB receptor activation. Neuroscience. 1995;68:465–478. doi: 10.1016/0306-4522(95)00172-f. [DOI] [PubMed] [Google Scholar]
- Jackson-Friedman C, Lyden PD, Nunez S, Jin A, Zweifler R. High dose baclofen is neuroprotective but also causes intracerebral hemorrhage: a quantal bioassay study using the intraluminal suture occlusion method. Exp. Neurol. 1997;147:346–352. doi: 10.1006/exnr.1997.6637. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Kantamneni S, Holman D, Wilkinson KA, Nishimune A, Henley JM. GISP increases neurotransmitter receptor stability by down-regulating ESCRT-mediated lysosomal degradation. Neurosci. Lett. 2009;452:106–110. doi: 10.1016/j.neulet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffray S, Tan K, Dulluc J, Bouali-Benazzouz R, Calver AR, Nagy F, Landry M. Dissociation and trafficking of rat GABAB receptor heterodimer upon chronic capsaicin stimulation. Eur. J. Neurosci. 2007;25:1402–1416. doi: 10.1111/j.1460-9568.2007.05398.x. [DOI] [PubMed] [Google Scholar]
- Lal S, Shuaib A, Ijaz S. Baclofen is cytoprotective to cerebral ischemia in gerbils. Neurochem. Res. 1995;20:115–119. doi: 10.1007/BF00970534. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Shigemoto R, Kulik A, Vida I, Fairén A, Luján R. Distribution of metabotropic GABA receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus during prenatal and postnatal development. Hippocampus. 2004;14:836–848. doi: 10.1002/hipo.10221. [DOI] [PubMed] [Google Scholar]
- Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7626–7631. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J. Physiol. 2000;524(Pt 1):91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JK, Lobner D, Monyer H, Choi DW. GABAA receptor activation attenuates excitotoxicity but exacerbates oxygen–glucose deprivation-induced neuronal injury in vitro. J. Cereb. Blood Flow Metab. 1996;16:1211–1218. doi: 10.1097/00004647-199611000-00015. [DOI] [PubMed] [Google Scholar]
- Ouyang C, Guo L, Lu Q, Xu X, Wang H. Enhanced activity of GABA receptors inhibits glutamate release induced by focal cerebral ischemia in rat striatum. Neurosci. Lett. 2007;420:174–178. doi: 10.1016/j.neulet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J. Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Schwartz-Bloom RD, Sah R. Gamma-aminobutyric acid(A) neurotransmission and cerebral ischemia. J. Neurochem. 2001;77:353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- Schwartz-Bloom RD, Miller KA, Evenson DA, Crain BJ, Nadler JV. Benzodiazepines protect hippocampal neurons from degeneration after transient cerebral ischemia: an ultrastructural study. Neuroscience. 2000;98:471–484. doi: 10.1016/s0306-4522(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Soundarapandian MM, Tu WH, Peng PL, Zervos AS, Lu Y. AMPA receptor subunit GluR2 gates injurious signals in ischemic stroke. Mol. Neurobiol. 2005;32:145–155. doi: 10.1385/MN:32:2:145. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Bernard LP, Nicklas WJ, Zeevalk GD. Attenuation of malonate toxicity in primary mesencephalic cultures using the GABA transport blocker, NO-711. J. Neurosci. Res. 2001;64:43–52. doi: 10.1002/jnr.1052. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Vollenweider F, Bendfeldt K, Maetzler W, Otten U, Nitsch C. GABA(B) receptor expression and cellular localization in gerbil hippocampus after transient global ischemia. Neurosci. Lett. 2006;395:118–123. doi: 10.1016/j.neulet.2005.10.079. [DOI] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. GABA(B) receptor activation desensitizes postsynaptic GABA(B) and A(1) adenosine responses in rat hippocampal neurones. J. Physiol. 2002;544:459–467. doi: 10.1113/jphysiol.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins ME, Li X, Smart TG. Tracking cell surface GABAB receptors using an alpha-bungarotoxin tag. J. Biol. Chem. 2008;283:34745–34752. doi: 10.1074/jbc.M803197200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Li C, Yu HM, Zhang F, Han D, Zhang GY. Neuroprotection of gamma-aminobutyric acid receptor agonists via enhancing neuronal nitric oxide synthase (Ser847) phosphorylation through increased neuronal nitric oxide synthase and PSD95 interaction and inhibited protein phosphatase activity in cerebral ischemia. J. Neurosci. Res. 2008;86:2973–2983. doi: 10.1002/jnr.21728. [DOI] [PubMed] [Google Scholar]