Abstract
Objective and methods
A loss-of-function cytosine (C) for thymidine (T) transition at nucleotide 8590 of CYP4A11 has been associated with increased blood pressure in humans. We tested the hypothesis that CYP4A11 T8590C genotype is associated with salt sensitivity in the International Hypertensive Pathotype cohort.
Results
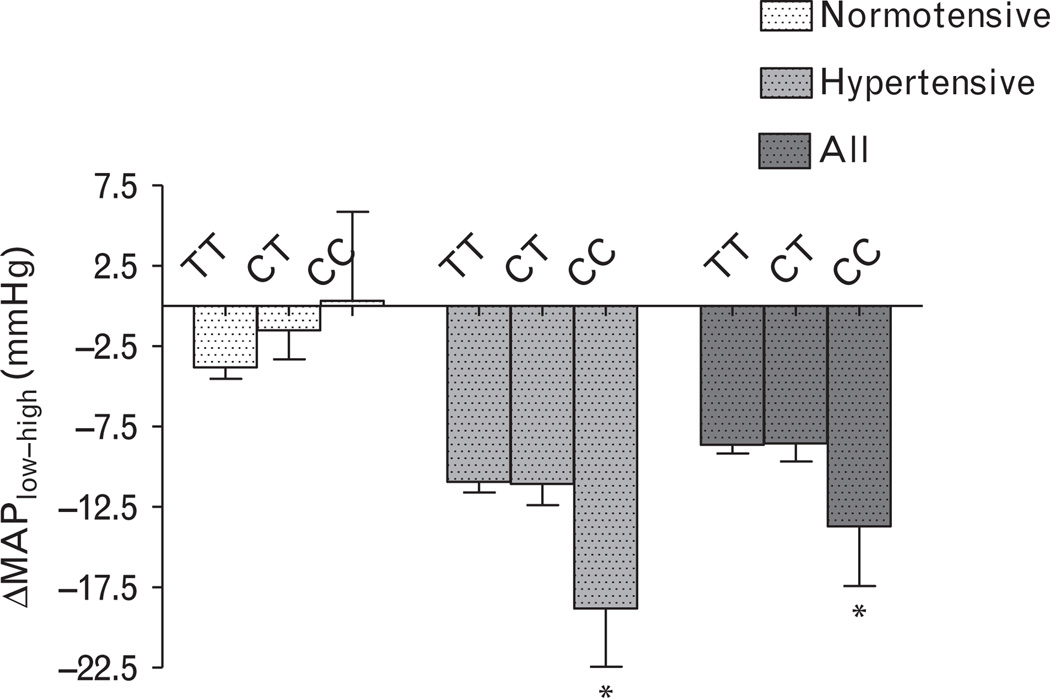
CYP4A11 T8590C genotype was associated with hypertension in whites. Among normotensive individuals, CYP4A11 T8590C genotype was associated with mean arterial pressure (MAP) during both high and low salt diets, such that there was no relationship between genotype and salt sensitivity of blood pressure. Among hypertensive individuals, CYP4A11 T8590C genotype did not associate with MAP during high salt intake, whereas MAP decreased with increasing number of C alleles during salt restriction. Consequently, among hypertensive individuals, change in MAP with salt restriction was greatest in individuals homozygous for the C allele (−10.9 ± 9.9, −11.1 ± 12.3, and −18.8 ± 12.0 mmHg in TT, CT, and CC groups, respectively, P = 0.02). In both normotensive and hypertensive individuals, individuals homozygous for the C allele exhibited an attenuated increase in renal blood flow during high salt. CYP4A11 genotype did not affect pressor responses to Angiotensin II in normotensive or hypertensive individuals.
Conclusion
The loss-of-function CYP4A11 8590C allele is associated with a diagnosis of hypertension and, in normotensive individuals, with higher blood pressure regardless of salt intake. Among hypertensive individuals, the C allele is associated with salt-sensitive blood pressure. Impaired renal vasodilation during high salt intake may contribute to salt sensitivity. Studies are needed to determine whether CYP4A11 T8590C genotype predicts responses to medications that affect sodium homeostasis in hypertensive individuals.
Keywords: blood pressure, CYP4A11, dietary salt intake, hypertension, renal blood flow, salt sensitivity
Introduction
Hypertension affects 74.5 million people in the United States and is estimated to contribute to 300 000 deaths per year [1]. Sixty percent of patients with hypertension have ‘salt-sensitive’ blood pressure (BP). Salt-sensitive hypertension is associated with an increased risk of cardiovascular events [2].
Studies in experimental models of hypertension implicate the P450 arachidonic acid monooxygenases in salt and water homeostasis. Epoxygenases CYP2C and CYP2J catalyze the conversion of arachidonic acid to epoxyeicosatrienoic acids (EETs), potent vasodilators and anti-inflammatory agonists, whereas the ω/ω-1 hydroxylases, CYP4A and CYP4F, catalyze the conversion of arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE) [3]. The 20-HETE metabolite can act in either a prohypertensive or antihypertensive manner dependent on its site-specific expression in the renal vasculature or tubule, respectively [4]. For example, 20-HETE promotes natriuresis by inhibiting tubular sodium transport [5,6]. 20-HETE causes vasoconstriction by inhibiting Ca2+-sensitive K+ channels [7] and enhances the effects of angiotensin (Ang) II and endothelin [8,9]. Opposing this vasoconstrictor effect, products of CYP4A induce expression of CYP2C and the formation of vasodilatory EETs in the kidney [10].
In the Dahl rat model, decreased urinary excretion of 20-HETE is associated with salt sensitivity and insulin resistance [11]. Genetic studies indicate that the ω/ω-1 hydroxylase CYP4A11 also regulates BP in humans. A loss-of-function CYP4A11 variant (8590C allele) characterized by reduced 20-HETE synthase activity has been associated with BP or hypertension in European and African–American populations, suggesting that 20-HETE exerts an antihypertensive effect in humans [12–15]. Nevertheless, the mechanism through which the loss-of-function C allele confers risk of hypertension is not known.
Laffer et al. [16] found no relationship between CYP4A11 T8590C genotype and salt or insulin sensitivity in a small study of 25 carefully phenotyped hypertensive individuals, but reported that the increase in urinary 20-HETE excretion in response to salt loading was diminished in salt-sensitive, insulin-resistant individuals with the C allele.
The current study tested the hypothesis that CYP4A11 T8590C genotype influences BP response to salt loading and vasoreactivity in a large group of well phenotyped individuals in the International Hypertension Pathotype (HyperPATH) study.
Methods
Individuals and protocol
Three hundred and thirty-two hypertensive and 147 normotensive individuals who had DNA available were drawn from the HyperPATH cohort [17,18]. A description of this cohort and study has been previously reported. Individuals were recruited from the general population of the Brigham and Women’s Hospital, Boston (n = 185); Hospital Broussais in France (n = 82); University of Utah (n = 134); Rome, Italy (n = 17); and Vanderbilt University (n = 61). The Institutional Review Board of each institution approved the study. All individuals gave written informed consent before enrollment and underwent a standardized protocol. Some characteristics of subsets of this population have been reported previously [17–19]. The present analyses of associations between phenotypes and CYP4A11 T8590C genotype are original, however.
All individuals had a screening history, physical and laboratory examination. Individuals with known or suspected secondary hypertension, diabetes, coronary artery disease, stroke, overt renal insufficiency (serum creatinine > 1.5 mg/dl), current tobacco or illicit drug use, alcohol intake more than 12 ounces per week, or other significant medical or psychiatric illnesses were excluded. Individuals with abnormal electrolytes or thyroid/liver function tests or ECG evidence of heart block, ischemia, or prior coronary events at the screening examination were excluded. Individuals were between ages 18 and 65 years and race was self-defined.
Hypertension was defined as seated DBP of at least 100 mmHg off antihypertensive medication, at least 90 mmHg with one medication, or treatment with at least two medications. Individuals with hypertension requiring more than four medications were excluded. Normotensive individuals, in addition to having BP less than 140/90 mmHg, reported no first-degree relatives diagnosed with hypertension before age 60. Individuals taking an angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or mineralocorticoid receptor antagonist were transitioned to amlodipine 3 months prior to study to minimize interference with assessment of the renin–angiotensin–aldosterone system. If needed, hydrochlorothiazide was added to control BP. All antihypertensive medications were discontinued 3 weeks before study.
Individuals then completed two dietary phases, a 5–7 day course of high sodium (≥200 mmol/day) and 7 days of low sodium (10 mmol/day) diets. Each diet was isocaloric, contained 100 mmol/day potassium, 20 mmol/day calcium, and was caffeine and alcohol-free. Sodium balance was confirmed by measuring sodium and creatinine in a 24-h urine collection (≥ 150 mmol urinary sodium for high sodium and ≤ 30 mmol for low sodium). On the final day of each diet, individuals were admitted to the General Clinical Research Center (GCRC) and remained fasting and supine overnight. Hemodynamic and hormone measurements were made between 0800 and 1000 h. During infusion studies, BP was measured at 5-min intervals using an automated device (DINAMAP, Critikon, Tampa, Florida, USA).
On the last day of high salt and low salt intake, the pressor, renal vasoconstrictor, and aldosterone responses to Ang II amide (3 ng/kg per min for 45 min) were measured in 432 individuals during high (TT:CT:CC = 270 : 97 : 14) and/or low salt (TT:TC:CC = 297 : 111 : 11) intake. The clearance of para-aminohippurate, infused as a bolus followed by a constant infusion, was used to assess renal plasma flow, as previously described [20]. The pressor response to graded doses norepinephrine was also measured in 181 of these individuals (130 : 41 : 10, respectively) during high salt intake, as a control for Ang II subtype 1 receptor-specific vasoconstriction.
Genotyping
Methods of laboratory analysis have been reported previously [20,21]. DNA was extracted from peripheral leukocytes using standard procedures. Genotyping was performed for the single single nucleotide polymorphism (SNP) T8590C (rs1126742) as a single plex on a Sequenom iPlex chemistry platform (Sequenom Inc., San Diego, California, USA). The genotyping success rate was 95.6% and repeat genotyping for 10% of the SNPs demonstrated concordance with the original genotype call. Genotyping results conformed to Hardy–Weinberg expectations in the study population.
Statistical analysis
Data are presented as means ± SD unless otherwise stated. Frequencies were compared among genotype groups using χ2-test or Fisher’s exact test. Continuous variables were compared among genotype groups using one-way analysis of variance or Kruskal–Wallis test. Within genotype group, the effect of salt was assessed using a paired t-test or signed-rank test, as appropriate. The pressor responses to Ang II or norepinephrine were analyzed using general linear model in which the within-subject variable was pressor dose and the between-subject variables were genotype, sex, quartile of age, and quartile of BMI. Analyses were completed using SPSS Statistics version 17.0 (SPSS Inc., Chicago, Illinois, USA). A P value less than 0.05 was considered significant.
Results
Baseline characteristics
Baseline characteristics appear in Table 1. Sex, age, and BMI were balanced across CYP4A11 T8590C genotype groups. The frequencies of the CYP4A11 8590 minor allele were similar to those previously reported for all racial groups. [12–15]. The frequency of the C allele was increased in black Americans compared with white Americans studied (29 versus 13%, P < 0.001). History of hypertension was associated with CYP4A11 T8590C genotype in white individuals (TT:CT:CC = 83.1 : 16.1 : 0.8% in normotensive individuals versus 72.4 : 25.7 : 2.0% in hypertensive individuals, P = 0.02) but not in black individuals studied. During high salt intake, mean arterial pressure (MAP) was 100.6 ± 18.7 and 100.7 ± 15.8 mmHg mmHg in the white American TT and CT genotype groups, respectively, versus 107.0 ± 13.6 mmHg in the CC genotype group. MAP was 104.0 ± 17.1, 104.1 ± 16.4, and 111.3 ± 24.9 mmHg in black American TT, CT, and CC genotype groups, respectively. Because the relationship between BP and CYP4A11 T8590C genotype trended in the same direction in blacks and whites in this study and in previous reports, [15] we grouped racial groups together for the remainder of the analyses. We also included the small number of Asian and individuals of other race to maximize power, although results of analyses were comparable if these individuals were excluded.
Table 1.
Subject characteristics by CYP4A11 T8590C genotype
| Characteristic | TT (n = 336) | CT (n = 128) | CC (n = 15) | P value genotype |
|---|---|---|---|---|
| Race (white:black:Asian:other) | 296:33:4:3 | 93:30:4:1 | 7:5:3:0 | <0.001 |
| Sex (M : F) | 175:161 | 76:52 | 7:8 | 0.32 |
| Age (years) | 45.7 ± 10.5 | 45.5 ± 10.5 | 44.7 ± 11.8 | 0.92 |
| BMI (kg/m2) | 27.4 ± 4.3 | 27.3 ± 4.0 | 27.8 ± 4.2 | 0.92 |
F, female; M, male.
Relationship between CYP4A11 T8590C genotype and blood pressure
Among normotensive individuals, CYP4A11 T8590C genotype was associated with BP during both high and low salt intake (Table 2). In normotensive individuals, the difference in BP measured during low salt intake compared with that measured during high salt intake (DMAPlow-high) was similar among genotype groups (Fig. 1). Among hypertensive individuals, CYP4A11 T8590C genotype did not statistically associate with MAP during high salt intake, whereas during low salt intake BP decreased as the number of C alleles increased. Thus, ΔMAPlow-high was significantly greater in individuals homozygous for the C allele (−10.9 ± 9.9, −11.1 ± 12.3, and −18.8 ± 12.0 mmHg in TT, CT, CC genotype groups, respectively, P = 0.02) among hypertensive individuals (Fig. 1). In addition, among hypertensive individuals, CYP4A11 T8590C genotype was significantly associated with salt-sensitive hypertension, defined as a decrease in MAP from high to low salt of at least 10 mmHg. Specifically, the prevalence of salt sensitivity was 50.2, 47.2, and 81.8% in hypertensive TT, CT, and CC genotype groups (P = 0.03 for CC genotype versus the other two genotype groups). When this analysis was stratified by race, the pattern was similar in blacks and whites with a prevalence of salt sensitivity of 45.5, 50.0, and 100% in black hypertensive TT, CT, and CC genotype groups and 51.3, 44.1, and 66.7% in white hypertensive TT, CT, and CC genotype groups, respectively.
Table 2.
Normotensive individuals
| TT (n = 109) |
CT (n = 34) |
CC (n = 4) |
P value genotype |
|
|---|---|---|---|---|
| SBP (mmHg) | ||||
| High salt | 110.5 ± 12.3* | 115.1 ± 12.2 | 118.8 ± 23.7 | 0.03 |
| Low salt | 106.6 ± 12.2 | 113.1 ± 12.8 | 113.3 ± 9.0 | 0.009 |
| DBP (mmHg) | ||||
| High salt | 66.2 ± 8.7* | 67.5 ± 11.0 | 68.0 ± 1.2 | 0.45 |
| Low salt | 63.7 ± 7.9 | 66.3 ± 8.7 | 64.8 ± 8.0 | 0.16 |
| MAP (mmHg) | ||||
| High salt | 81.7 ± 9.4* | 85.7 ± 12.3 | 84.9 ± 10.0 | 0.06 |
| Low salt | 77.7 ± 8.6 | 83.7 ± 10.4 | 85.3 ± 2.8 | 0.001 |
| Heart rate (beats/min) | ||||
| High salt | 58.9 ± 8.4* | 60.5 ± 11.9 | 56.0 ± 5.3 | 0.74 |
| Low salt | 60.7 ± 8.7 | 61.2 ± 10.7 | 61.8 ± 7.3 | 0.73 |
| 24-h sodium (mmol/day) | ||||
| High salt | 240.2 ± 77.9* | 242.2 ± 77.9* | 246.6 ± 118.9* | 0.87 |
| Low salt | 10.1 ± 8.1 | 8.5 ± 7.7 | 12.2 ± 10.2 | 0.60 |
| 24-h potassium (mmol/day) | ||||
| High salt | 77.0 ± 28.2 | 69.4 ± 24.5 | 51.7 ± 27.4 | 0.06 |
| Low salt | 72.4 ± 21.6 | 75.3 ± 24.4 | 80.6 ± 19.9 | 0.41 |
| Plasma renin activity (ng/min per ml) | ||||
| High salt | 0.40 ± 0.39* | 0.37 ± 0.29* | 0.22 ± 0.11* | 0.55 |
| Low salt | 4.36 ± 4.46 | 4.22 ± 2.90 | 5.10 ± 4.13 | 0.95 |
| Aldosterone (ng/dl) | ||||
| High salt | 4.08 ± 3.22* | 3.83 ± 2.00* | 2.51 ± 0.03* | 0.36 |
| Low salt | 19.24 ± 12.00 | 21.98 ± 12.57 | 19.98 ± 11.81 | 0.54 |
| RPF (ml/min per 1.73m2) | ||||
| High salt | 601.4 ± 141.5* | 573.5 ± 126.8 | 518.5 ± 53.0 | 0.24 |
| Low salt | 552.4 ± 134.2 | 544.3 ± 109.1 | 569.0 ± 106.1 | 0.89 |
MAP, mean arterial pressure; RPF, renal plasma flow.
P < 0.05 versus low salt.
Fig. 1.
Relationship between CYP4A11 T8590C genotype and change in mean arterial pressure (MAP) during low versus high salt intake. Data are presented as mean ± SEM. C indicates cytosine and T indicates thymidine. *P < 0.05 versus TT or CT genotype groups.
Relationship between CYP4A11 T8590C genotype and renal blood flow
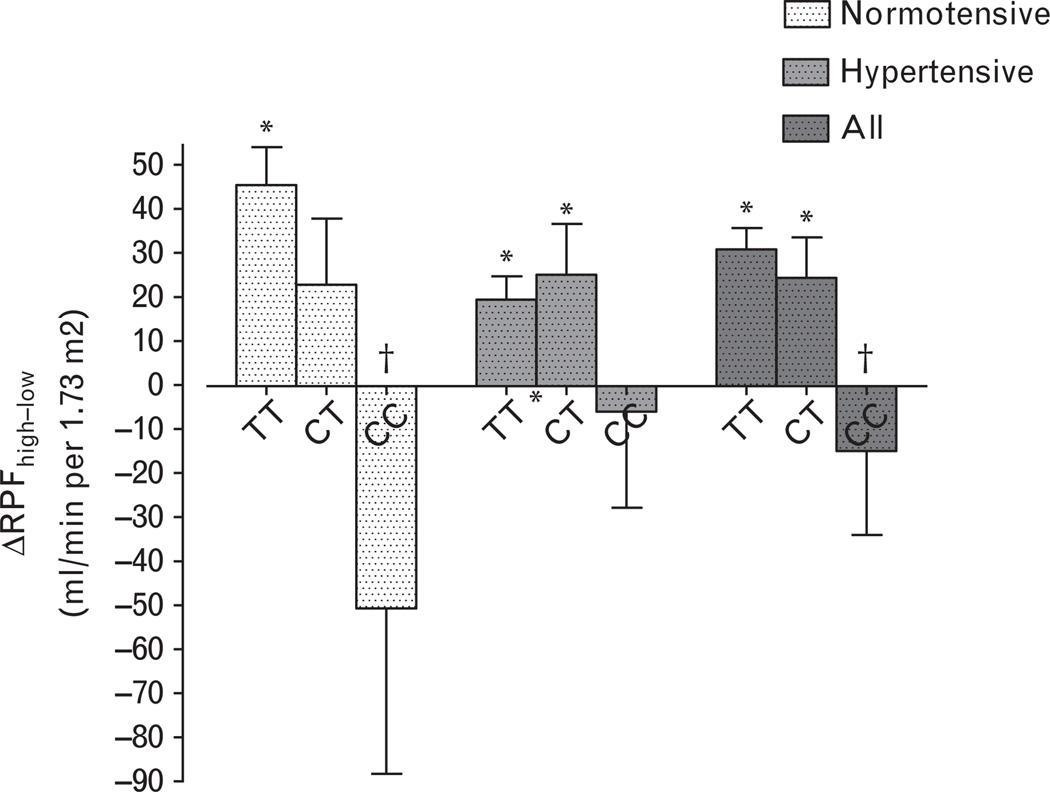
Twenty-four hour urine sodium excretion provided evidence of dietary compliance and was comparable among the three genotype groups in normotensive and hypertensive individuals (Tables 2 and 3). Aldosterone and plasma renin activity (PRA) were suppressed during high salt intake compared with low salt intake and comparable among genotype groups. Renal plasma flow was statistically similar among the genotype groups; however, CYP4A11 T8590C genotype affected the relationship between salt intake and renal plasma flow (Tables 2 and 3, Fig. 2). Renal plasma flow was significantly greater during high salt intake compared with during low salt intake in TT normotensive individuals and in TT and TC hypertensive individuals, but this pattern was reversed in normotensive or hypertensive individuals homozygous for the C allele (Fig. 2).
Table 3.
Hypertensive individuals
| TT (n = 227) |
CT (n = 94) |
CC (n = 11) |
P value genotype |
|
|---|---|---|---|---|
| SBP (mmHg) | ||||
| High salt | 148.7 ± 20.8* | 147.5 ± 19.7* | 151.0 ± 18.9* | 0.87 |
| Low salt | 133.7 ± 19.3 | 130.5 ± 20.2 | 123.9 ± 12.2 | 0.05 |
| DBP (mmHg) | ||||
| High salt | 89.6 ± 12.3* | 86.2 ± 11.6* | 91.2 ± 11.8* | 0.13 |
| Low salt | 81.1 ± 11.7 | 77.5 ± 11.7 | 77.8 ± 9.4 | 0.02 |
| MAP (mmHg) | ||||
| High salt | 110.1 ± 14.0* | 107.0 ± 13.2* | 113.0 ± 14.5* | 0.32 |
| Low salt | 99.4 ± 13.3 | 96.3 ± 13.7 | 94.2 ± 9.3 | 0.03 |
| Heart rate (beats/min) | ||||
| High salt | 65.4 ± 10.0 | 64.3 ± 10.6 | 62.3 ± 9.0 | 0.26 |
| Low salt | 66.2 ± 10.3 | 63.9 ± 10.1 | 63.7 ± 7.9 | 0.07 |
| 24-h sodium (mmol/day) | ||||
| High salt | 221.5 ± 74.8* | 226.7 ± 85.9* | 225.6 ± 74.5* | 0.62 |
| Low salt | 15.7 ± 11.7 | 15.9 ± 10.2 | 10.5 ± 8.3 | 0.62 |
| 24-h potassium (mmol/day) | ||||
| High salt | 71.1 ± 25.2 | 66.6 ± 20.9 | 65.3 ± 23.8 | 0.12 |
| Low salt | 72.4 ± 20.0 | 71.4 ± 21.2 | 76.6 ± 23.9 | 0.92 |
| Plasma renin activity (ng/min per ml) | ||||
| High salt | 0.53 ± 0.57* | 0.50 ± 0.53* | 0.45 ± 0.30* | 0.61 |
| Low salt | 2.93 ± 3.18 | 2.75 ± 2.19 | 3.49 ± 3.91 | 0.99 |
| Aldosterone (ng/dl) | ||||
| High salt | 5.56 ± 4.31* | 5.45 ± 3.78* | 4.67 ± 2.87* | 0.59 |
| Low salt | 16.44 ± 11.50 | 17.02 ± 12.00 | 18.35 ± 10.00 | 0.55 |
| RPF (ml/min per 1.73m2) | ||||
| High salt | 517.8 ± 102.9* | 519.0 ± 117.2* | 508.8 ± 90.1 | 0.93 |
| Low salt | 493.3 ± 104.3 | 497.7 ± 117.6 | 519.9 ± 101.1 | 0.55 |
MAP, mean arterial pressure; RPF, renal plasma flow.
P < 0.05 versus low salt.
Fig. 2.
Relationship between CYP4A11 T8590C genotype and change in renal plasma flow (ΔRPF) during high versus low salt intake. Data are presented as mean ± standard error of the mean. C indicates cytosine and T indicates thymidine. *P < 0.05 for the comparison of RPF during high salt versus during low salt. †P < 0.05 versus TT and CT genotype groups combined.
CYP4A11 T8590C genotype and the pressor effects of Ang II and norepinephrine
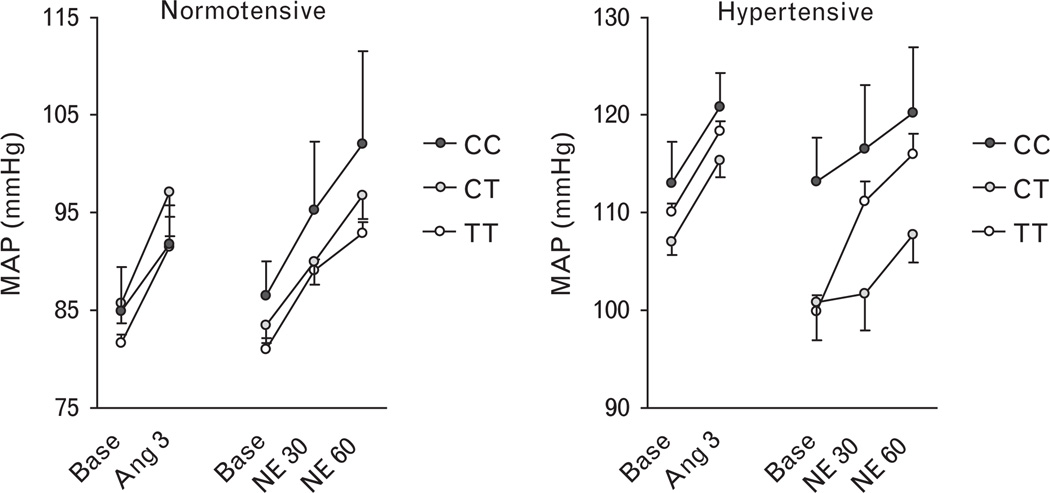
Ang II significantly increased MAP during both high salt (Fig. 3) and low salt (not shown) conditions (P < 0.001 for effect of Ang II in either normotensive or hypertensive individuals), as previously described [22]. CYP4A11 T8590C genotype did not affect the pressor response to Ang II in either normotensive or hypertensive individuals, regardless of salt intake (all P > 0.05 for genotype × Ang II interaction). CYP4A11 genotype also did not affect the renal vasoconstrictor response to Ang II (shown for high salt in supplemental figure, http://links.lww.com/HJH/A112) or the aldosterone response to Ang II (shown for low salt in supplemental figure, http://links.lww.com/HJH/A112).
Fig. 3.
Relationship between CYP4A11 T8590C genotype and the pressor response to intravenous angiotensin at a dose of 3 ng/min (Ang 3) or norepinephrine at doses of 30 and 60 µg/min (NE30 and NE60) during high salt (200 mmol/day) in normotensive (left panel) and hypertensive (right panel) individuals. Data are presented as mean ± SEM. C indicates cytosine and T indicates thymidine.
Norepinephrine also significantly increased MAP in normotensive and hypertensive individuals (Fig. 3). CYP4A11 T8590C genotype did not affect the pressor response to norepinephrine in either normotensive or hypertensive individuals, regardless of salt intake salt intake (P = 0.138 for genotype × norepinephrine interaction, P = 0.067 for CT versus TT genotype).
Discussion
The loss-of-function CYP4A11 8590C allele has been associated with higher BP or a diagnosis of hypertension in population studies [12–15]. In this study, we confirm that the CYP4A11 T8590C genotype is associated with hypertension, and with BP among normotensive individuals. We demonstrate for the first time that, among hypertensive individuals, homozygosity for the C allele is associated with salt-sensitive BP. We also report that homozygosity for the C allele is associated with relative renal vasoconstriction during high salt intake. Of note, we found no relationship between CYP4A11 T8590C genotype and Ang II or norepinephrine-induced vasoconstriction.
These findings resemble observations in the Dahl salt-sensitive rat model of hypertension. CYP4A genotype segregates with salt-sensitive hypertension in this rodent model [11]. Decreased ω/ω-1 hydroxylase activity is associated with salt-sensitive hypertension, whereas induction of CYP4A increases pressure natriuresis in the Dahl salt-sensitive rat [23]. Conversely, pharmacological inhibition of 20-HETE production in the outer medulla of the kidney in Lewis rats renders them salt-sensitive [24].
Interestingly, we found that the relationship between CYP4A11 T8590C genotype and salt sensitivity of BP differed in the normotensive and hypertensive individuals studied. Among normotensive individuals without a family history of hypertension, CYP4A11 genotype was associated with BP and independent of salt intake. In hypertensive individuals, sensitivity of BP resulted from a greater decrease in BP during low salt intake with an increasing number of C alleles. This difference in salt-sensitivity between normotensive and hypertensive individuals resembles that described by Weinberger et al. [25], who reported that normotensive and hypertensive individuals responded similarly to salt loading, but that hypertensive individual exhibited a greater decrease in BP during salt restriction. An important implication of this finding in the present study is that the relationship between CYP4A11 T8590C genotype and BP in large epidemiological studies may depend on the population studied and on sodium intake.
It is important to note that although the CYP4A11 CC genotype was associated with salt-sensitive hypertension, approximately one-half of individuals in the hypertensive TT and CT genotype groups also had salt-sensitive BP. Likewise, the prevalence of salt-sensitive hypertension in the population [2] far exceeds the frequency of the CC genotype (3.3% of all hypertensive individual in this study). Recent studies suggest, however, that even rare variants can contribute substantially to common complex traits such as salt-sensitive BP [26].
20-HETE also inactivates calcium-sensitive potassium channels in vascular smooth muscle [7], causes renal vasoconstriction in rodent models, and enhances vascular reactivity to constrictors such as Ang II, endothelin, and norepinephrine [9,27,28]. We found no effect of CYP4A11 T8590C genotype on the constrictor response to systemic Ang II or norepinephrine infusion. Instead, we found that renal plasma flow was not increased appropriately during salt loading in individuals homozygous for the C allele. Although 20-HETE acts as a vasoconstrictor, metabolites of CYP4A induce CYP2C and the renal formation of vasodilatory EETs in rodent models [10] Our data suggest that the predominant effect of the loss-of-function CYP4A11 8590C allele in humans is relative renal vasoconstriction, consistent with decreased formation of EETs.
Laffer et al. [16] previously reported an association between CYP4A11 T8590C genotype and the aldoster-one-to-renin ratio in individuals carrying the C allele compared with those with the TT genotype. In the present study, aldosterone and PRA were appropriately suppressed during high salt intake in all groups, consistent with increased volume, but were not significantly different among the genotype groups. Although we too noted a somewhat higher aldosterone-to-renin ratio in homozygotes for the C allele during high salt intake, this was attributable to a trend toward decreased PRA in carriers of the C allele and was not significant.
A few limitations merit notation. The relatively small number of black individuals makes it impossible to draw conclusions regarding the relationship between CYP4A11 T8590C genotype and hypertension in this group, although one other study has shown an association [15]. In addition, because 20-HETE and the EETs undergo oxidative degradation over time and because urine samples had been stored for several years, we did not assess the effect of salt loading or CYP4A11 T8590C genotype on 20-HETE or EET excretion. Ward et al. [29] have reported previously that the C allele is associated with decreased urinary 20-HETE excretion. On the contrary, the ability to assess genotype–phenotype relationships in a large number of individuals who had been carefully phenotyped with respect to salt-sensitivity and vascular reactivity outweighs these limitations.
In conclusion, prior reports indicate an association of BP and hypertension with the loss-of-function variant 8590C of CYP4A11 in humans. The present study confirms this finding and indicates that homozygosity for the C allele results in altered renal vascular dilation during high dietary salt intake. In addition, among hypertensive individuals, homozygosity for the C allele is associated with salt-sensitive BP. Studies are needed to determine whether treatment strategies that improve renal vascular function and/or that affect natriuresis have preferential benefit in hypertensive individuals who are homozygous for the CYP4A11 8590C allele.
Acknowledgements
This work was supported by National Institutes of Health grants DK038226, HL060906, RR024975, HL084236, LM008748, RR025758, RR002635, and HL055000.
Abbreviations
- 20-HETE
20-hydroxyeicosatetraenoic acid
- C allele
cytosine allele
- CC genotype
homozygous for cytosine on each of two inherited alleles
- CT genotype
heterozygous, with one cytosine and one thymidine allele
- EETs
epoxyeicosatrienoic acids
- HyperPATH
hypertension pathotype
- MAP
mean arterial pressure
- T allele
thymidine allele
- TT genotype
homozygous for thymidine on each of two inherited alleles
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, et al. Executive summary: heart disease and stroke statistics – 2010 update – a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 Part 2):429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 3.Capdevila JH, Falck JR. The CYP P450 arachidonic acid monooxygenases: from cell signaling to blood pressure regulation. Biochem Biophys Res Commun. 2001;285:571–576. doi: 10.1006/bbrc.2001.5167. [DOI] [PubMed] [Google Scholar]
- 4.Sarkis A, Lopez B, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids in hypertension. Curr Opin Nephrol Hypertens. 2004;13:205–214. doi: 10.1097/00041552-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Imig JD. Epoxygenase metabolites. Epithelial and vascular actions. Mol Biotechnol. 2000;16:233–251. doi: 10.1385/MB:16:3:233. [DOI] [PubMed] [Google Scholar]
- 6.Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, Roman RJ. Inhibition of the formation of EETs and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R58–R68. doi: 10.1152/ajpregu.00713.2003. [DOI] [PubMed] [Google Scholar]
- 7.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, et al. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270(1 Pt 2):R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 8.Oyekan A, Balazy M, McGiff JC. Renal oxygenases: differential contribution to vasoconstriction induced by ET-1 and ANG II. Am J Physiol. 1997;273(1 Pt 2):R293–R300. doi: 10.1152/ajpregu.1997.273.1.R293. [DOI] [PubMed] [Google Scholar]
- 9.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, et al. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman RJ, Alonso-Galicia M, Wilson TW. Renal P450 metabolites of arachidonic acid and the development of hypertension in Dahl salt-sensitive rats. Am J Hypertens. 1997;10(5 Pt 2):63S–67S. [PubMed] [Google Scholar]
- 12.Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, et al. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 13.Mayer B, Lieb W, Gotz A, Konig IR, Aherrahrou Z, Thiemig A, et al. Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg echocardiographic substudy. Hypertension. 2005;46:766–771. doi: 10.1161/01.HYP.0000182658.04299.15. [DOI] [PubMed] [Google Scholar]
- 14.Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, et al. The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension. 2008;52:373–380. doi: 10.1161/HYPERTENSIONAHA.108.114199. [DOI] [PubMed] [Google Scholar]
- 15.Gainer JV, Lipkowitz MS, Yu C, Waterman MR, Dawson EP, Capdevila JH, et al. Association of a CYP4A11 variant and blood pressure in black men. J Am Soc Nephrol. 2008;19:1606–1612. doi: 10.1681/ASN.2008010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laffer CL, Gainer JV, Waterman MR, Capdevila JH, Laniado-Schwartzman M, Nasjletti A, et al. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension. 2008;51:767–772. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins WS, Hunt SC, Williams GH, Tolpinrud W, Jeunemaitre X, Lalouel JM, et al. Genotype-phenotype analysis of angiotensinogen polymorphisms and essential hypertension: the importance of haplotypes. J Hypertens. 2010;28:65–75. doi: 10.1097/HJH.0b013e328332031a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun B, Williams JS, Pojoga L, Chamarthi B, Lasky-Su J, Raby BA, et al. Renin gene polymorphism: its relationship to hypertension, renin levels and vascular responses. J Renin Angiotensin Aldosterone Syst. 2011 doi: 10.1177/1470320311405873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gumieniak O, Perlstein TS, Williams JS, Hopkins PN, Brown NJ, Raby BA, et al. Ala92 type 2 deiodinase allele increases risk for the development of hypertension. Hypertension. 2007;49:461–466. doi: 10.1161/01.HYP.0000256295.72185.fd. [DOI] [PubMed] [Google Scholar]
- 20.Schnurr E, Lahme W, Kuppers H. Measurement of renal clearance of inulin and PAH in the steady state without urine collection. Clin Nephrol. 1980;13:26–29. [PubMed] [Google Scholar]
- 21.Williams GH, Dluhy RG, Lifton RP, Moore TJ, Gleason R, Williams R, et al. Nonmodulation as an intermediate phenotype in essential hypertensives. Hypertension. 1992;20:788–796. doi: 10.1161/01.hyp.20.6.788. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins PN, Hunt SC, Jeunemaitre X, Smith B, Solorio D, Fisher NDL, et al. Angiotensinogen genotype affects renal and adrenal responses to angiotensin II in essential hypertension. Circulation. 2002;105:1921–1927. doi: 10.1161/01.cir.0000014684.75359.68. [DOI] [PubMed] [Google Scholar]
- 23.Roman RJ, Ma YH, Frohlich B, Markham B. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension. 1993;21(6 Pt 2):985–988. doi: 10.1161/01.hyp.21.6.985. [DOI] [PubMed] [Google Scholar]
- 24.Moreno C, Maier KG, Hoagland KM, Yu M, Roman RJ. Abnormal pressure-natriuresis in hypertension: role of cytochrome P450 metabolites of arachidonic acid. Am J Hypertens. 2001;14(6 Pt 2):90S–97S. doi: 10.1016/s0895-7061(01)02075-1. [DOI] [PubMed] [Google Scholar]
- 25.Weinberger MH, Miller TZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8 Suppl II:127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 26.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, et al. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet. 2008;40:592–599. doi: 10.1038/ng.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35(1 Pt 2):307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Roman RJ, Falck JR, de la Cruz L, Lombard JH. Effects of high-salt diet on CYP450-4A omega-hydroxylase expression and active tone in mesenteric resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1557–H1565. doi: 10.1152/ajpheart.00755.2004. [DOI] [PubMed] [Google Scholar]
- 29.Ward NC, Tsai IJ, Barden A, van Bockxmeer FM, Puddey IB, Hodgson JM, et al. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;51:1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]