Abstract
Chronic low-grade inflammation has emerged as a key contributor to the pathogenesis of Polycystic Ovary Syndrome (PCOS). A dietary trigger such as glucose is capable of inciting oxidative stress and an inflammatory response from mononuclear cells (MNC) of women with PCOS, and this phenomenon is independent of obesity. This is important because MNC-derived macrophages are the primary source of cytokine production in excess adipose tissue, and also promote adipocyte cytokine production in a paracrine fashion.
The proinflammatory cytokine tumor necrosis factor-α (TNFα) is a known mediator of insulin resistance. Glucose-stimulated TNFα release from MNC along with molecular markers of inflammation are associated with insulin resistance in PCOS. Hyperandrogenism is capable of activating MNC in the fasting state, thereby increasing MNC sensitivity to glucose; and this may be a potential mechanism for promoting diet-induced inflammation in PCOS.
Increased abdominal adiposity is prevalent across all weight classes in PCOS, and this inflamed adipose tissue contributes to the inflammatory load in the disorder. Nevertheless, glucose ingestion incites oxidative stress in normal weight women with PCOS even in the absence of increased abdominal adiposity.
In PCOS, markers of oxidative stress and inflammation are highly correlated with circulating androgens. Chronic suppression of ovarian androgen production does not ameliorate inflammation in normal weight women with the disorder. Furthermore, in vitro studies have demonstrated the ability of pro-inflammatory stimuli to upregulate the ovarian theca cell steroidogenic enzyme responsible for androgen production. These findings support the contention that inflammation directly stimulates the polycystic ovary to produce androgens.
Keywords: Hyperandrogenism, inflammation, oxidative stress, insulin resistance, ovarian dysfunction, abdominal adiposity
Introduction
Polycystic Ovary Syndrome (PCOS) is characterized by hyperandrogenism, chronic oligo- or anovulation and polycystic ovaries [1]. Hyperandrogenism in particular, is a hallmark feature of PCOS because it is strongly implicated in the genesis of the disorder [2]; and is also associated with metabolic derangements that contribute to the underlying pathophysiology [3]. Consequently, the AE-PCOS Society maintains that the presence of hyperandrogenism is required to diagnose PCOS in consort with either chronic oligo- or anovulation or the presence of polycystic ovarios [4].
It is now clear that PCOS is a proinflammatory state, and emerging data suggests that chronic low-grade inflammation underpins the development of metabolic aberration and ovarian dysfunction in the disorder [5,6]. Most importantly, there is a strong association between hyperandrogenism and inflammation in PCOS that has been the focus of ongoing investigation [5,7-10]. Novel data presented herin suggests that in PCOS, diet-induced inflammation may directly invoke hyperandrogenism.
Insulin resistance and defective insulin signaling in PCOS
Insulin resistance is a common feature of PCOS affecting 50-70% of women with the disorder. The compensatory hyperinsulinemia is considered to be a promoter of the hyperandrogenism and chronic oligo- or anovulation. Android obesity is evident in ~52%-64% of women with PCOS, and is independently associated with metabolic abnormalities such as insulin resistance [1,11]. Abdominal adiposity in particular is present in ~30% of normal weight women with PCOS [12].
Circulating levels of the proinflammatory cytokine tumor necrosis factor-α (TNFα) are elevated in obesity, and are also elevated in PCOS independent of obesity [13,14]. In fact, the discovery of TNFα elevations in PCOS served as the initial clue that PCOS is a proinflammatory state. In obesity-related diabetic syndromes, TNFα is a known mediator of insulin resistance by causing increased serine phosphorylation of insulin receptor substrate-1 (IRS-1) in insulin sensitive tissues [15]. This leads to decreased expression of GLUT 4, the insulin sensitive glucose transport proteín [16].
The insulin receptor in PCOS is genetically and functionally normal. Insulin resistance in PCOS is also caused by a post-receptor defect in insulin signaling with increased serine phosphorylation implicated as the cause of decreased insulin-stimulated IRS-1 activation and decreased GLUT 4 expression [17,18]. Thus, the ability of TNFα to stimulate increased serine phosphorylation makes it an ideal candidate for initiating these molecular events in PCOS.
Although insulin resistance per se is considered to be the responsible entity for hyperandrogenism in PCOS, this mechanism does not explain the hyperandrogenism evident in women with the disorder without insulin resistance and/or excess adiposity. Inflammation may be the common thread in the induction of insulin resistance that is related to PCOS per se, or to superimposed excess adiposity. This concept raises the possibility that inflammation may be capable of directly inducing hyperandrogenism in PCOS.
Chronic low-grade inflammation in PCOS
There is a genetic basis for the chronic low-grade inflammation observed in PCOS. Several proinflammatory genotypes including those that encode TNF-α, and the type 2 TNF receptor as well as interleukin-6 (IL-6) and its signal transducer are associated with PCOS [19-21].
The majority of studies addressing the status of chronic low-grade inflammation in PCOS have focused on the measurement of circulating C-reactive protein (CRP) using high-sensitivity assays. CRP is an acute phase reactant produced by the liver following stimulation by IL-6, the endocrine cytokine originating from adipose tissue in this instance [22]; CRP is also directly produced by adipose tissue [23]. CRP levels >3 mg/L are equally predictive of a cardiovascular event compared to the ATP III criteria for metabolic syndrome [24]. CRP also plays a functional role by promoting the uptake of lipids into foamy macrophages within atherosclerotic plaques [25].
A recent meta-analysis revealed that CRP is the most reliable circulating marker of chronic low-grade inflammation in PCOS [26]. However, the CRP elevation in normal weight women with PCOS (<3.0 mg/L) is still much less compared to the obese (>3.0 mg/L) regardless of whether or not they have PCOS [27,28]. Thus, CRP elevations attributable to PCOS are obscured in the presence of obesity, and are below the range to predict metabolic or cardiovascular risk. This suggests that in PCOS, a single static circulating marker may not be reflective of inflammation at the molecular level.
The role of diet-induced inflammation in PCOS
Circulating mononuclear cells (MNC) and MNC-derived macrophages in tissue produce proinflammatory cytokines such as TNFα and IL-6 [29]. While TNFα is a known mediator of insulin resistance, the impact of IL-6 on insulin resistance is variable [15,30,31]. Moreover, IL-6 is clearly involved in the promotion of atherogenesis [32].
Circulating mononuclear cells utilize glucose during glycolysis for mitochondrial respiration. Some glucose is diverted to the hexose monophosphate shunt to generate nicotinamide adenine dinucleotide phosphate (NADPH) [33]. Membrane-bound NADPH oxidase is activated by translocation of a cytosol component known as p47phox to the cell membrane [34,35]. Oxidation of NADPH by NADPH oxidase generates superoxide, a reactive oxygen species (ROS) that induces oxidative stress [36]. This in turn activates the transcription factor, nuclear factor κB (NFκB) by its dissociation from the inhibitory proteín, inhibitory κB (IκB). Activated NFκB translocates to the nucleus to promote TNFα and IL-6 gene transcription [15,37].
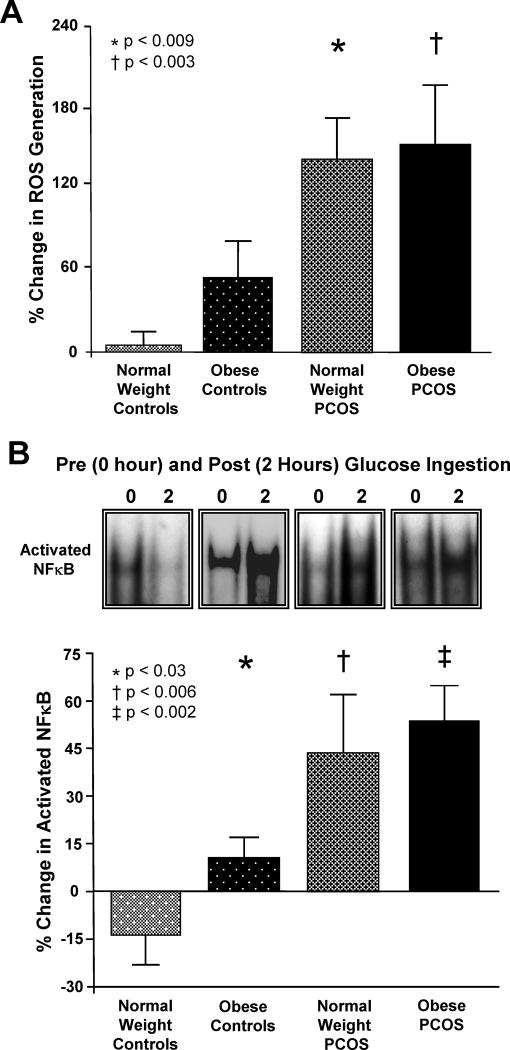
In PCOS, glucose ingestion induces an inflammatory response as evidenced by increased ROS-related oxidative stress, and increased NFκB activation that are independent of obesity (Fig. 1A-B) [5,9]. The release of TNFα and IL-6 from circulating MNC is also altered in PCOS by glucose ingestion in vivo, and by glucose exposure in vitro [7,8]. In addition, these markers of oxidative stress and inflammation are associated with glucose-challenged measures of insulin sensitivity and/or fasting measures of insulin resistance [5,8,9]. Thus, diet-induced inflammation in PCOS culminates in proinflammatory signaling known to be involved in the development of insulin resistance and atherogenesis.
Figure 1.
(A) The change from baseline (%) in ROS generation from mononuclear cells (MNC) when fasting samples (pre) were compared to the samples collected 2 hours after glucose ingestion (post). * the percent (%) change in ROS generation in normal weight women with PCOS was greater than that of normal weight ovulatory controls, P < 0.009. † the % change in ROS generation in obese women with PCOS was greater than that of normal weight ovulatory controls, P < 0.003. (B) Representative EMSA bands from the 4 study groups showing the change in quantity of NFκB in nuclear extracts from MNC when fasting samples (pre) were compared to the samples collected 2 hours after glucose ingestion (post). Densitometric quantitative analysis of intranuclear NFκB protein content in MNC. Compared to normal weight ovulatory controls, the % change in NFκB activation was significantly greater in obese ovulatory controls (*, P<0.03), in normal weight women with PCOS (†, P<0.006), and in obese women with PCOS (‡, P<0.002). Adapted from González et al. [5,9], with permission. Copyright The Endocrine Society, 2006.
The influence of adipose tissue on inflammation in PCOS
The proinflammatory state of obesity contributes to the promotion of insulin resistance and atherogenesis when present in PCOS. Hypoxia-related adipocyte death in response to adipose tissue expansion promotes an influx of MNC into the stromal-vascular compartment [38]. These MNC alter morphologically to become resident macrophages. MNC-derived macrophages are the prime source of TNFα and IL-6 production in adipose tissue, and also stimulate cytokine production in adipocytes through paracrine mechanisms [39].
The expression of molecular markers of inflammation is similar in adipose tissue of overweight women regardless of PCOS status, and there is lower expression in normal weight women with PCOS compared to overweight individuals [40]. Thus, the inflammatory load derived from adipose tissue in PCOS is in proportion to body mass, but is not uniquely greater compared to that of individuals without PCOS. Even in the absence of frank obesity, increased abdominal adiposity is prevalent across all weight classes in PCOS [12].
Until recently, it remained unclear whether increased abdominal adiposity was the cause of the proinflammatory state in normal weight women with PCOS. However, it is now known that markers of oxidative stress such as MNC-derived ROS generation and p47phox protein content increase in response to glucose ingestion in normal weight women with PCOS without increased abdominal adiposity [41]. This population is also insulin resistant, and exhibits higher testosterone levels and lower CRP levels compared to normal weight women with PCOS who have increased abdominal adiposity. Nevertheless, markers of oxidative stress are still greater in normal weight women with PCOS who have increased abdominal adiposity. There are also associations between CRP and abdominal adiposity, and between markers of oxidative stress and circulating androgens in normal weight women with PCOS. Thus, glucose-stimulated oxidative stress is independent of increased abdominal adiposity in normal weight women with PCOS, but increased abdominal adiposity contributes to the inflammatory load in the disorder. In addition, testosterone production in PCOS is greater in the absence of increased abdominal adiposity while CRP elevations are mostly an adiposity-related phenomenon.
The relationship between inflammation and hyperandrogenism in PCOS
Circulating and molecular markers of oxidative stress and inflammation are highly correlated with circulating androgens [5,7-10]. These findings raise the possibility that in PCOS, either hyperandrogenemia pre-activate MNC to account for the hyperglycemia-induced inflammation, or conversely that glucose-stimulated inflammation promotes ovarian androgen production in PCOS. There is data to support that both mechanisms may occur [42-44].
Induction of hyperandrogenism in normal weight ovulatory women
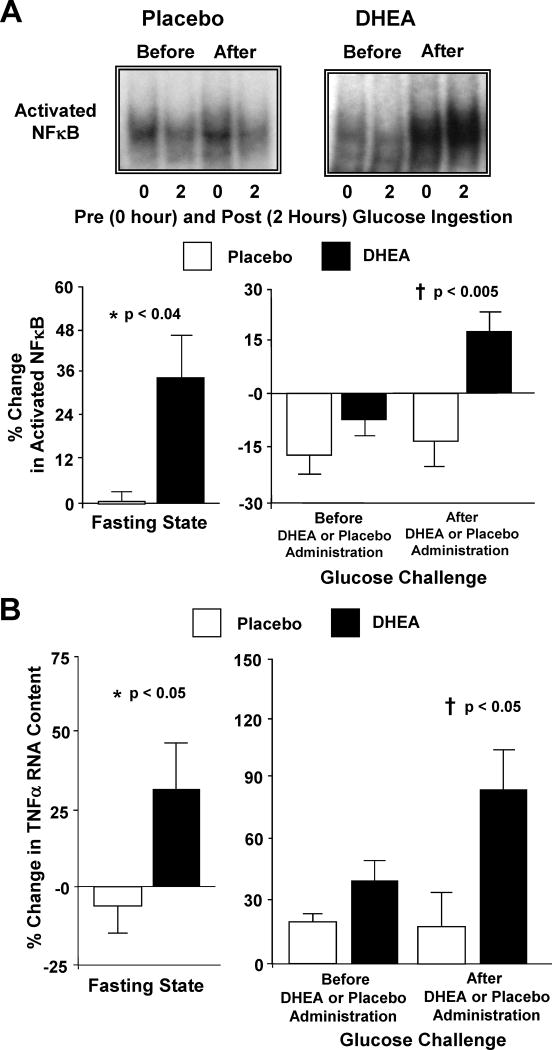
In PCOS, MNC are pre-activated as evidenced by increased ROS generation and activated NFκB in the fasting state [45-46]. This accounts for the increased MNC sensitivity to glucose ingestion in the disorder. In contrast, MNC of normal weight ovulatory women are not sensitive to hyperglycemia, and do not exhibit an inflammatory response to glucose ingestion [5,7-9]. Acute oral androgen administration raises circulating androgen levels in normal weight ovulatory women to the range observed in PCOS. In the process, ROS-related oxidative stress, activated NFκB and TNFα RNA content from MNC increase in the fasting state, and in response to glucose ingestion (Fig. 2A-B) [42,43]. Thus, hyperandrogenemia to the degree present in PCOS, promotes MNC activation and increases MNC sensitivity to glucose ingestion. This suggests that hyperandrogenism, the hallmark feature of PCOS, is the progenitor of diet-induced inflammation in the disorder.
Figure 2.
(A) Representative EMSA bands from the two study groups showing the quantity of NFκB in nuclear extracts from mononuclear cells (MNC) in samples collected in the fasting state (0) and 2 hours post-glucose ingestion (2), before and after treatment with DHEA or placebo. Densitometric quantitative analysis comparing the change from baseline (%) in MNC-derived activated NFκB between the two study groups for fasting samples before and after (before versus after, 0) DHEA or placebo administration (left panel); and for fasting and 2 hour post-glucose ingestion samples for each OGTT (before, 0 versus 2; after, 0 versus 2) as a measure of the response to glucose challenge before and after DHEA or placebo administration (right panel). After DHEA administration, the % change in activated NFκB was significantly greater compared to placebo in the fasting state (*, P<0.04), and in response to glucose ingestion (†, P<0.005). (B) Comparison between groups of the change from baseline (%) in TNFα mRNA content in MNC for fasting samples before and after (before versus after, 0) DHEA or placebo administration (left panel); and for fasting and 2 hour post-glucose ingestion samples for each OGTT (before, 0 versus 2; after, 0 versus 2) as a measure of the response to glucose challenge before and after DHEA or placebo administration (right panel). Values are normalized to 28S rRNA expression. After DHEA administration, the percent (%) change in TNFα mRNA transcripts significantly increased compared to placebo in the fasting state (*, P<0.05), and in response to glucose ingestion (†, P<0.05). Adapted from González et al. [43], with permission. Copyright The American Physiological Society, 2011.
Suppression of androgens in women with PCOS and ovulatory women
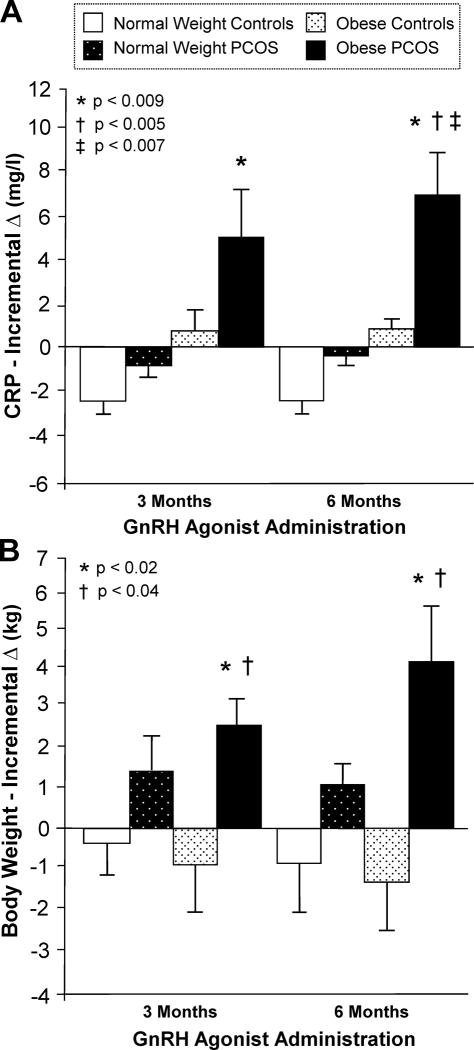
Alteration in circulating CRP reflects exacerbation or amelioration of inflammation in clinical practice making it a useful measurement of inflammatory load [47-49]. CRP and body weight increase in obese women with PCOS, but remain unaltered in normal weight women with PCOS in response to chronic gonadotropin releasing hormone agonist (GnRH) agonist-induced androgen suppression. CRP is also unaltered in obese ovulatory women, but decreases in lean ovulatory women without significant weight change in either group during similar treatment (Fig. 3A-B) [44].
Figure 3.
The incremental change (Δ) from baseline in (A) serum C-reactive protein (CRP) levels and (B) body weight after 3 and 6 months of gonadotropin-releasing hormone (GnRH) agonist administration. The incremental Δ in CRP was significantly (*, P<0.009) higher in obese women with PCOS compared to normal weight ovulatory controls after 3 and 6 months of GnRH agonist treatment; and compared to obese ovulatory controls (†, P<0.005) and normal weight ovulatory controls (‡, P<0.007) after 6 months of treatment. The incremental Δ in body weight was significantly higher in obese women with PCOS compared to obese ovulatory controls (*, P<0.02) and normal weight ovulatory controls (†, P<0.04) after 3 and 6 months of GnRH agonist treatment. González et al. [44].
The ability of elevated circulating androgens to promote lipolysis may be responsible for the rise in CRP following chronic GnRH agonist administration in obese women with PCOS [50]. Testosterone in particular, is known to stimulate catecholamine-induced hormone sensitive lipase activity, which in turn, limits adipose tissue expansion [51]. A decrease in lipolysis following androgen suppression to castrate levels may explain the progressive weight gain in obese women with PCOS that most likely represents expansion of the adipose tissue compartment during chronic of GnRH agonist administration. In fact, circulating free fatty acids (FFA) decline in obese women with PCOS during chronic GnRH agonist administration [43]. Subsequent increases in IL-6 production from inflamed adipose tissue could result in the progressive rise in CRP observed in these individuals during this period.
Circulating androgens have a limited effect on lipolysis in normal weight women with PCOS and obese ovulatory women. Moreover, catecholamine resistance of subcutaneous adipose tissue precluding adequate induction of hormone-sensitive lipase activity is well documented in these individuals [52]. This phenomenon can limit expansion of inflamed adipose tissue to explain their unaltered levels of CRP and FFA and lack of significant change in weight during GnRH agonist administration. Thus, the factors responsible for limitation of fat mass in normal weight women with PCOS and obese ovulatory women are not dependent on the release of control by circulating androgens. In contrast, the decline in CRP in normal weight ovulatory women following GnRH agonist-induced androgen suppression corroborates the studies showing an increase in inflammatory load following androgen administration in this population [42].
These data demonstrate that hyperandrogenism in PCOS exerts an anti-inflammatory effect when obesity is present, but does not promote inflammation in the disorder. These unique observations support the contention that androgens have a pleiotropic effect on inflammation dependent on the combination of PCOS and weight status present in a given individual.
Induction of androgen production capacity by inflammation
Inflammation may be the promoter of hyperandrogenism in the disorder. Infiltration of the ovary by MNC-derived macrophages has been previously demonstrated [53]. In vitro studies show that CYP17, the ovarian steroidogenic enzyme responsible for androgen production is upregulated by proinflammatory stimuli, and inhibited by anti-inflammatory agents such as resveratrol and statins [6,54]. TNFα is a proinflammatory cytokine capable of stimulating in vitro proliferation of androgen producing theca cells [55]. It is possible that MNC recruited into the polycystic ovary may cause a local inflammatory response that stimulates ovarian androgen production in women with PCOS.
Conclusions
In PCOS, a dietary trigger such as glucose is capable of inducing oxidative stress to stimulate an inflammatory response even in the absence of excess adiposity. Hyperandrogenism may be the progenitor of chronic low-grade inflammation. Diet-induced inflammation in particular may be the underpinning of insulin resistance in the disorder. Inflammation directly stimulates excess ovarian androgen production. Increased abdominal adiposity contributes to the inflammatory load in PCOS, and its development may be controlled by the severity of hyperandrogenism.
In Polycystic Ovary Syndrome.
a prooxidant, proinflammatory state exists that is independent of excess adiposity,
inflammation triggered by glucose ingestion is associated with insulin resistance,
hyperandrogenism may be the progenitor of diet-induced inflammation,
oxidative stress and inflammation promotes hyperandrogenism, and
superimposed excess adiposity augments the inflammatory load.
Acknowledgments
Supported by National Institutes of Health Grant HD-048535 to F.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Rotterdam ESHRE/ASRM-Sponsored PCOS Conference Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;1:167–72. doi: 10.1016/s0015-0282(01)02947-8. [DOI] [PubMed] [Google Scholar]
- 3.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of the cardiovascular risk and prevention of cardiovascular disease in women with polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;96:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 5.González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor kB triggers inflammation and insulin resistance in polycystic ovary syndrome. Journal of Clin Endocrinol Metab. 2006;91:1508–12. doi: 10.1210/jc.2005-2327. [DOI] [PubMed] [Google Scholar]
- 6.Piotrowski PC, Rzepczynska IJ, Kwintkiewicz J, Duleba AJ. Oxidative stress induces expression of CYP11A, CYP17, STAR and 3bHSD in rat theca-interstitial cells. J Soc Gynecol Invest. 2005;12(2 Suppl):319A. [Google Scholar]
- 7.González F, Minium J, Rote NS, Kirwan JP. Hyperglycemia alters tumor necrosis factor-a release from mononuclear cells in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:5336–42. doi: 10.1210/jc.2005-0694. [DOI] [PubMed] [Google Scholar]
- 8.González F, Rote NS, Minium J, Kirwan JP. In vitro evidence that hyperglycemia stimulates tumor necrosis factor-a release in obese women with polycystic ovary syndrome. J Endocrino. 2006;188:521–9. doi: 10.1677/joe.1.06579. [DOI] [PubMed] [Google Scholar]
- 9.González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–40. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 10.González F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism. 2009;58:954–62. doi: 10.1016/j.metabol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodarzi MO, Korenman SG. The importance of insulin resistance in polycystic ovary syndrome. Fertil Steril. 2002;77:255–58. doi: 10.1016/s0015-0282(03)00734-9. [DOI] [PubMed] [Google Scholar]
- 12.Carmina E, Bucchierri S, Esposito A, Del Puente A, Mansueto P, Orio F, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of it relation to insulin resistance. J Clin Endocrinol Metab. 2007;92:2500–5. doi: 10.1210/jc.2006-2725. [DOI] [PubMed] [Google Scholar]
- 13.Dandona P, Weinstock R, Love J, Thusu K, Aljada A, Wadden T. Elevated tumor necrosis factor a in serum of obese patients: fall with weight loss. J Clin Endocrinol Metab. 1998;83:2907–10. doi: 10.1210/jcem.83.8.5026. [DOI] [PubMed] [Google Scholar]
- 14.González F, Thusu K, Rahman EH, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor a in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–41. doi: 10.1016/s0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- 15.Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, et al. Insulin/IGF-1 and TNF-a stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–9. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephans JM, Pekala JH. Transcriptional repression of the C/EBP-alpha and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. Regulations is coordinate and independent of protein synthesis. J Biol Chem. 1992;267:13580–4. [PubMed] [Google Scholar]
- 17.Rosenbaum D, Haber RS, Dunaif A. Insulin resistance in polycystic ovary syndrome: Decreased expression of GLUT 4 glucose transporters in adipocytes. Am J Physiol. 1993;264:E197–202. doi: 10.1152/ajpendo.1993.264.2.E197. [DOI] [PubMed] [Google Scholar]
- 18.Corbould A, Kim YB, Youngren JF, Pender C, Kahn BB, Lee A, Dunaif A. Insulin resistance in the skeletal muscle of women with PCOS involves intrinsic and acquired defects in insulin signaling. Am J Physiol Endocrinol Metab. 2005;288:E1047–54. doi: 10.1152/ajpendo.00361.2004. [DOI] [PubMed] [Google Scholar]
- 19.Escobar-Morreale HF, Calvo RM, Villuendas G, Sancho J, San Millan JL. Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obes Res. 2003;11:987–96. doi: 10.1038/oby.2003.136. [DOI] [PubMed] [Google Scholar]
- 20.Peral B, San Millan JL, Castello R, Moghetti P, Escobar-Morreale HF. The methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. J Clin Endocrinol Metab. 2002;87:3977–83. doi: 10.1210/jcem.87.8.8715. [DOI] [PubMed] [Google Scholar]
- 21.Villuendas G, San Millan JL, Sancho J, Escobar-Morreale HF. The -597 G->A and -174 G->C polymorphisms in the promoter of the IL-6 gene are associated with hyperandrogenism. J Clin Endocrinol Metab. 2002;87:1134–41. doi: 10.1210/jcem.87.3.8309. [DOI] [PubMed] [Google Scholar]
- 22.Moshage HJ, Roelofs HM, van Pelt JF, Hazenberg BP, van Leeuwen MA, et al. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun. 1988;155:112–117. doi: 10.1016/s0006-291x(88)81056-8. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14,719 initially healthy American women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 25.Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]
- 26.Escobar-Morreale HF, Luque-Ramírez M, González F. Serum inflammatory markers in polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 95:1048–58. doi: 10.1016/j.fertnstert.2010.11.036. 011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulman N, Leiba LR, Shachar S, Linn R, Zinder O, Blumenfeld Z. Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160–5. doi: 10.1210/jc.2003-031096. [DOI] [PubMed] [Google Scholar]
- 28.Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89:5592–6. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 29.Loms Ziegler-Heitbrock HW. The biology of the monocyte system. Eur J Cell Biol. 1989;49:1–12. [PubMed] [Google Scholar]
- 30.Holmes AG, Mesa JL, Neill BA, Chung J, Carey AL, Steinberg GR, et al. Prolonged interleukin-6 administration enhances glucose tolerance and increases skeletal muscle PPARalpha and UCP2 expression in rats. J Endocrinol. 2008;198:367–74. doi: 10.1677/JOE-08-0113. [DOI] [PubMed] [Google Scholar]
- 31.Franckhauser S, Elias I, Rotter Sopasakis V, Sopasakis V, Ferré T, Nagaev I, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. 2008;51:1306–16. doi: 10.1007/s00125-008-0998-8. [DOI] [PubMed] [Google Scholar]
- 32.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 33.Tan AS, Ahmed N, Berridge MV. Acute regulation of glucose transport after activation of human peripheral blood neutrophils by phorbol myristate acetate, fMLP, and granulocyte-macrophage colony-stimulation factor. Blood. 1998;91:649–55. [PubMed] [Google Scholar]
- 34.Groemping Y, Lapouge K, Smerdon SJ, Rittenger K. Understanding activation of NADPH oxidase: a structural characterization of p47phox. Biophys J. 2003;84:356A. [Google Scholar]
- 35.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic determination of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 36.Chanock SJ, el Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J Biol Chem. 1994;269:24919–22. [PubMed] [Google Scholar]
- 37.Barnes PJ, Karin M. Nuclear factor-kB: pivitol transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 38.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fain JN, Bahouth SW, Madan AK. TNFa release by nonfat cells of adipose tissue. Int J Obes. 2004;28:616–22. doi: 10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- 40.Lindholm A, Blomquist C, Bixo M, Dahlbom I, Hansson T, Sundström Poromaa I, et al. No difference in markers of adipose tissue inflammation between overweight women with polycystic ovary syndrome and weight-matched controls. Hum Reprod. 2011;26:1478–85. doi: 10.1093/humrep/der096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González F, Shepard MK, Rote NS, Minium J. Diet-induced oxidative stress is independent of abdominal adiposity in normal weight women with polycystic ovary syndrome. Proceedings of the 9th meeting of the Androgen Excess and Polycystic Ovary Syndrome Society. 2011;9:64. [Google Scholar]
- 42.González F, Daniels JK, Blair HE, Nair KS. Androgen administration stimulates reactive oxygen species generation from leukocytes of normal reproductive-age women. Reprod Sci. 2010;17(3 Suppl):265A. [Google Scholar]
- 43.González F, Nair KS, Daniels JK, Basal E, Schimke JM. Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. Am J Physiol Endocrinol Metab. 2011 Nov 1; doi: 10.1152/ajpendo.00416.2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.González F, Nair KS, Coddington CC, Blair HE. Anti-inflammatory effect of hyperandrogenism in polycystic ovary syndrome. Reprod Sci. 2010;17(3 Suppl):249A–250A. [Google Scholar]
- 45.González F, Rote NS, Minium J, Kirwan JP. Hyperandrogenism is related to reactive oxygen species generation from pre-activated leukocytes in polycystic ovary syndrome. Reprod Sci. 2007;14(2 Suppl):215A. [Google Scholar]
- 46.González F, Rote NS, Minium J, Kirwan JP. Insulin sensitivity and hyperandrogenism in polycystic ovary syndrome are related to activated nuclear factor kB from mononuclear cells in the fasting state. Proceedings of the 89th meeting of the Endocrine Society. 2007;89:142. [Google Scholar]
- 47.Dessein PH, Joffe BI, Stanwix E. High sensitivity C-reactive protein as a disease activity marker in rheumatoid arthritis. J Rheumatol. 2004;31:1095–7. [PubMed] [Google Scholar]
- 48.Linares LF, Gomez-Reino JJ, Carreria PE, Morillas L, Ibero I. C-reactive protein (CRP) levels in systemic lupus erythematosus (SLE) Clin Rheumatol. 1986;5:66–9. doi: 10.1007/BF02030970. [DOI] [PubMed] [Google Scholar]
- 49.Rahmati MA, Craig RG, Homel P, Kaysen GA, Levin NW. Serum markers of periodontal disease status and inflammation in hemodialysis patients. Am J Kidney Dis. 2002;40:983–9. doi: 10.1053/ajkd.2002.36330. [DOI] [PubMed] [Google Scholar]
- 50.Wahrenberg H, Ek I, Revnisdottir S, Carlström K, Bergqvist A, Arner P. Divergent effects of weight reduction and oral anticonception treatment on adrenegic lypolysis regulation in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1999;84:2182–7. doi: 10.1210/jcem.84.6.5794. [DOI] [PubMed] [Google Scholar]
- 51.Xu, De Pergola G, Björntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126:1229–34. doi: 10.1210/endo-126-2-1229. [DOI] [PubMed] [Google Scholar]
- 52.Ek I, Arner P, Bergqvist A, Carlström K, Wahrenberg H. Impaired adipocyte lypolysis in nonobese women with polycystic ovary syndrome: a possible link to insulin resistance? J Clin Endocrinol Metab. 1997;84:2182–7. doi: 10.1210/jcem.82.4.3899. [DOI] [PubMed] [Google Scholar]
- 53.Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11:790–7. doi: 10.1093/oxfordjournals.humrep.a019256. [DOI] [PubMed] [Google Scholar]
- 54.Ortega I, Cress AB, Villanueva AJ, Sokalska A, Stanley SD, Duleba AJ. Resveratrol potentiates effects of simvastatin on inhibition of rat ovarian theca-interstitial cell steroidogenesis. Fertil Steril. 2011;96(3 Suppl):S40–1. doi: 10.1186/1757-2215-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spazynsky RZ, Arici A, Duleba AJ. Tumor necrosis factor alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–8. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]