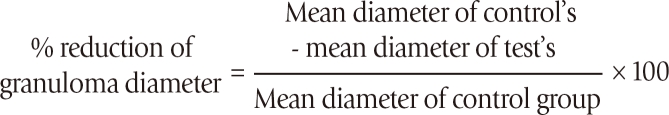Abstract
The aim of the study is to characterize the phenotypes of CD4+ CD25+ T regulatory cells within the liver granulomas and association with both Foxp-3 gene expression and splenic cytokines. Naïve C57BL/6 mice were intravenously injected with multiple doses of the soluble egg antigen (SEA) 7 days before cercarial infection. The immunized and infected control groups were sacrificed 8 and 16 weeks post-infection (PI). Histopathology, parasitological parameters, splenic phenotypes for T regulatory cells, the FOXP-3 expression in hepatic granuloma using real-time PCR, and the associated splenic cytokines were studied. Histopathological examination of the liver revealed remarkable increase in degenerated ova within hepatic granuloma which decreased in diameter at weeks 8 and 16 PI (P<0.01). The percentage of T regulatory cells (CD4+ CD25+) increased significantly (P<0.01) in the immunized group compared to the infected control at weeks 8 and 16 PI. The FOXP-3 expression in hepatic granulomas increased from 10 at week 8 to 30 fold at week 16 PI in the infected control group. However, its expression in the immunized group showed an increase from 30 at week 8 to 70 fold at week 16 PI. The splenic cytokine levels of pro-inflammatory cytokines, IFN-γ, IL-4, and TNF-α, showed significant decreases (P<0.05) compared to the infected control group. In conclusion, the magnitude and phenotype of the egg-induced effects on T helper responses were found to be controlled by a parallel response within the T regulatory population which provides protection in worm parasite-induced immunopathology.
Keywords: Schistosoma mansoni, T regulatory cell, soluble egg antigen, Foxp-3 gene
INTRODUCTION
Chronic helminth infections are associated with impaired immune responsiveness [1], and helminth parasites have been widely attributed with the ability to manipulate and down-modulate protective immune responses [2]. Schistosoma mansoni infection, a chronic disease of the tropics is caused by disseminated worm eggs that induce CD4+ T-cell mediated granulomatous inflammation, fibrosis, and occasional death [3]. In the murine model of the disease, the acute stage (8-10 weeks) of the vigorous granulomatous response dominated by type 2 cytokine production is spontaneously down-modulated at the chronic stage (16-20 weeks) with diminished granuloma development [3]. The factors relevant to immune-modulation of granuloma size include CD8+ suppressor effector cells, CD4+ suppressor inducer and effector cells, macrophages, cross-regulation of Th1 and Th2 cells, antiidiotypic antibodies (Schistosoma japonicum) and antiidiotypic T cells (S. mansoni and S. japonicum) [4].
Immunization using soluble egg antigen (SEA) from S. mansoni has been shown to provide immunity in mice, thus protecting the mice from challenge by S. mansoni cercariae. This protective immunity was characterized as a SEA-specific T-cell proliferation accompanied by IFN-γ and IL-2 production and cytotoxic CD8+ T-cell activation, which contributed to a marked reduction in the number of granulomas and the amount of fibrosis, leading to survival of the mice [5]. Taking advantage of the naturally occurring regulatory system for the purpose of reducing the excessive granulomatous inflammation in schistosomiasis has been achieved by Sadler et al. [6]. In the same context, the induction of granuloma hyporesponsiveness has been achieved by repeated injection with eggs or SEA in S. mansoni infection and in S. japonicum infection [7].
In recent years, a new category of CD4+ CD25+ T regulatory (Treg) lymphocytes have been identified that maintain immune tolerance to self and they are involved in immune regulation of various conditions, such as autoimmune diseases [8], graft organ transplantation, and infectious diseases [9]. In infectious diseases, Treg may be induced in antigen-specific manner and may suppress tissue destruction resulting from immune responses [10]. Several publications indicated that the CD4+ CD25+ Treg subset, which spontaneously arises in the thymus [11], can also be peripherally induced by antigen [12] and functions in the regulation of parasite-induced immunopathology [13].
More recently, the Foxp-3 gene which encodes a forkhead-winged helix transcription factor Scurfin [14] was found to be expressed by and required for the generation of CD4+ CD25+ Treg [15]. The aim of this study is to localize CD4+ CD25+ T cells within the liver granulomas of both infected and immunized mice. It was of interest to characterize their function and association with Foxp-3 gene expression and changes in the dynamics of splenic cytokine profiles in order to clarify their role in the regulation of egg-induced immunopathology.
MATERIALS AND METHODS
Animals and parasites
C57BL/6 mice (6-8 weeks old) were obtained from the Schistosome Biological Supply Program, Theodor Bilharz Research Institute (SBSP, TBRI, Giza, Egypt) and kept under standard housing conditions. All procedures related to animal experimentation met the International Guiding Principles for Biomedical Research Involving Animals as issued by the International Organizations of Medical Sciences.
Preparation of S. mansoni soluble egg antigen (SEA)
S. mansoni SEA was purchased from SBSP, TBRI. The crude SEA preparation was purified, sterilized by filtration through 0.45 µm filters (Nalgene Brand Product, Sybran Corp., Rochester, New York, USA) and the protein content was estimated using the Bio-Rad kit (Bio-Rad Laboratories, Hercules, California, USA) according to Bradford [19].
Experimental design
The mice were divided into 3 groups (20 per group). One group was immunized 7 days before infection; animals were intravenously injected with 4 doses (10 µg of the SEA each in 10 µl PBS) given 2 days apart (SEA group). The second group (infected group) received no immunization before infection. All animals in the previously mentioned groups were infected by tail immersion with 25 cercariae of an Egyptian strain of S. mansoni. A third group, untreated and not infected, was used as uninfected control. Mice were sacrificed at weeks 8 and 16 post-infection (PI).
Parasitological parameters
Worm burden
Perfusion of adult worms from the liver and porto-mesenteric system was performed 8 and 16 weeks after infection according to Duvall and Dewitt [20].
Tissue egg load
The number of eggs per gram tissue (liver and intestine) was studied according to the procedure by Cheever [21].
Oogram pattern
The percentages of immature, mature, and dead ova in the small intestines were computed from a total of 100 eggs per intestinal segment and classified according to the categories previously defined by Pellergrino et al. [22].
Histopathological study
Granuloma measurement
Livers of mice were fixed in 10% buffered formalin, processed into paraffin blocks, serially cut at 4 µm thickness, and stained with hematoxylin and eosin. Hepatic granuloma measurements were done according to Von Lichtenberg [23] using an ocular micrometer for those containing a central ovum only.
The percent reduction in granuloma diameter relative to the infected controls was calculated as follows:
Counting was carried out in 5 successive microscopic fields (10×10) in serial tissue sections of more than 250 µm apart.
Granuloma isolation and cell separation
Hepatic granulomas were isolated and enzymatically dispersed according to Ragheb and Boros [24]. Granuloma cells were used for the CD4+ CD25+ T regulatory cell separation. Some of the granuloma samples were mixed with Trizol (Invitrogen, Carlsbad, California, USA) and immediately frozen in liquid nitrogen for RNA preparation.
Flow cytometric enumeration of Tregs cells in hepatic granuloma
Granuloma cells were isolated from the liver tissue of infected mice as described by Ragheb and Boros [24]. Briefly, livers were perfused with sterile PBS to remove blood cells. The tissue was passed through a sterile stainless sieve and washed twice with cold PBS. The pellet of granulomas was resuspended in RPMI 1640 medium containing 1 mg/ml collagenase D (Roche) and 4 U/ml DNase I (Invitrogen) and digested for 20-30 min at 37℃. The digested material was passed through a cell strainer (100 µm) and washed twice with RPMI 1640 medium. Erythrocytes were lysed with ACK buffer (PharMingen, Country), and cells were washed twice with RPMI 1640 medium. Cell suspension were stained by adding 10 µl of anti-CD4 FITC labeled and 10 µl of anti-CD25 PE and then sorted for the 2 target populations by flow cytometry using FACS Calibur Sorter (BD Biosciences, Sunnyvale, California, USA).
Measurement of FOXP-3 by real-time PCR
RNA was extracted from the frozen liver samples using Trizol (Invitrogen). Isolated pure RNA for reverse transcriptase-polymerase chain reaction (RT-PCR) was prepared by treating RNA samples with RNase free DNAse I (Promega Corporation, Madison, Wisconsin, USA). Further, samples were extracted with buffered phenol and then chloroform: isoamylalcohol (24:1). The recovered RNA was ethanol precipitated and stored in RNAse-free distilled water. The purity of RNA samples was assessed spectrophotometrically. The presence of relative mRNA species was evaluated by the SYBR Green method using the ABI Prism 7700 Sequence Detector System (Applied Biosystems, Foster City, California, USA). Primers were designed for PCR by using Gene Runner Software (Hasting Software, Inc., Hasting, New York, USA). The RNA sequences used for primer design were obtained from the Gene Bank (forward, 5'-GGC CCT TCT CCA GGA CAG A-3' and reverse 5'-GCT GAT CAT GGC TGG GTT GT-3'). All primer sets had a calculated annealing temperature of 60°. RT-PCR was performed in duplicate in a 25 µl reaction volume containing 2X SYBR Green PCR Master Mix (Applied Biosystems), 900 nm of each primer, 2-3 µl of cDNA and the volume was adjusted with pure distilled water. The RT-PCR amplifications were 2 min at 50° followed by 40 cycles of denaturation for 15 sec and annealing/extension at 60° for 1 min. Real-Time PCR data were analyzed using v.1.7 Sequence Detection Software (PE Biosystems, Foster City, California, USA). Relative expression of the genes was calculated by using the comparative CT method as described earlier by Gerard et al. [25]. Relative transcript levels were normalized to the 18s rRNA genes. For comparison, transcript levels of naive, uninfected mice were set at 1 and data from infected mice are reported as fold increase over 1 baseline. Dissociation curves were used to verify the specificity of the PCR products.
Cytokine measurements
Splenic cells were cultured at a concentration of 1×106 cells/ml in 24-well microtitre plates (Corning Glass Works, Corning, New York, USA) in the presence of 5 µg/ml SEA. The culture supernatants were harvested at 24 hr to assay IL-4 and IL-10, TNF-α and TGF-β, and at 72 hr for INF-γ measurement [26]. The supernatant was stored at -20℃ until assayed. Cytokines were assayed by sandwich ELISA, using a cytokine detection kit (Genzyme Immunobiologicals, Cambridge, Massachusetts, USA). The level of cytokines was calculated by reference to standard curves constructed with unknown amounts of all the studied cytokines in (pg).
Statistical analysis
Data are expressed as the mean±SD. Comparison between the mean values of different parameters in the studied groups was performed using 1-way ANOVA test, with post-hoc using LSD test. The data were considered significant if P-value was ≤0.05. Statistical analysis was performed with the aid of the SPSS computer program (version 16 windows).
RESULTS
Parasitological and histopathological parameters
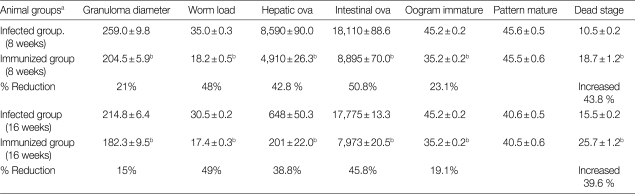
The results in Table 1 show significant reduction in the granuloma diameter (21%) on comparing SEA immunized group with the infected control group. Moreover, a significant reduction was also noticed in the mean number of S. mansoni adult worms, the mean number of ova/gram tissue (liver and intestine) and The percent of immature ova at both at weeks 8 and 16 PI in the group immunized with SEA compared to the infected control groups (P<0.01). In contrast, the percent of dead ova was higher at weeks 8 and 16 PI in the immunized group compared to the infected control.
Table 1.
Parasitological parameters detected at week 8 post-infection in animal groups
a10 mice in each group.
bSignificant difference from infected controls (P<0.01).
Flow cytometric enumeration of Treg cells in hepatic granuloma
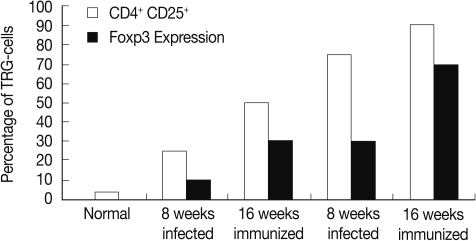
The percentage of T regulatory cells (CD4+ CD25+) was increased significantly (P<0.01) in the immunized group compared to the infected control at weeks 8 and 16 PI (Fig. 1).
Fig. 1.
Percentage of CD4+ CD25+ T regulatory cells subset and in Foxp-3 expression granulomas of the different studied groups.
FOXP-3 expression in granulomatous livers of the different studied groups using quantitative real time PCR
The FOXP-3 expression in hepatic granuloma was increased from 10 fold at 8 weeks to 30 fold at week 16 PI in the infected control group. However, its expression in the immunized group showed increase from 30 fold at week 8 to 70 fold at week 16 PI (Fig. 1).
Splenic cytokine measurements
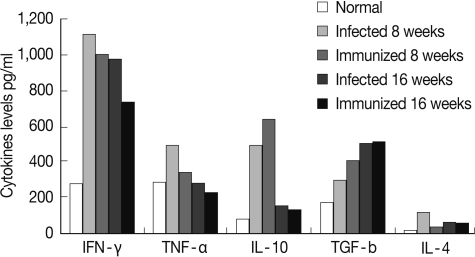
At week 8 PI, the profiles of pro-inflammatory splenic cytokines levels, IFN-γ, TNF-α, and IL-4, were decreased significantly (P<0.05, P<0.05, and P<0.01, respectively), while the levels of the anti-inflammatory cytokine, IL-10 and TGF-β, were significantly increased (P<0.05) in immunized SEA immunized group when compared with their corresponding S. mansoni-infected group (Fig. 2). However, at week 16 PI, there is no significance difference between the levels of the different splenic cytokines in both infected control and immunized groups.
Fig. 2.
Splenic cytokine levels in the different studied groups.
DISCUSSION
An early paradigm of anti-helminth immune responses was based upon Th1/Th2 dichotomy, with resistance to schistosome infection being associated with Th2 responses [27]. Because it is now evident that the Th1/Th2 dichotomy does not sufficiently explain the balance between susceptibility and resistance to helminth infections [28], there is increasing interest in the ability of regulatory T cells to modulate effector T cell responses. The beneficial nature of such regulation is suggested by previous findings that during infection with schistosomes, both Th1 and excessive Th2 responses directed against egg Ag can have highly deleterious consequences [29]. A lot of trials have been conducted to find a possible way for ameliorating schistosome infection severity or morbidity by inhibition of host reaction around S. mansoni eggs [30].
Natural Treg cells have a major role in the control of immune responses in multiple settings, including thymic development, autoimmunity, atopic allergy, transplantation, and infectious diseases. In human paracoccidiomycosis, CD4+ CD25+ Treg cells accumulate in characteristic granulomatous lesions and exert strong suppressive activity on effector cells, implying that Treg cells might contribute to the regulation of these lesions. In experimental schistosomiasis and the schistosome egg injection model, Treg is reported to control both Th1 and Th2 aspects of the immune response and play important roles in minimizing pathology during schistosome infections [31].
In murine schistosomiasis, the granuloma evolves through 3 stages, the primary response, the secondary vigorous and the secondary modulated stages [32]. Based on this categorization, the dynamics of immunoregulatory responses were studied at weeks 8 and 16 PI. Repeated intravenous injection with antigens before infection intiated primary and secondary responses earlier than in natural infection.
In this work, phenotypic T cell subsets showed significantly increased percentage of T regulatory cells (CD4+ CD25+) in the SEA immunized infected group compared to the corresponding infected control group at weeks 8 and 16 PI. Similar increase in CD4+ CD25+ cells following egg antigen immunization was previously noticed in S. mansoni and S. japonicum infection [33]. Following T-cell receptor engagement, CD4+ CD25+ T cells can suppress the activation and proliferation of other CD4+ and CD8+ T cells in an antigen non-specific manner [34] partly by inhibiting IL-2 production [35].
The regulatory activity of Treg cells appears to stem from their expression of Foxp-3, a transcription factor that acts as an important regulator of the Treg phenotype [36]. Indeed, we found that Foxp-3 was highly expressed in CD4+ CD25+ T cells from S. mansoni egg antigen-immunized mice compared to infected control group at both weeks 8 and 16. The differences in T cell subset profile within the hepatic granuloma might be reflected by the functional activity of T cells. Thus, the reduction in granuloma diameter was concurrently associated with increased CD4+ CD25+ T cells and elevated Foxp-3 transcripts. Our results was with agreement with Maher et al. [37] who stated that at 16 weeks of the infection, the livers of mice bearing the down-modulated granulomas had greatly elevated Foxp-3 transcripts. Moreover, the percentage of CD4+ CD25+ cells greatly increased within the down-modulated granulomas. Our data also revealed decrease of IL-4, a Th2 cytokine, in SEA immunized group compared to the infected control group at week 8 PI. This observation was consistent with a significant increase in CD4+ CD25+ T cells [38].
In this study, administration of SEA prior to infection resulted in decreased worm load, hepatic and intestinal ova together with change in oogram pattern. This could be due to enhancement of immune response or would be acting as assort of primary infection that somewhat hinders the challenge one. Similarly, immunization with SEA of lung stage schistosomula prior to infection induced protective effect, manifested by reduction in parasitological parameters, increased levels of specific immunoglobulins as well as raised hepatic mRNA expression of TNF-α and TGF-β [39]. In the present work, at week 8 PI, the levels of IFN-γ and TNF-α in SEA immunized group were significantly reduced compared to the infected controls, showing the most pronounced reduction of granuloma diameter. The cytokines play an important role in regulation of the inflammatory granulomatous response in schistosomiasis [40]. IFN-γ and TNF-α appears to play an important role in the generation and maintenance of egg induced granuloma [41]. The diminished focal and systemic production of IFN-γ and TNF-α may be implicated in the downmodulation of the granulomatous response [42] It is possible that the acceleration of destruction of ova may have resulted in decreased liberation of egg antigens into circulation to a level unable to stimulate splenic cells to secrete these cytokines.
The requirement for IL-10 is critical in several important human diseases, including schistosomiasis, wherein marked increases in host morbidity and mortality are observed when IL-10 levels are low or absent [43]. In murine S. mansoni infection, IL-10 reduces hepatocyte damage induced by the eggs of the parasite, is essential for maintenance of a non-lethal chronic infection and inhibits inappropriate immune responses in experimental models [43]. CD4+ CD25+ T cells secrete immunosuppressive cytokines, like IL-10 and TGF-β [44]. Our data revealed significantly elevated IL-10 and TGF-β in SEA immunized group compared to the infected control group at week 8 PI. Thus, in this study on murine S. mansoni infection, CD4+ CD25+ T cells were, at least in part, an important contributor to IL-10 production. Thus, blocking IL-10 or modulating CD4+ CD25+ T cells could control helminth infection [41].
In summary, the present study has shown that the magnitude and phenotype of the egg-induced effect on T helper response were found to be controlled by a parallel response within the T regulatory population. In agreement with previous publications CD4+ CD25+ Treg cells provide protection not only in autoimmune but also in worm parasite-induced immuopathology. Artificially enhanced induction of Treg cell activity may thus be considered as a modality in the suppression of the schistosome egg-induced immunopathology.
References
- 1.World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helmimthiasis. Geneva, Switzerland: World Health Organization; 2002. [PubMed] [Google Scholar]
- 2.Cheever A, Hoffmann K, Wyn T. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465–466. doi: 10.1016/s0167-5699(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 3.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 4.Boros DL. T helper cell populations, cytokine dynamics and pathology of the schistosomal egg granuloma. Microbes Infect. 1999;1:511–516. doi: 10.1016/s1286-4579(99)80090-2. [DOI] [PubMed] [Google Scholar]
- 5.Cheever AW. Differential Regulation of Granuloma Size and Hepatic Fibrosis in Schistosome Infections. Mem Inst Oswaldo Cruz. 1997;92:689–692. doi: 10.1590/s0074-02761997000500024. [DOI] [PubMed] [Google Scholar]
- 6.Sadler CH, Rutitzky LI, Stadecker MJ, Wilson RA. IL-10 is crucial for the transition from acute to chronic disease state during infection of mice with Schistosoma mansoni. Eur J Immunol. 2003;33:880–888. doi: 10.1002/eji.200323501. [DOI] [PubMed] [Google Scholar]
- 7.Pancré V, Delacre M, Herno J, Auriault C. Schistosomal egg antigen-responsive CD8 T-cell population in Schistosoma mansoni-infected BALB/c mice. Immunology. 1998;98:525–534. doi: 10.1046/j.1365-2567.1999.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahi H, Osman A, Cook RM, LoVerde PT, Stadecker MJ. Schistosoma mansoni phosphoenolpyruvate carboxykinase, a novel egg antigen: Immunological properties of the recombinant protein and identification of a T-cell epitope. Infect Immun. 2000;68:3385–3393. doi: 10.1128/iai.68.6.3385-3393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stadecker MJ. The shrinking schistosomal egg granuloma: How accessory cells control T cell-mediated pathology. Exp Parasitol. 1994;79:198–201. doi: 10.1006/expr.1994.1080. [DOI] [PubMed] [Google Scholar]
- 10.Hassanein H, Akl M, Shaker Z, el-Baz H, Sharmy R, Rabie I. Induction of hepatic egg granuloma hyporesponsiveness in murine schistosomiasis mansoni by intravenous injection of small doses of soluble egg antigen. APMIS. 1997;105:773–783. doi: 10.1111/j.1699-0463.1997.tb05083.x. [DOI] [PubMed] [Google Scholar]
- 11.Shevach EM. CD4+ CD25+ suppressor T cells: More questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe K, Mwinzi PMN, Black CL, Muok EMO, Karanja DMS, Secor WE, Secor DG. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am J Trop Med Hyg. 2007;77:676–682. [PMC free article] [PubMed] [Google Scholar]
- 13.Cavassani KA, Campanelli AP, Moreira AP, Vancim JO, Vitali LH, Mamede RC, Martinez R, Silva JS. Systemic and local characterization of regulatory T cells in a chronic fungal infection in humans. J Immunol. 2006;177:5811–5818. doi: 10.4049/jimmunol.177.9.5811. [DOI] [PubMed] [Google Scholar]
- 14.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 15.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+ CD4+ regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 16.Singh K, Gerard H, Hudson A, Reddy T, Boros D. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology. 2005;114:410–417. doi: 10.1111/j.1365-2567.2004.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunkow ME, Jeffrey EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 18.Fontenot JD, Gavin MA, Rudensky A. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Duvall RH, Dewitt WB. An improved perfusion technique for recovering adult Schistosoma from laboratory animals. Am J Trop Med Hyg. 1967;16:483–484. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- 21.Cheever AW. Condition affecting the accuracy of potassium hydroxide digestion technique for counting S. mansoni eggs in tissues. Bull WHO. 1968;39:328–331. [PMC free article] [PubMed] [Google Scholar]
- 22.Pellegrino J, Oliveira CA, Faria J, Cumha AS. New approach to the screening of drugs in experimental Schistosoma mansoni in mice. Am J Trop Med Hyg. 1962;11:201–215. doi: 10.4269/ajtmh.1962.11.201. [DOI] [PubMed] [Google Scholar]
- 23.Von Lichtenberg FC. Host response to eggs of Schistosoma mansoni. I. Granuloma formation in the sensitized laboratory mouse. Am J Pathol. 1962;41:711–731. [PMC free article] [PubMed] [Google Scholar]
- 24.Ragheb S, Boros DL. Characterizations of granuloma T lymphocyte function from Schistosoma mansoni-infected mice. J Immunol. 1989;142:3239–3246. [PubMed] [Google Scholar]
- 25.Gerard HC, Branigan PJ, Schumacher HR, Jr, Hudson AP. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25:734–742. [PubMed] [Google Scholar]
- 26.Grzych JM, Pearce E, Cheever A, Caulada ZA, Caspar P, Heiny S, Lewis F, Sher A. A Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 27.Capron, Dombrowicz D, Capron M. Regulation of the immune response in experimental and human schistosomiasis: The limits of an attractive paradigm. Microbes Infect. 1999;1:485–490. doi: 10.1016/s1286-4579(99)80086-0. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe K, Carter JM, Neely-Burnam M, Colley DG. Relative imbalance between T regulatory cells and activated T cells in mice with differential morbidity in chronic Schistosoma mansoni infections. Immunology. 2009;31:440–446. doi: 10.1111/j.1365-3024.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 29.Stadecker MJ, Asahi H, Finger E, Hernandez HJ, Rutitzky LI, Sun J. The immunobiology of Th1 polarization in high-pathology schistosomiasis. Immunol Rev. 2004;201:168–179. doi: 10.1111/j.0105-2896.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 30.Zouain CS, Gustavson S, Silva-Teixeira DN, Contigili C, Rodrigues V, Leite MF, Goes AM. Human immune response in schistosomiasis: The role of p24 in the modulation of cellular reactivity to S. mansoni antigens. Hum Immunol. 2002;63:647–656. doi: 10.1016/s0198-8859(02)00422-6. [DOI] [PubMed] [Google Scholar]
- 31.Weinstock JV. The pathogenesis of granulomatous inflammation and organ injury in schistosomiasis: Interactions between the schistosome ova and the host. Immunol Invest. 1992;21:455–475. doi: 10.3109/08820139209069384. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Zhao J, Yang Y, Zhang L, Yang X, Zhu X, Ji M, Sun N, Su C. Schistosoma japonicum egg antigens stimulate CD4+ CD25+ T cells and modulate airway inflammation in a murine model of asthma. Immunology. 2006;120:8–18. doi: 10.1111/j.1365-2567.2006.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kursar M, Bonhagen K, Fensterle J, Kohler A, Hurwitz R, Kamradt T, Kaufmann SH, Mittrucker HW. Regulatory CD4+ CD25+ T cells restrict memory CD8+ T cell responses. J Exp Med. 2002;196:1585–1592. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tournoy KG, Kips JC, Schou C, Pauwels RA. Airway eosinophilia is not a requirement for allergen-induced airway hyperresponsiveness. Clin Exp Allergy. 2000;30:79–85. doi: 10.1046/j.1365-2222.2000.00772.x. [DOI] [PubMed] [Google Scholar]
- 35.Ramsdell F. Foxp3 and natural regulatory T cells: Key to a cell lineage? Immunity. 2003;19:165–168. doi: 10.1016/s1074-7613(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 36.Wang, Jiang J, Guan Q, Lan Z, Wang H, Nguan CYC, Jevnikar A M, Du C. Reduction of Foxp3-expressing regulatory T cell infiltrates during the progression of renal allograft rejection in a mouse model. Transplant Immunology. 2008;19:93–102. doi: 10.1016/j.trim.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Maher KM, El-Shennawy AM, Ibrahim RB, Mahmoud S. Protective and antipathology potential of immunization with Schistosoma mansoni-secretory/excretory product. New Egypt J Med. 2003;28:173–182. [Google Scholar]
- 38.Garraud O, Nutman TB. The role of cytokines in human B-cell differentiation into immunoglobulin-secreting cells. Bull Inst Pasteur. 1996;94:285–309. [Google Scholar]
- 39.Hoffmann KF, Caspar P, Cheever AW, Wynn TA. IFN-gamma, IL-2 and TNF-alpha are required to maintain reduced liver pathology in mice vaccinated with Schistosoma mansoni eggs and IL-2. J Immunol. 1998;161:4201–4210. [PubMed] [Google Scholar]
- 40.Singh KP, Gerard HC, Hudson AP, Boros DL. Expression of matrix metalloproteinases and their inhibitors during the desorption of schistosome egg induced fibrosis in praziquantel-treated mice. Immunology. 2004;111:343–352. doi: 10.1111/j.0019-2805.2004.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wille U, Nishi M, Lieberman L, Wilson EH, Roos DS, Hunter CA. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. Parasite Immunol. 2004;26:229–236. doi: 10.1111/j.0141-9838.2004.00704.x. [DOI] [PubMed] [Google Scholar]
- 42.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 43.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol. 2005;174:7368–7375. doi: 10.4049/jimmunol.174.11.7368. [DOI] [PubMed] [Google Scholar]