Abstract
Treatment of hydatid disease is mainly surgical, with medical treatment being reserved as a coadjuvant treatment. Use of effective scolicidal agents during surgery of cystic echinococcosis is essential to reduce the recurrence rate. The goal of this study was to evaluate the in vitro scolicidal effects of hydroalcoholic extracts of Satureja khuzestanica leaves and aqueous extracts of Olea europaea leaves on hydatid cyst protoscolices. Echinococcus granulosus protoscolices were collected from the liver of sheep infected with the hydatid cyst. Various concentrations of plant extracts were used in different exposure times for viability assay of protoscolices. Among the olive leaf extracts tested, 0.1% and 0.01% concentrations had strong scolicidal effects in 120 min. S. khuzestanica 0.1% had very strong scolicidal effects in 30, 60, and 120 min of exposure times and the mortality rate decreased with the lower concentration. The finding have shown that the scolicidal activity of S. khuzestanica against cystic echinococosis protoscolices were more effective, while the O. europaea extract showed less effects.
Keywords: Echinococcus granulosus, Olea europaea, Satureja khuzestanica, protoscolicidal activity, hydatid cyst
INTRODUCTION
Hydatidosis caused by Echinococcus spp. is a major zoonotic infection that is detrimental to both humans and animal husbandries in many countries. Cystic echinicoccosis affects mainly the intermediate host's viscera, including the liver, lungs, and less frequently, the spleen, kidneys, bone, brain, and other organs [1]. Currently the basic approaches for treatment of hydatid disease are surgery and chemotherapy. However, operative leakage may lead to dissemination of viable protoscolices to adjacent tissues and thus to intrapritoneal hydatid disease [2,3].
The olive (Olea europaea) tree, a plant which can survive for hundreds of years, is known to naturally possess strong resistance to microbial attack [4]. Natural olive leaf and olive leaf extracts are now marketed as anti-aging agents, immunostimulators, and even antibiotics. Olive leaf extracts have been used throughout history for their medical properties, for instance, treatment of infections. Several studies have shown a decreased risk of bacterial and parasitic protozoan diseases with an increasing consumption of olive products [4,5]. Although antiprotozoal activities of the competent Oleuropein (O. europaea) have been examined, anthelmintic potential of olive leaf extracts have not been reported.
Satureja khuzestanica Jamzad is an endemic plant that is widely distributed in the southern part of Iran. It is famous for its medical uses as an analgesic and antiseptic in folk medicine [6]. Recently, antiviral, antibacterial, antifungal, and antiprotozoal effects were investigated from various species of Satureja [7-15].
Therefore, the aim of this investigation was to examine the activity of Iranian medical plants, including O. europaea and S. khuzestanica, against protoscolices of hydatid cysts and to determine the exposure time and concentrations of the extracts providing scolicidal activities.
MATERIALS AND METHODS
Plant material
Leaves of O. europaea and the aerial parts of S. khuzestanica were collected in June 2010 from Khorram Abad (center of Lorestan province) in southwestern Iran, and identified by Botanical Section, Lorestan University of Medical Sciences. The plants were dried in open air and shady conditions until completely dried and then ground to a powder. All experiments were performed with 1 batch of olive leaf and S. khuzestanica extracts, separately.
Preparation of aqueous plant extracts
A 200 g based on dry weight powdered leaves added to adequate amount water (1,000 ml) to concentration of 20% (w/v). The products were squeezed through gauze cloth to remove the practice and the extract was passed through a 0.2 µm filter (Millipore™ Membrane Filter, USA). The procedure of extraction and filtration were operated at room temperature. The extract was stored at 4℃ until use.
Preparation of hydroethanolic plant extract
S. khuzestanica extracts were prepared according to the method of Zarrin et al. [16]. Briefly, about 10 g powdered leaves of S. khuzestanica was extracted with adding 100 ml of 80% ethanol (1:10 w/v). After 72 hr at room temperature, the suspension was filtered though a Whatman paper No.1 and the crude ethanol extracts were evaporated at 37℃. One gram of extract was dissolved in 1 ml of 100% dimethylsulfoxide (DMSO), and the final concentration of each extract was adjusted to 1,000 mg/ml.
Collection of protoscolices
Sixty hydatid cysts were collected from the liver of infected sheep slaughtered at Khorram Abad and carried to the Parasitology Laboratory at the Department of Parasitology and Mycology, Lorestan University of Medical Sciences. The protoscolices were obtained from the hydatid fluid and washed 3 times in PBS (pH 7.2). The concentration of protoscolices was confirmed as the number of protoscolices per ml of the hydatid fluid in a saline solution (0.9% NaCl solution) containing 5×103 protoscolices in 1 ml with more than 90% viability was used in further use [2].
Viability assay
A 0.1 mg of eosin stain was dissolved in 100 ml distilled water at a 0.1% (w/v) concentration. The viability was checked microscopically after adding 10 µl of eosin solution to 10 µl of protoscolices for 15 min. Stained protoscolices were considered as dead while unstained protoscolices were recorded as alive. Non-treated protoscolices (with plant extracts) were considered as the control group.
Statistical analysis
The statistical analysis was performed with the use of SPSS version 15.0.1. The protoscolicidal activity was calculated as means±SD.
RESULTS
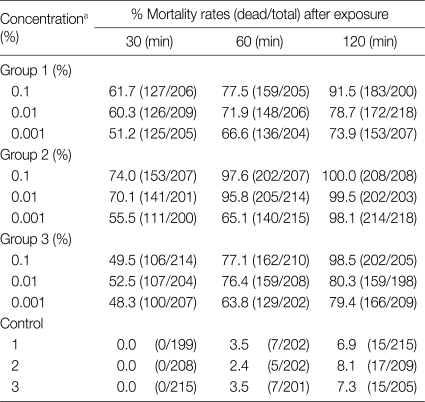
The mortality rates of protoscolices of hydatid cysts after exposure to different concentrations of O. europaea extracts in various time periods are demonstrated in Table 1. Olive leaf extracts 0.1% had strong scolicidal effects in 120 min, and 0.01% also revealed the same effects at the same time. A 96.7% of protoscolices lost viability at 120 min (0.01% diluted). The mortality rate at 0.001% decreased to 53.1% at 30 min, while many of the protoscolices died at 0.1% at 120 min (Table 2).
Table 1.
Protoscolicidal effects of various concentrations of Olea europaea leaf extract in different time periods
aConcentration of all of the plant extract perpetrated with 1 batch.
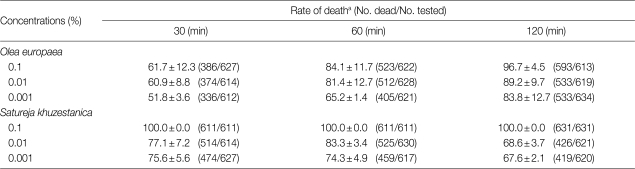
Table 2.
The protoscolicidal activity of different extracts of Olea europaea and Satureja khuzestanica at 30, 60, and 120 min of exposure times
aResults are expressed as mean±SD.
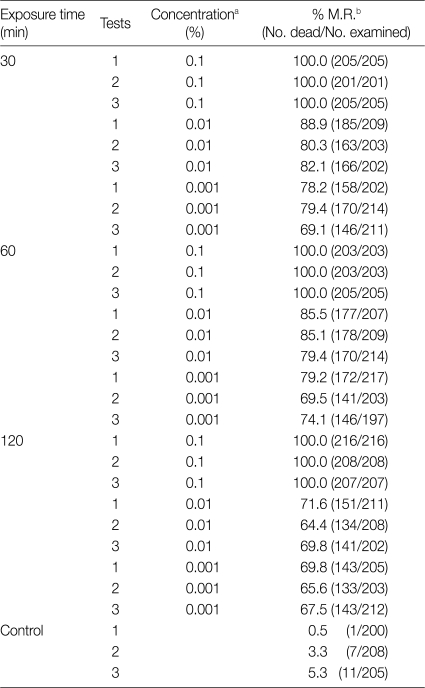
The experiment conducted with S. khuzestanica showed all of the protoscolices died in 0.1% concentrations. On the other hand, the mortality rate was low by increasing the time of exposure and decreasing concentration. The effects of different concentrations of S. khuzestanica extracts on the viability of E. granulosus protoscolices in different exposure times is in shown Table 3.
Table 3.
Scolicidal effects of different concentrations of Satureja khuzestanica after 30, 60, and 120 min of application
aConcentration of all of the plant extract perpetrated with 1 batch.
bMortality rate (%)
DISCUSSION
The surgical treatment of E. granulosus cyst is still the currently most effective method. It can be performed successfully in majority of the patients, if a cyst does not have a risky localization or if the disease is not too far advanced. It has been traditional to inject protoscolicidal agents into hydatid cysts perioperatively. However, lack of objective evidence about the efficacy and the presence of toxicity associated with the protoscolicidal agents led many surgeons to abandon this routine step in the operative management of cystic echinococosis [17,18]. However, the cystic fluid contains a large number of protoscolices and they have the potential to grow into new hydatid cysts [19].
In recent years, there has been a considerable interest in finding natural scolicidal agents from plant materials to replace synthetic ones [2,3,20-22]. Information from previous studies has shown that the plant contains a large variety of substances that possess antimicrobial activity. Hydroalcoholic extracts of Satureja had antiprotozoal properties on Trypanosoma cruzi and Plasmodium falciparum [14,15]. In addition, O. europaea aqueous extracts have been used as herbal medicine for several years for different medical purposes, and an in vitro study focused solely on the antimicrobial and antifungal properties of olive leaf extracts [4].
In the present investigation, protoscolicidal effects of the 2 herbal agents were observed, separately. We found that the protoscolicidal efficacies of S. khuzestanica extracts in various concentrations and different time periods were appropriate, whereas olive leaf extracts had less effects on protoscolices of hydatid cysts. These plants are known to have antimicrobial effects and naturally grow in the Lorestan province of Iran.
We realized that S. khuzestanica killed all of the protoscolices and its 0.1% dilution revealed strong scolicidal activity at the 30, 60, and 120 min, although 0.01% diluted S. khuzestanica did not reveal enough protoscolicidal effects at the same time. The results of our study proved that protoscolices killed with S. khuzestanica (0.001% diluted) showed a decrease in the mortality rate to 67.6% at 120 min exposure time. Low concentrations (0.1%) of O. europaea leaf extracts had effective protoscolicidal efficacy. It is remarkable that increasing exposure time showed more scolicidal activities.
In conclusion, the present study is the first report demonstrating the effectiveness of S. khuzestanica and O. europaea on protoscolices. S. khuzestanica had the greatest scolicidal effect against cystic echinococosis. This plant may be useful as an agent in the PAIR (Puncture-Aspiration-Injection-Reaspiration) method for cystic echinococcosis because of its rapid and strong scolicidal effects. It seems that O. europaea leaf extracts have a less scolicidal activity, but it could be used as an agent with surgery techniques. However, more research is necessary to evaluate mode of actions and in vivo effects of these plant extracts, and also possible side effects on animals and humans.
ACKNOWLEDGMENTS
The technical assistance of Ms. Yousra Hosseini and Mr. Arash B. Jamshidi is acknowledged with pleasure. This study was partially funded by a grant (No. HRC-1170) from Razi Herbal Medicines Research Center, Lorestan University of Medical Sciences, Iran. The authors declare that there is no conflict of interest.
References
- 1.Ammann RW, Eckert J. Cestodes. Echinococcus. Gastroenterol Clin North Am. 1996;25:655–689. doi: 10.1016/s0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- 2.Sadjjadi SM, Zoharizadeh MR, Panjeshahin MR. In vitro screening of different Allium sativum extracts on hydatid cysts protoscoleces. J Invest Surg. 2008;21:318–322. doi: 10.1080/08941930802348261. [DOI] [PubMed] [Google Scholar]
- 3.Moazeni M, Nazer A. In vitro effectiveness of garlic (Allium sativum) extract on scolices of hydatid cyst. World J Surg. 2010;34:2677–2681. doi: 10.1007/s00268-010-0718-7. [DOI] [PubMed] [Google Scholar]
- 4.Markin D, Duek L, Berdicevsky I. In vitro antimicrobial activity of olive leaves. Mycoses. 2003;46:132–136. doi: 10.1046/j.1439-0507.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- 5.Juven B, Henis Y. Studies on the antimicrobial activity of olive phenolic compounds. J Appl Bacteriol. 1970;33:721–732. doi: 10.1111/j.1365-2672.1970.tb02255.x. [DOI] [PubMed] [Google Scholar]
- 6.Haeri S, Minaie B, Amin G, Nikfar S, Khorasani R, Esmaily H, Salehnia A, Abdollahi M. Effect of Satureja khuzestanica essential oil on male rat fertility. Fitoterapia. 2006;77:495–499. doi: 10.1016/j.fitote.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki K, Nakano M, Kawahata T, Mori H, Otake T, Ueba N, Oishi I, Inami R, Yamane M, Nakamura M, Murata H, Nakanishi T. Anti-HIV-1 activity of herbs in Labiatae. Biol Pharm Bull. 1998;21:829–833. doi: 10.1248/bpb.21.829. [DOI] [PubMed] [Google Scholar]
- 8.Abad MJ, Bermejo P, Gonzales E, Iglesias I, Irurzun A, Carrasco L. Antiviral activity of Bolivian plant extracts. Gen Pharmacol. 1999;32:499–503. doi: 10.1016/s0306-3623(98)00214-6. [DOI] [PubMed] [Google Scholar]
- 9.Sahin F, Karaman I, Güllüce M, Oğütçü H, Sengül M, Adigüzel A, Oztürk S, Kotan R. Evaluation of antimicrobial activities of Satureja hortensis L. J Ethnopharmacol. 2003;87:61–65. doi: 10.1016/s0378-8741(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 10.Skocibusić M, Bezić N. Phytochemical analysis and in vitro antimicrobial activity of two Satureja species essential oils. Phytother Res. 2004;18:967–970. doi: 10.1002/ptr.1489. [DOI] [PubMed] [Google Scholar]
- 11.Sonboli A, Fakhari A, Kanani MR, Yousefzadi M. Antimicrobial activity, essential oil composition and micromorphology of trichomes of Satureja laxiflora C. Koch from Iran. Z Naturforsch C. 2004;59:777–781. doi: 10.1515/znc-2004-11-1202. [DOI] [PubMed] [Google Scholar]
- 12.Tampieri MP, Galuppi R, Macchioni F, Carelle MS, Falcioni L, Cioni PL, Morelli I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia. 2005;159:339–345. doi: 10.1007/s11046-003-4790-5. [DOI] [PubMed] [Google Scholar]
- 13.Boyraz N, Ozcan M. Inhibition of phytopathogenic fungi by essential oil, hydrosol, ground material and extract of summer savory (Satureja hortensis L.) growing wild in Turkey. Int J Food Microbiol. 2006;107:238–242. doi: 10.1016/j.ijfoodmicro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Sülsen V, Güida C, Coussio J, Paveto C, Muschietti L, Martino V. In vitro evaluation of trypanocidal activity in plants used in Argentine traditional medicine. Parasitol Res. 2006;98:370–374. doi: 10.1007/s00436-005-0060-4. [DOI] [PubMed] [Google Scholar]
- 15.van Baren C, Anao I, Leo Di Lira P, Debenedetti S, Houghton P, Croft S, Martino V. Triterpenic acids and flavonoids from Satureja parvifolia. Evaluation of their antiprotozoal activity. Z Naturforsch C. 2006;61:189–192. doi: 10.1515/znc-2006-3-406. [DOI] [PubMed] [Google Scholar]
- 16.Zarrin M, Amirrajab N, Sadeghi-Nejad B. In vitro antifungal activity of Satureja khuzestanica Jamzad against Cryptococcus neoformans. Pak J Med Sci Q. 2010;26:880–882. [Google Scholar]
- 17.Belghiti J, Benhamou JP, Houry S, Grenier P, Huguier M, Fékété F. Caustic sclerosing cholangitis: a complication of the surgical treatment of hydatid disease of the liver. Arch Surg. 1986;121:1162–1165. doi: 10.1001/archsurg.1986.01400100070014. [DOI] [PubMed] [Google Scholar]
- 18.Prasad J, Bellamy PR, Stubbs RS. Instillation of scolicidal agents into hepatic hydatid cysts: can it any longer be justified? N Z Med J. 1991;104:336–337. [PubMed] [Google Scholar]
- 19.Besim H, Karayalçin K, Hamamci O, Güngör C, Korkmaz A. Scolicidal agents in hydatid cyst surgery. HPB Surg. 1998;10:347–351. doi: 10.1155/1998/78170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseini SV, Ghanbarzadeh K, Barzin Z, Sadjjadi SM, Tanideh N, Mehrabani D. In vitro protoscolicidal effects of hypertonic glucose on protoscolices of hydatid cyst. Korean J Parasitol. 2006;44:239–242. doi: 10.3347/kjp.2006.44.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciftci IH, Esme H, Sahin DA, Solak O, Sezer M, Dilek ON. Effect of octenidine dihydrochloride on viability of protoscoleces in hepatic and pulmonary hydatid diseases. J Natl Med Assoc. 2007;99:674–677. [PMC free article] [PubMed] [Google Scholar]
- 22.Moazeni M, Larki S. In vitro effectiveness of acidic and alkline solutions on scolices of hydatid cyst. Parasitol Res. 2010;106:853–856. doi: 10.1007/s00436-010-1723-3. [DOI] [PubMed] [Google Scholar]