Abstract
Congenital Neospora caninum infection was diagnosed in two Saanen goat kids from two distinct herds with a history of abortion and weak newborn goat kids in the Southern region of the State of Minas Gerais, Brazil. The first kid was weak at birth, had difficulty to rise and was unable to nurse. Gross lesions of porencephaly and hydrocephalus ex vacuo were seen. Multifocal necrosis, gliosis and non-supurative encephalitis were observed in the brain. Several parasitic cysts with a thick wall that reacted strongly only with polyclonal antiserum to Neospora caninum were seen in the cerebral cortex, brain stem and cerebellum. The second kid was born from a Neospora caninum seropositive mother that aborted in the last pregnancy. It was born without clinical signs. The diagnosis of neosporosis was based on antibody titer of 1:800 to N. caninum by indirect fluorescence antibody test obtained from blood collected before the goat kid ingested the colostrum and Neospora caninum DNA was detected by polymerase chain reaction and sequenced from placenta. This is the first report of neosporosis in goats in the southeast region of Brazil.
Keywords: Neospora caninum, histopathology, goat, parasitic infection
INTRODUCTION
Neospora caninum is an intracellular protozoan parasite that was first described in 1984 in dogs [1], but was not isolated and named until 1988 [2]. Neosporosis is considered the main cause of abortion in dairy cattle in several countries [3]. The fetus may die in utero, be resorbed, mumified, stillborn, born alive with clinical signs such as recumbency, underweight and neurologic signs, or born clinically normal but chronically infected [4]. Natural infection in goats is uncommon and only a few cases of abortion or congenital disease have been reported [5-8]. Gross lesions are rare [4]. Microscopic lesions may be present in many organs but are most common in the central nervous system (CNS), heart and liver [9]. In the CNS, the lesions consist of non-suppurative encephalomyelitis, with or without multifocal necrosis, glial proliferation, and presence of N. caninum in tissue sections [3]. In Brazil, the significance of the disease in goats has been poorly investigated. In one previous report in caprine herds from the southern region of Minas Gerais State, the mean prevalence rates of animals that tested positive by indirect fluorescence antibody test (IFAT) for N. caninum were 10.7% and for Toxoplasma gondii 21.4% and the relationship between occurrence of abortion and seroprevalence of N. caninum and T. gondii was significant by Fisher's exact test and chi-square test [10]. This report describes congenital neosporosis in two goat kids from two distinct herds with cases of abortion and newborn weak goat kids.
CASES REPORT
In the first case, the brain and few specimens of heart, lung, kidney and intestines fixed in 10% formalin, of a one-day-old male Saanen goat kid was submitted to the Veterinary Pathology Laboratory at the Universidade Federal de Lavras (UFLA), Minas Gerais State, Brazil for histopathology examination. The kid had been weak at birth, was unable to nurse, had difficulty to rise, and died few hours later. It was from a small herd with 19 does and one buck in the municipality of Lavras. One month later, serum samples from the herd were tested by IFAT for N. caninum and T. gondii antibodies using tachyzoites of the strain NC-1 and RH as antigens, respectively, and a commercial fluorescein isothiocyanate conjugate anti-goat IgG (Sigma, St. Louis, Missouri, USA) as a secondary antibody [10,11]. In the herd, 31.6% does were positive (>1:50) for N. caninum. The highest titer (1:400) was of the doe that gave birth to the N. caninum-infected goat kid. The doe did not have any antibodies for T. gondii. The animals were fed concentrate with a mineral mix containing 2 mg/kg Cu, 0.1 mg/kg Co, 30 mg/kg Fe, 0.1 mg/kg I, 0.1 mg/kg Se, 8 mg/kg Mg, 20 mg/kg Zn, and good quality forage.
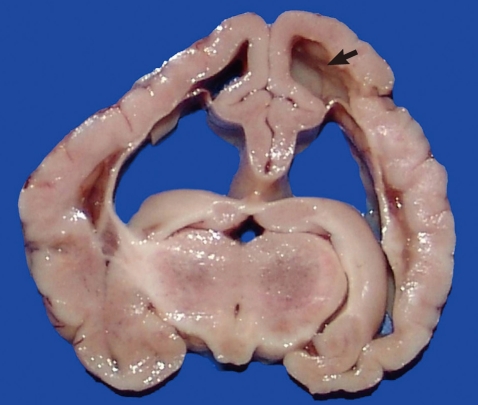
The cerebral hemispheres of the goat kid were asymmetrical, the gyri were swollen and flattened and, on cut surface there was porencephaly, characterized by fluid-filled cavities in areas normally occupied by the white matter. Due to the lack of brain substance, there was expansion of the lateral ventricles (hydrocephalus ex vacuo) (Fig. 1). Gross lesions were not observed in the cerebellum and brain stem. The tissues were processed for routine histopathologic examination.
Fig. 1.
Goat kid with congenital neosporosis. Transverse section of the brain. Note cavities in areas normally occupied by white matter (porencephaly) (arrow) and expansion of the lateral ventricles (hydrocephalus ex vacuo).
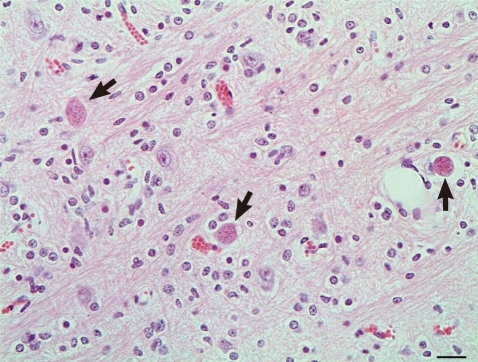
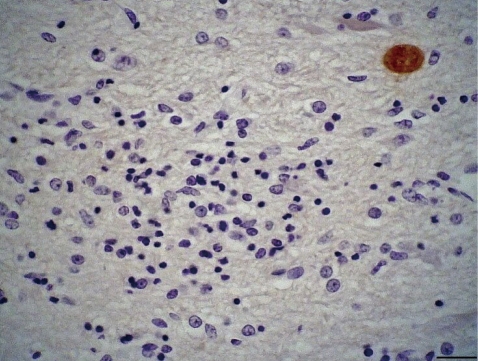
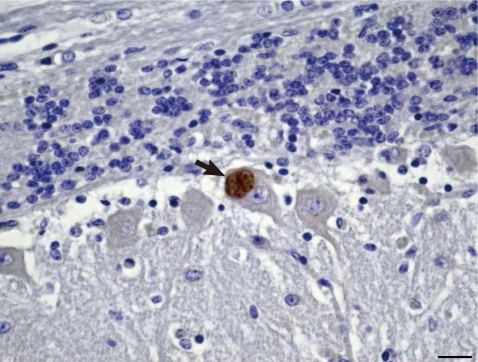
Microscopically, in the cortical gray matter there were mild to moderate perivascular cuffs of lymphocytes and plasma cells, multiple foci of gliosis and rare protozoal cysts. Also, there was absence of the white mater. The grossly observed cavitated areas, where surrounded, in some places, by glial cells. Small foci of necrosis in the brain stem and cortical gray matter were observed. Multiple foci of intense gliosis and numerous protozoal cysts were also in the cerebellum and brain stem (Fig. 2), either in the neuroparenchyma in areas of gliosis (Fig. 3) or in the cytoplasm of neurons (Fig. 4). The parasitic cysts ranged in size from 9.8×9.8 µm to 20.5×18.5 µm. Cyst walls were 1.0 µm thick. Additional brain sections were submitted to periodic acid-Schiff (PAS) staining and to immunohistochemistry (IHC) using antibodies anti-N. caninum (VMRD, Pullman, Washington, USA) and anti-T. gondii (VMRD, Pullman, Washington, USA) as previously described [6] and a streptavidin-biotin-peroxidase labeling kit (Dako, Carpinteria, California, USA) for the detection. Immunohistochemical labeling for Sarcocystis neurona (VMRD, Pullman, Washington, USA) was performed at the Diagnostic Center for Population and Animal Health at Michigan State University, Lansing, MI as previously described [12] using the Enhanced V-Red Detection system (Ventana Medical Systems, Tucson, Arizona, USA). Positive controls consisted of tissues that contained the different parasites. For the negative controls, the primary antibodies were replaced with homologous non immune sera. The cysts containing slender bradyzoites stained strongly with PAS and only with the anti-N. caninum serum (Figs. 3, 4). Tachyzoites were not observed. Alterations in other tissues consisted of mild nonsuppurative myocarditis with rare intralesional cysts stained positively by IHC for N. caninum, and a single similar protozoal cyst embedded in the pulmonary parenchyma. Associated tissue reaction was lacking in the lung. No lesions were observed in the other tissues. Serum samples of all animals from the herd were tested by agar gel immunodiffusion for bluetongue virus (BTV) antibodies [13] at the Laboratory of Animal Virology at the Universidade Federal de Minas Gerais, Minas Gerais State. The goats were all negative. Soil samples were collected in ten different sites of the pasture where the animals were kept and analyzed for Cu, Zn, Fe, B, Mg and S by Atomic Absorption Spectrometer and for Mo by Inductively Coupled Plasma - Optical Emission Spectrometry. The samples provided normal mineral parameters: Mo 0.199 mg/kg, Cu 8.5 mg/dm3, Zn 12.4 mg/dm3, Fe 50.6 mg/dm3, Mn 29.0 mg/dm3, B 0.1 mg/dm3, and S 14.3 mg/dm3. The liver from the porencephaly affected kid was not available for Cu analysis.
Fig. 2.
Goat kid with congenital neosporosis. Histologic section of the brain stem with numerous protozoal cysts (arrows). Hematoxylin and eosin stain. ×400. Bar=20 µm.
Fig. 3.
Brain stem with gliosis and intralesional positively labeled protozoal cyst. Immunohistochemical stain with anti-Neospora caninum antibody, Mayer's hematoxylin counterstain. ×400. Bar= 20 µm.
Fig. 4.
Cerebellum with positively labeled protozoal cysts (arrow) located in the cytoplasm of a Purkinje neuron. Immunohistochemical stain with anti-Neospora caninum antibody, Mayer's hematoxylin counterstain. ×400. Bar=20 µm.
In the second case, a pregnant goat was referred to UFLA for an assisted delivery because it aborted in the last pregnancy. Serum samples from the mother and from the newborn kid, collected prior to the ingestion of colostrum, were subjected to IFAT [11] for N. caninum and T. gondii antibodies. The kid and mother titers for N. caninum were 1:800 and 1:400, respectively. Both were negative for T. gondii (<1:64). Placenta samples for N. caninum PCR were submitted to the Virology Laboratory of the Veterinary Medicine Department at UFLA.
Genomic DNA was extracted from the placenta using a commercial kit (Invitek, Berlin, Germany) according to the manufacturer's instructions. Amplification of the gene flanking the ribosomal N. caninum fragment was carried out using primers based on published sequence, 5'-CGGAAGGATCATTCACACG-3' (forward direction) [14] and 5'-CCCACTGAAACAGACGTACC-3' (reverse direction) [15]. The PCR mixture consisted of 100 ng DNA, 1X PCR buffer, 2.5 mM MgCl2, 0.3 mM dNTPs, 3U Taq DNA polymerase, from a commercial kit (Promega, Madison, Wisconsin, USA) and 0.4 mM of each primer completed to a total volume of 20 µl with sterilized water [14]. Reactions were performed using a PT100 (MJ Research Incorporation, Waltham, Massachusetts, USA) thermocycler with the protocol: denaturation at 95℃ for 5 min, 35 cycles of 95℃ for 30 sec, 55℃ for 60 sec, 72℃ for 60 sec, and extension at 72℃ for 7 min. PCR amplicons were separated by electrophoresis on 1.5% agarose gels, purified using a commercial kit (Sigma, St. Louis, Missouri, USA) according to the manufacturer's instructions, and sequenced with a MegaBACETM sequencer (Amersham Biosciences, Amersham, UK). DNA sequence data were processed for the removal of gaps and primers, and the sequences compared using BlastN [16]. Sequence alignment and phylogenetic analysis was performed using ClustalW and MEGA4.
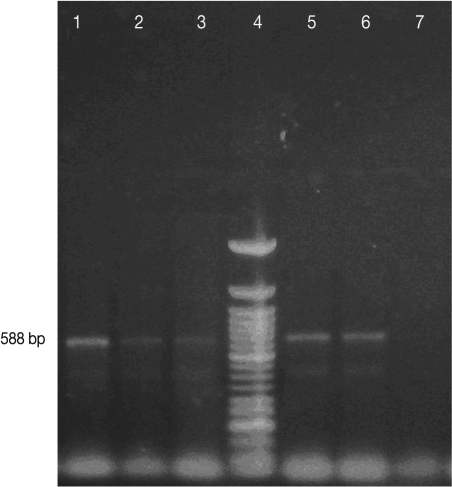
A 588 bp amplicon was obtained (Fig. 5) with the ribosomal N. caninum sequence primers and the amino acid sequence was deposited in GenBank (accession number HQ323749.1). The amino acid sequence showed more than 90% homology with other N. caninum sequences deposited in Genbank.
Fig. 5.
PCR for Neospora caninum using TIM11-LAV1 primers (TIM11 by Payne & Ellis, 1996. LAV1 produced by the authors). Lanes (1) Positive control, NC-1 isolate in Vero cells (588 bp); (2) and (3) Fetal bovine brain from a case of neosporosis unrelated to this report; (4) 50-bp DNA ladder (Invitrogen); (5) and (6) Caprine placenta from the case reported in these paper; (7) Negative control, MDBK cells DNA.
Placenta samples were fixed on 10% neutral buffered formalin, routinely processed and embedded in paraffin. Histologic examination of the placenta revealed no lesions and no parasites were seen by IHC procedures.
DISCUSSION
Two cases of congenital neosporosis in goats were reported here. In the first case porencephaly was seen. Gross lesions associated with N. caninum infection are rare, although hydrocephalus has been described in caprine [7]. Porencephaly in goats can be caused by copper deficiency and BTV infection. The majority of cases of copper deficiency described in goats have a delayed onset (delayed swayback), with a high incidence of cerebellar and peripheral motor axon degeneration [17]. Since the liver from kid number one was not submitted for testing, tissue Cu levels could not be determined. However, the soil of the farm where the goat herd was held showed adequate levels of copper and of its antagonists [18] and no other lesions of copper deficient were seen in the herd. Furthermore, the goats were fed with commercial feed supplemented with copper. Although it is not possible to completely rule out the diagnosis of copper deficiency, it is unlikely that deficiency of this trace mineral is involved in the lesions observed in the kid of this report. Bluetongue can cause hydranencephaly and porencephaly mainly in lambs and calves [19]. In Brazil, only one outbreak of this disease was described thus far and affected mainly sheep [20]. The goats in this study did not have any antibodies to BTV. Nevertheless, the exact etiology of the porencephaly remains undetermined. In spite of the concurrence of porencephaly and cerebral neosporosis it is not possible to ascertain the participation of the protozoan organism in the development of this brain lesion.
The histopathologic lesions of multifocal nonsuppurative encephalitis and gliosis associated with N. caninum in the goat kids are similar to those induced by T. gondii and Sarcocystis infection in ruminant fetuses and stillborn animals [7,21-24]. Sarcocystis species is identified by its specific endothelial tropism [23,24]. Sarcocystis is PAS-negative and characterized by a rosetting array of organisms that reflects its form of asexual reproduction [23-25]. N. caninum is very similar in appearance to T. gondii and therefore needs to be differentiated in tissues by immunoperoxidase using specific antisera [21,24] and by PCR [8,12]. A preliminary way to distinguish N. caninum from T. gondii is the thickness of the tissue cyst wall. The T. gondii tissue cysts have a very thin wall, that is <0.5 µm thick [7], which is negative by PAS in contrast to the bradyzoites, which are positive [26]. N. caninum cyst wall is thicker (≥1 µm), bradyzoites are PAS-positive and the cyst wall stains variably with PAS (26). In this study, the 1.0 µm thick cyst wall is consistent with descriptions of N. caninum, as it is thicker than that described for the cyst wall of T. gondii.
The presence of several cysts positive by PAS (bradyzoites and cyst wall) and anti-N. caninum antibodies accompanied by microscopic inflammatory and reactive lesions in the brain and heart of the goat kid, associated with the anti-N. caninum-IFAT titer (1:400) and no titer to T. gondii in the dam is consistent with the diagnosis of congenital neosporosis in that animal. The finding of several tissue cysts and no tachyzoites associated with the perivascular lymphoplasmacytic cell response and the presence of focal gliosis suggests that the infection was chronic in the goat kid.
In the second case, the diagnosis of congenital neosporosis was based on positive serology (1:800) obtained before the ingestion of colostrum and PCR of placental genetic material. These findings are similar to those in cattle where the majority of calves infected via placenta are born clinically healthy but perpetuate the infection within the herd [21]. The findings demonstrate that N. caninum infects goats in the state of Minas Gerais, Brazil, and should be considered in the differential diagnosis with other diseases responsible for abortion and weak newborn kids.
ACKNOWLEDGMENT
This work was supported by the Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Minas Gerais, Brazil.
References
- 1.Bjerkas I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd. 1984;70:271–274. doi: 10.1007/BF00942230. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc. 1988;192:1269–1285. [PubMed] [Google Scholar]
- 3.Dubey JP, Buxton D, Wouda W. Pathogenesis of bovine neosporosis. J Comp Pathol. 2006;134:267–289. doi: 10.1016/j.jcpa.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. 2003;41:1–16. doi: 10.3347/kjp.2003.41.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barr BC, Anderson ML, Woods LW, Dubey JP, Conrad PA. Neospora-like protozoal infections associated with abortions in goats. J Vet Diagn Invest. 1992;4:365–367. doi: 10.1177/104063879200400331. [DOI] [PubMed] [Google Scholar]
- 6.Corbellini LG, Colodel EM, Driemeier D. Granulomatous encephalitis in a neurologically impaired goat kid associated with degeneration of Neospora caninum tissue cysts. J Vet Diagn Invest. 2001;13:416–419. doi: 10.1177/104063870101300509. [DOI] [PubMed] [Google Scholar]
- 7.Dubey JP, Morales JA, Villalobos P, Lindsay DS, Blagburn BL, Topper MJ. Neosporosis-associated abortion in a dairy goat. J Am Vet Med Assoc. 1996;208:263–265. [PubMed] [Google Scholar]
- 8.Eleni C, Crotti S, Manuali E, Costarelli S, Filippini G, Moscati L, Magnino S. Detection of Neospora caninum in an aborted goat foetus. Vet Parasitol. 2004;123:271–274. doi: 10.1016/j.vetpar.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Dubey JP, Schares G. Diagnosis of bovine neosporosis. Vet Parasitol. 2006;140:1–34. doi: 10.1016/j.vetpar.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 10.Varaschin MS, Guimarães AM, Hirsch C, Mesquita LP, Abreu CC, Rocha CMBM, Wouters F, Moreira MC. Factors associated to seroprevalence of Neospora caninum and Toxoplasma gondii in caprine herds in southern Minas Gerais state, Brazil. Pesq Vet Bras. 2011;31:53–58. [Google Scholar]
- 11.Figliuolo LPC, Rodrigues AAR, Viana RB, Aguiar DM, Kasai N, Gennari SM. Prevalence of anti-Toxoplasma gondii and anti-Neospora caninum antibodies in goats from São Paulo State, Brazil. Small Ruminant Res. 2004;55:29–32. [Google Scholar]
- 12.Soldati S, Kiupel M, Wise A, Maes R, Botteron C, Robert N. Meningoencephalomyelitis caused by Neospora caninum in a juvenile fallow deer (Dama dama) J Vet Med A Physiol Pathol Clin Med. 2004;51:280–283. doi: 10.1111/j.1439-0442.2004.00646.x. [DOI] [PubMed] [Google Scholar]
- 13.Costa JRR, Lobato ZIP, Herrmann GP, Leite RC, Haddad JPA. Prevalence of bluetongue virus antibodies in cattle and sheep in southwest and southeast regions of Rio Grande do Sul, Brazil. Arq Bras Med Vet Zootec. 2006;58:273–275. [Google Scholar]
- 14.dos Santos DS, Andrade MP, Varaschin MS, Guimarães AM, Hirsch C. Neospora caninum in bovine fetuses of Minas Gerais, Brazil: genetic characteristics of rDNA. Rev Bras Parasitol Vet. 2011;20:281–288. doi: 10.1590/s1984-29612011000400005. [DOI] [PubMed] [Google Scholar]
- 15.Payne S, Ellis J. Detection of Neospora caninum DNA by the polymerase chain reaction. Int J Parasitol. 1996;26:347–351. doi: 10.1016/0020-7519(96)00030-6. [DOI] [PubMed] [Google Scholar]
- 16.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 17.Jubb KVF, Huxtable CR. The Nervous System. In: Jubb KVF, Kennedy PC, Palmer N, editors. Pathology of Domestic Animals (Vol. 1) 4th ed. San Diego, USA: Academic Press; 1993. pp. 267–439. [Google Scholar]
- 18.Underwood EJ, Suttle NF. The mineral nutrition of livestock. 3th ed. CAB International; 1999. pp. 1–614. [Google Scholar]
- 19.Zachary JF. Nervous System. In: MacGavin MD, Zachary JF, editors. Pathologic Basis of Veterinary Disease. St. Louis, Missouri, USA: Mosby, Elsevier; 2007. pp. 833–971. [Google Scholar]
- 20.Clavijo A, Sepulveda L, Riva J, Pessoa-Silva M, Tailor-Ruthes A, Lopez JW. Isolation of bluetongue virus serotype 12 from an outbreak of the disease in South America. Vet Rec. 2002;151:301–302. doi: 10.1136/vr.151.10.301. [DOI] [PubMed] [Google Scholar]
- 21.Dubey JP, Lindsay DS. A review of Neospora caninum and neosporosis. Vet Parasitol. 1996;67:1–59. doi: 10.1016/s0304-4017(96)01035-7. [DOI] [PubMed] [Google Scholar]
- 22.Dubey JP. Epizootic toxoplasmosis associated with abortion in dairy goats in Montana. J Am Vet Med Assoc. 1981;178:661–670. [PubMed] [Google Scholar]
- 23.Hong CB, Giles RC, Jr, Newman LE, Fayer R. Sarcocystosis in an aborted bovine fetus. J Am Vet Med Assoc. 1982;181:585–588. [PubMed] [Google Scholar]
- 24.Barr BC, Anderson ML, Blanchard PC, Daft BM, Kinde H, Conrad PA. Bovine fetal encephalitis and myocarditis associated with protozoal infections. Vet Pathol. 1990;27:354–361. doi: 10.1177/030098589002700508. [DOI] [PubMed] [Google Scholar]
- 25.Dubey JP, Davis SW, Speer CA, Bowman DD, de Lahunta A, Granstrom DE, Topper MJ, Hamir AN, Cummings JF, Suter MM. Sarcocystis neurona n. sp. (Protozoa: Apicomplexa), the etiologic agent of equine protozoal myeolencephalitis. J Parasitol. 1991;77:212–218. [PubMed] [Google Scholar]
- 26.Dubey JP, Barr BC, Barta JR, Bjerkås I, Björkman C, Blagburn BL, Bowman DD, Buxton D, Ellis JT, Gottstein B, Hemphill A, Hill DE, Howe DK, Jenkins MC, Kobayashi Y, Koudela B, Marsh AE, Mattsson JG, McAllister MM, Modrý D, Omata Y, Sibley LD, Speer CA, Tress AJ, Uggla A, Upton SJ, Williams DJL, Lindsay DS. Redescription of Neospora caninum and its differentiation from related coccidia. Int J Parasitol. 2002;32:929–946. doi: 10.1016/s0020-7519(02)00094-2. [DOI] [PubMed] [Google Scholar]