Abstract
Toxoplasma gondii can modulate host cell gene expression; however, determining gene expression levels in intermediate hosts after T. gondii infection is not known much. We selected 5 genes (ALDH1A2, BEX2, CCL3, EGR2 and PLAU) and compared the mRNA expression levels in the spleen, liver, lung and small intestine of genetically different mice infected with T. gondii. ALDH1A2 mRNA expressions of both mouse strains were markedly increased at day 1-4 postinfection (PI) and then decreased, and its expressions in the spleen and lung were significantly higher in C57BL/6 mice than those of BALB/c mice. BEX2 and CCR3 mRNA expressions of both mouse strains were significantly increased from day 7 PI and peaked at day 15-30 PI (P<0.05), especially high in the spleen liver or small intestine of C57BL/6 mice. EGR2 and PLAU mRNA expressions of both mouse strains were significantly increased after infection, especially high in the spleen and liver. However, their expression patterns were varied depending on the tissue and mouse strain. Taken together, T. gondii-susceptible C57BL/6 mice expressed higher levels of these 5 genes than did T. gondii-resistant BALB/c mice, particularly in the spleen and liver. And ALDH1A2 and PLAU expressions were increased acutely, whereas BEX2, CCL3 and EGR2 expressions were increased lately. Thus, these demonstrate that host genetic factors exert a strong impact on the expression of these 5 genes and their expression patterns were varied depending on the gene or tissue.
Keywords: Toxoplasma gondii, RT-PCR, mouse, ALDH1A2, BEX2, CCL3, EGR2, PLAU
INTRODUCTION
The intracellular protozoan parasite Toxoplasma gondii is a ubiquitous pathogen of warm-blooded animals, with approximately one to 2 billion humans infected worldwide. Furthermore, this parasite can infect and replicate in virtually any nucleated cells, and is able to penetrate several important barriers [1]. It causes a large range of clinical manifestations in humans, from abortion and congenital infection to eye disease and fatal encephalitis [1]. During host cell invasion, T. gondii uses a specialized set of secretory organelles to inject parasite-derived effector molecules into host cells. This parasite displays a high level of sophistication in its ability to manipulate host responses and their underlying signal transduction cascades [2-5]. Expression-profiling of a Toxoplasma-infected host transcriptome showed a significant change in host gene transcripts as compared with uninfected cells at 24 hr post-infection [2]. Similarly, quantitative analysis of the host proteome during Toxoplasma infection showed that the host cell proteome responded in a dramatic way to T. gondii invasion in terms of both expression levels and protein modifications, revealing a complex and intimate molecular relationship between host and parasite [5]. Although previous data demonstrated that T. gondii-infected cells modulate various molecules including cytokines or chemokines [2-5], the transcriptional profiles of host genes after T. gondii-infection remain to be elucidated.
Toxoplasma can modify host cell signaling and cell cycle control, and also modulates host cell gene expression [5,6]. However, determining the expression levels of specific genes in intermediate hosts after T. gondii infection is not know much. Recently, we examined the transcriptional profile of T. gondii-infected THP-1 macrophages using a cDNA microarray, and we selected 5 genes that showed significantly increased mRNA expression at more than one timepoint in T. gondii-infected THP-1 cells as compared with uninfected cells. The following genes were included: ALDH1A2 (aldehyde dehydrogenase1 family, member A2), BEX2 (brain expressed X-linked 2), CCL3 (chemokine C-C motif ligand 3), EGR2 (early growth response 2) and PLAU (plasminogen activator, urokinase). Mice have a similar genetic background and immune-related organs as compared with humans, which makes them an appropriate model for investigation of immune responses and host-pathogen interactions against T. gondii [7]. Therefore, to compare the mRNA expression profiles of these 5 genes in genetically different mice, T. gondii-resistant BALB/c and T. gondii-susceptible C57BL/6 mice were infected with the Me49 strain of T. gondii, and mRNA levels of these genes in the spleen, liver, lung and small intestine of both mouse strains were determined using reverse transcription polymerase chain reaction (RT-PCR).
MATERIALS AND METHODS
Mice
Female 6- to 8-week-old BALB/c (resistant to T. gondii infection) and C57BL/6 (susceptible to T. gondii infection) mice were used. All mice were maintained in pathogen-free conditions within our animal facility. Animal studies were performed under the authority of the Chungnam National University Animal Ethnics Committee.
Parasites and T. gondii infection
Cysts of T. gondii strain ME49 were harvested from the brains of chronically infected mice. Mice were sacrificed and their brains were homogenized in phosphate buffered saline (PBS; pH 7.2). Ten microliters of brain suspension was placed on a glass slide and the number of cysts was counted in 5 separate fields of view using a light microscope. For experimental infections, BALB/c and C57BL/6 mice were orally inoculated with 5 T. gondii Me49 cysts by gavage.
Preparation of mouse tissues and oligonucleotides
Mice were sacrificed on days 0, 1, 4, 7, 15, and 30 after peroral infection with T. gondii ME49. The spleen, liver, lung and small intestine from both BALB/c and C57BL/6 mice were removed, washed in PBS, and total RNA was extracted.
We selected 5 genes ALDH1A2, PLAU, BEX2, EGR2, and CCL3 from the transcriptional profile of T. gondii-infected THP-1 macrophages using a cDNA microarray. These genes were shown to have significantly increased mRNA expression at more than one time point in T. gondii-infected THP-1 cells among 4 times of checking point (1, 4, 6, and 16 hr PI) as compared with uninfected cells. To examine the mRNA expression profiles of 5 genes in the tissues of mice, we prepared oligonucleotide primers specific for each of these 5 genes (Table 1). Primers for PCR were designed from sequences selected from GenBank (National Center for Biotechnology Information; http://www.ncbi.nlm.nih.gov) using the Primer3 program at an annealing temperature of 60℃.
Table 1.
Primers sequences used for reverse transcriptase-polymerase chain reaction (RT-PCR)
aALDH1A2, Aldehyde dehydrogenase1 family, member A2; PLAU, Plasminogen activator, Urokinase; BEX2, Brain expressed X-linked 2; EGR2, Early growth response 2; CCL3, Chemokine (C-C motif) Ligand 3.
bF, forward primer sequences; R, reverse primer sequences.
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from the spleen, liver, lung and small intestine of BALB/c and C57BL/6 mice using Trizol reagent (Invitrogen, Carlsbad, California, USA), according to the manufacturer's instructions. For RNA extraction, 80-100 mg of tissue was cut into small pieces and homogenized in 1 ml of Trizol reagent. Homogenized samples were incubated for 5 min at room temperature (RT), 0.2 ml chloroform was added, and the mixture was then incubated for 3 min at RT, followed by centrifugation at 12,000×g for 15 min at 4℃. Isopropyl alcohol (0.5 ml) was added to the supernatant to precipitate RNA. After washing with 75% ethanol, the RNA pellet was dried at RT and resuspended in 100 µl diethyl pyrocarbonate-treated RNase-free water (DEPC). The concentration and purity of RNA were determined using a spectrophotometer.
The cDNA reaction mixture contained the following: 5 µg total RNA; 10 pmol oligo (dT)15 primer (Bioneer, Daejon, Korea), 2 µl; 10× M-MLV RT reaction buffer (0.5 M Tris-HCl; pH 8.3, 0.75 M KCl, 30 mM MgCl2, 0.1 M DTT, Enzynomics, Daejeon, Korea) 2 µl; 0.25 mM dNTP mixture (TaKaRa, Otsu, Shiga, Japan), 2 µl; ribonuclease inhibitor (20 units, Enzynomics), 0.25 µl; and M-MLV reverse transcriptase (200 units/µl, Enzynomics), 1 µl. Reverse transcriptase (RT) reactions were incubated for 60 min at 42℃, and the reaction stopped by incubation at 70℃ for 10 min. PCR reactions were performed in a 25 µl volume using 1 µl cDNA as a template, 10× Taq polymerase buffer, 2.5 µl; 2.5 mM dNTP, 2 µl; 5' and 3' primers (10 pmol/µl), 1.5 µl: (Table 1) 10× Taq polymerase, 0.125 µl (Enzynomics); and cDNA template, 1 µl. β-Actin cDNA was used as an internal standard. PCR conditions were as follows: 95℃ for 2 min; followed by 30-35 cycles of denaturation at 95℃ for 40 sec, annealing at 60℃ for 30 sec, and extension at 72℃ for 30 sec; followed by a final extension at 72℃ for 10 min. To determine the necessary number of amplification cycles, we checked the amounts of amplification products of each gene at various numbers of PCR cycles. The PCR products were increased linearly within the numbers of PCR cycle of each gene (30-35 cycles). Twelve microliter of the PCR product were subjected to electrophoresis in a 1% agarose gel with 0.5% TBE buffer containing 0.5 g ethidium bromide. A UV transilluminator (Gel Doc, Bio-Rad Laboratories Ltd, Hemel, Hempstead, UK) was used to visualize PCR products and quantify the band intensities.
Statistical analysis
Results are expressed as the mean±standard error (SE) for each group. Statistical differences in mRNA expression were determined using the Kruskal-Wallis test. Differences among the various groups were considered significant when P<0.05.
RESULTS
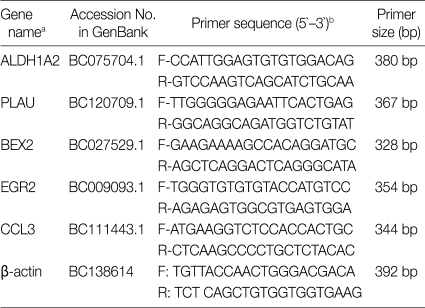
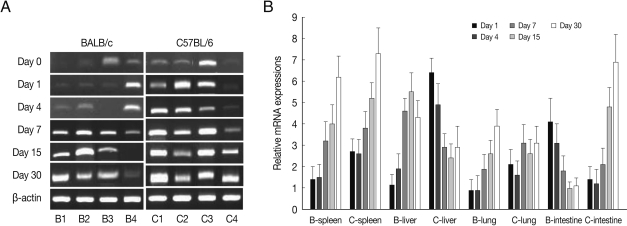
As shown in Fig. 1, ALDH1A2 mRNA expressions in the spleen, liver, lung and small intestine of both mouse strains were markedly increased at day 1-4 PI as compared with uninfected controls (mRNA expression level was set as 1; P<0.01), and decreased thereafter to the basal level at day 15-30 PI. At day 1-4 PI, ALDH1A2 mRNA levels in the spleen and lung of C57BL/6 mice were significantly higher as compared with those of BALB/c mice, however there were not significant differences of its expressions between liver and small intestine.
Fig. 1.
ALDH1A2 mRNA expressions in the various tissues of mice infected with cyst of Me49 strain of Toxoplasma gondii. (A) Cellular RNA was then extracted, and the mRNA expression of ALDH1A2 was analysed by RT-PCR, (B) Kinetics of mRNA expression of AHDH1A2. Data are presented as mean±SE of 5 mice per group and are representative of 2 separate experiments. B1-4; spleen, liver, lung and small intestine of T. gondii-infected BALB/c mice, respectively. C1-4; spleen, liver, lung and small intestine of T. gondii-infected C57BL/6 mice, respectively.
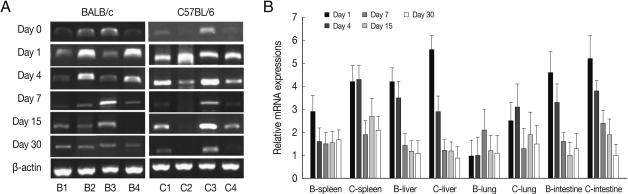
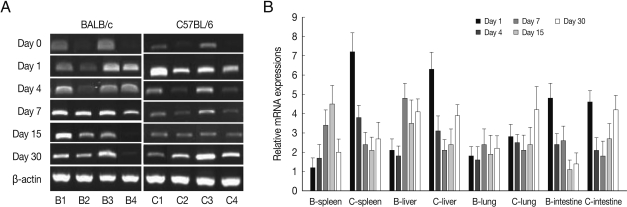
BEX2 mRNA expressions in the spleen, liver, lung and small intestine of both mouse strains were significantly increased from day 7 PI in comparison to uninfected control and peaked at day 15-30 PI, especially high in the spleen and small intestine of C57BL/6 mice (Fig. 2). BEX2 mRNA expressions in the spleen and small intestine were significantly higher in the C57BL/6 mice than those of BALB/c mice, however there were not significant differences of BEX2 expressions in the liver and lung between both mouse strains.
Fig. 2.
BEX2 mRNA expressions in the various tissues of mice infected with cyst of Me49 strain of T. gondii. (A) Cellular RNA was then extracted, and the mRNA expression of BEX2 was analyzed by RT-PCR, (B) Kinetics of mRNA expression of BEX2. Data are presented as mean±SE of 5 mice per group and are representative of 2 separate experiments.
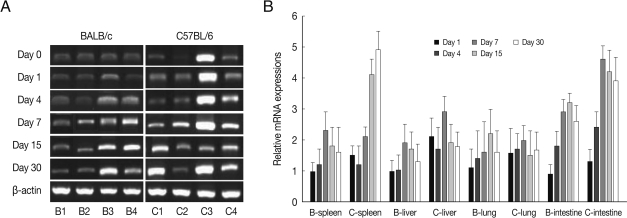
CCL3 mRNA expressions in the spleen, liver, lung and small intestine of both mouse strains were significantly increased from 7-15 days after T. gondii infection as compared to uninfected control, and then peaked at day 30 PI (P<0.05), and its expressions in the spleen and liver were prominently high (Fig. 3). CCL3 mRNA expressions in the small intestine were significantly higher in C57BL/6 mice as compared to that of BALB/c mice, however its expressions in the spleen, liver or lung were not significantly different from each mouse strain.
Fig. 3.
CCL3 mRNA expressions in the various tissues of mice infected with cyst of Me49 strain of T. gondii. (A) Cellular RNA was then extracted, and the mRNA expression of CCL3 was analyzed by RT-PCR, (B) Kinetics of mRNA expression of CCL3. Data are presented as mean±SE of 5 mice per group and are representative of 2 separate experiments.
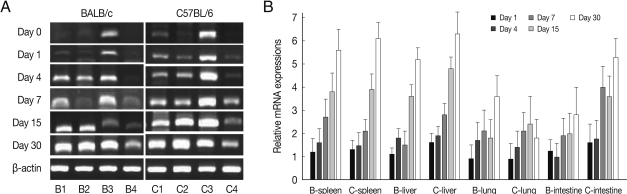
As shown in Fig. 4, EGR2 mRNA expressions in the spleen and lung of both mouse strains were gradually increased after T. gondii infection, and peaked at day 30 PI (P<0.01). However EGR2 expression patterns were different in the liver and small intestine between BALB/c and C57BL/6 mice. EGR2 mRNA expressions in the liver of BALB/c mice was initially low and peaked at day 15 or 30 PI (P<0.01), whereas its expression in C57BL/6 mice were markedly high at day 1 PI and decreased thereafter (P<0.01). EGR2 mRNA expression in the small intestine of BALB/c mice was peaked at day 1 PI (P<0.01) and then decreased gradually to the basal, whereas its expression of C57BL/6 mice were significantly increased from day 7 PI and peaked at day 30 PI.
Fig. 4.
EGR2 mRNA expressions in the various tissues of mice infected with cyst of Me49 strain of Toxoplasma gondii. (A) Cellular RNA was then extracted, and the mRNA expression of EGR2 was analyzed by RT-PCR, (B) Kinetics of mRNA expression of EGR2. Data are presented as mean±SE of 5 mice per group and are representative of 2 separate experiments.
PLAU mRNA expressions in the spleen and liver of BALB/c mice were significantly increased from day 7 PI as compared with those of uninfected control (P<0.05) and peaked at day 15-30 PI, however its expressions in C57BL/6 mice were peaked at day 1 (P<0.01), and then abruptly decreased. PLAU mRNA expressions did not change significantly in the lung of both mouse strains during experimental period (Fig. 5). PLAU mRNA expression in the small intestine of BALB/c and C57BL/6 mice were peaked at day 1 PI (P<0.01) and then decreased (P<0.01).
Fig. 5.
PLAU mRNA expressions in the various tissues of mice infected with cyst of Me49 strain of T. gondii. (A) Cellular RNA was then extracted, and the mRNA expression of PLAU was analyzed by RT-PCR, (B) Kinetics of mRNA expression of PLAU. Data are presented as mean±SE of 5 mice per group and are representative of 2 separate experiments.
DISCUSSION
Host genetic factors strongly determine the course of various infectious diseases. BALB/c mice are known to be genetically resistant to T. gondii infection, whereas C57BL/6 mice are susceptible [8]. We evaluated the role of host genetic factors on the expressions of ALDH1A2, BEX2, EGR2, CCL3 and PLAU genes in various mouse tissues. Five gene mRNA expression levels were higher in T. gondii-infected C57BL/6 mouse as compared with BALB/c mouse, especially high in the spleen and liver. And ALDH1A2 and PLAU expression were increased acutely, whereas BEX2, CCL3 and EGR2 expressions were increased lately. Thus, these demonstrate that host genetic factors exert a strong impact on the expression of these 5 genes in a murine model and their expression patterns were varied depending on the gene or tissue.
Host cell transcription is a major process affected by infection, and changes in gene expression occur at various times following infection [2,3,9]. The EGR1 and EGR2 genes are well-known immediate early response genes in growth factor-stimulated cells and encode transcription factors that regulate cell survival and growth [10]. In this study, EGR2 mRNA expression was rapidly upregulated in both strains of mice after T. gondii infection, especially high in the spleen and small intestine of T. gondii-infected C57BL/6 mouse as compared with those of BALB/c mice. These results are similar to previous studies suggesting that EGR1 and EGR2 transcription factors, as well as their downstream targets, were rapidly upregulated in Toxoplasma-infected cells [2,11], and that T. gondii increases the activity of transcription factors, including STAT3/6, hypoxia inducible factor and EGR2 [12]. Both our work and previous studies demonstrate that viable T. gondii acts as a powerful inducer of the transcriptional factor EGR2, especially in C57BL/6 mice, but the generated expression patterns differ from those induced by host factors.
ALDH1A2 is the primary enzyme responsible for the synthesis of retinoic acid (RA) from retinaldehyde. RA, the active derivative of vitamin A (retinol), is a signaling molecule that plays a major role in regeneration and differentiation in normal and disease states [13]. BEX2 belongs to the brain-expressed X-linked gene family and is known to play a role in cell cycle progression and neuronal differentiation [14], and it regulates mitochondrial apoptosis and G1 cell cycle in breasr cancer [15]. In this study, ALDH1A2 and BEX2 mRNA expressions in both mouse strains were upregulated after infection, and its expression was higher in C57BL/6 than BALB/c mice, especially in the spleen and small intestine. These data suggest that high production of ALDH1A2 and BEX2 mRNA in the spleen and small intestine of C57BL/6 mice may be related to a mechanism to compensate the pathophysiological changes such as apoptosis after T. gondii infection [13].
PLAU encodes a serine protease involved in degradation of the extracellular matrix. PLAU protein is capable of converting plasminogen to plasmin and plays a key role in cell adhesion, migration, and proliferation [16]. In the current study, PLAU mRNA expression levels were higher in C57BL/6 mice than those of BALB/c mice, especially high in the spleen and liver. These phenomena indicate that T. gondii-infected C57BL/6 mice may induce more tissue reactions such as inflammation, thrombolysis, wound healing, and tissue regeneration than in BALB/c mice [17].
CCL3 is produced by several types of immune cells and glial cells and selectively promotes adhesion and chemotaxis of various leukocyte subtypes [18]. We observed that CCL3 mRNA expression was significantly increased in both mouse strains after T. gondii infection, and was especially high in the spleen, liver and small intestine of C57BL/6 mice. Previous studies demonstrated that mRNA expression levels of CCL2, CCL3, CCL5, CXCL2, CXCL9 and CXCL10 were significantly increased on day 8 PI in freshly isolated IECs collected from T. gondii-infected mice [19], and T. gondii triggered neutrophil synthesis of CCL3, CCL4, CCL5, and CCL20 [20]. Both our and previous data suggest that CCL3 is strongly expressed in mice after T. gondii infection; however, its expression was higher in a susceptible mouse strain.
Here, we evaluated the mRNA expression profile of ALDH1A2, BEX2, CCL3, EGR2, and PLAU genes to identify the impact of host genetic factors in T. gondii infection. Levels of mRNA expressions were high in the spleen, liver and small intestine of C57BL/6 mice; however, mRNA expressions in the lung of BALB/c mice were low. High expression of these genes in T. gondii-infected C57BL/6 mice may serve as a compensatory mechanism, preventing the pathophysiologic consequences of T. gondii infection. Thus, our data demonstrate that host genetic factors exert a strong impact on the expression of certain genes in tissue- and gene-specific manners, which allow the host to resist parasite infection.
ACKNOWLEDGMENTS
This study was financially supported by research fund of Chungnam National University in 2008. The authors had no commercial interest in the study.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem. 2001;276:24223–24231. doi: 10.1074/jbc.M100951200. [DOI] [PubMed] [Google Scholar]
- 3.Gail M, Gross U, Bohne W. Transcriptional profile of Toxoplasma gondii-infected human fibroblasts as revealed by gene-array hybridization. Mol Genet Genomics. 2001;265:905–912. doi: 10.1007/s004380100487. [DOI] [PubMed] [Google Scholar]
- 4.Okomo-Adhiambo M, Beattie C, Rink A. cDNA microarray analysis of host-pathogen interactions in a porcine in vitro model for Toxoplasma gondii infection. Infect Immun. 2006;74:4254–4265. doi: 10.1128/IAI.00386-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson MM, Jones AR, Carmen JC, Sinai AP, Burchmore R, Wastling JM. Modulation of the host cell proteome by the intracellular apicomplexan parasite Toxoplasma gondii. Infect Immun. 2008;76:828–844. doi: 10.1128/IAI.01115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lüder CG, Stanway RR, Chaussepied M, Langsley G, Heussler VT. Intracellular survival of apicomplexan parasites and host cell modification. Int J Parasitol. 2009;39:163–173. doi: 10.1016/j.ijpara.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Tsaparas P, Mariño-Ramírez L, Bodenreider O, Koonin EV, Jordan IK. Global similarity and local divergence in human and mouse gene co-expression networks. BMC Evol Biol. 2006;6:70. doi: 10.1186/1471-2148-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strack A, Schlüter D, Asensio VC, Campbell IL, Deckert M. Regulation of the kinetics of intracerebral chemokine gene expression in murine Toxoplasma encephalitis: Impact of host genetic factors. Glia. 2002;40:372–377. doi: 10.1002/glia.10104. [DOI] [PubMed] [Google Scholar]
- 9.Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-κB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IκB to the parasitophorous vacuole membrane. J Cell Sci. 2003;116:4359–4371. doi: 10.1242/jcs.00683. [DOI] [PubMed] [Google Scholar]
- 10.Wiley M, Teygong C, Phelps E, Radke J, Blader IJ. Serum response factor regulates immediate early host gene expression in Toxoplasma gondii-infected host cells. PLoS One. 2011;6:e18335. doi: 10.1371/journal.pone.0018335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelps ED, Sweeney KR, Blader IJ. Toxoplasma gondii rhoptry discharge correlates with activation of the early growth response 2 host cell transcription factor. Infect Immun. 2008;76:4703–4712. doi: 10.1128/IAI.01447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollard AM, Knoll LJ, Mordue DG. The role of specific Toxoplasma gondii molecules in manipulation of innate immunity. Trends Parasitol. 2009;25:491–494. doi: 10.1016/j.pt.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudas LJ. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim Biophys Acta. 2012;1821:213–221. doi: 10.1016/j.bbalip.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez E, Zhou W, Witta SE, Freed CR. Characterization of the Bex gene family in humans, mice, and rats. Gene. 2005;357:18–28. doi: 10.1016/j.gene.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Naderi A, Liu J, Bennett IC. BEX2 regulates mitochondrial apoptosis and G1 cell cycle in breast cancer. Int J Cancer. 2010;126:1596–1610. doi: 10.1002/ijc.24866. [DOI] [PubMed] [Google Scholar]
- 16.Ragno P. The urokinase receptor: a ligand or a receptor? Story of a sociable molecule. Cell Mol Life Sci. 2006;63:1028–1037. doi: 10.1007/s00018-005-5428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blasi F, Carmeliet P. uPAR: A versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 18.Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 19.Gopal R, Birdsell D, Monroy FP. Regulation of chemokine responses in intestinal epithelial cells by stress and Toxoplasma gondii infection. Parasite Immunol. 2011;33:12–24. doi: 10.1111/j.1365-3024.2010.01248.x. [DOI] [PubMed] [Google Scholar]
- 20.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]