Abstract
Particulate matter (PM) is an important metric for studying the health effects of household air pollution. There are limited data on PM exposure for children in homes that use biomass fuels, and no previous study has used direct measurement of personal exposure in children younger than 5 years of age. We estimated PM2.5 exposure for 1,266 children in The Gambia by applying the cookhouse PM2.5-CO relationship to the child’s CO exposure. Using this indirect method, mean PM2.5 exposure for all subjects was 135 ± 38 μg/m3; 25% of children had exposures of 151 μg/m3 or higher. Indirectly-estimated exposure was highest among children who lived in homes that used firewood (collected or purchased) as their main fuel (144 μg/m3) compared to those who used charcoal (85 μg/m3). To validate the indirect method, we also directly measured PM2.5 exposure on 31 children. Mean exposure for this validation dataset was 65 ± 41 μg/m3 using actual measurement and 125 ± 54 μg/m3 using the indirect method based on CO exposure. The correlation coefficient between direct measurements and indirect estimates was 0.01. Children in The Gambia have relatively high PM2.5 exposure. There is a need for simple methods that can directly measure PM2.5 exposure in field studies.
Keywords: Indoor air pollution, biomass fuels, child survival, global health, Africa, particulate matter, exposure assessment, statistical model
Introduction
Biomass fuels and coal are the primary source of energy for cooking and heating for approximately onehalf of the world’s population, and 80% of the population of sub-Saharan Africa [1]. In most developing countries, biomass is burned in traditional open fires leading to high concentrations of multiple pollutants [1–3]. Women and young children may spend hours near cooking fires, and hence have high exposures. There is increasing evidence that biomass smoke is a risk factor for pneumonia, the leading cause of child death worldwide [4].
Air pollution is a complex mixture of solid and gaseous pollutants. Although multiple components of combustion air pollution are associated with adverse health outcomes, particulate matter (PM) has been consistently, independently, and coherently related to various diseases affected by air pollution [5–8]. Measuring personal exposure of children to PM is difficult because current PM monitors are too large and heavy to be carried by a small child for many hours. As a result, there is very little data on personal exposure to PM from biomass smoke among children. Previous research has used two indirect methods to assess personal PM exposure of children: measuring personal exposure to carbon monoxide (CO) as a proxy pollutant to estimate PM exposure [9–13] and micro-environment PM monitoring combined with time-location-activity budgets [14–17]. A recent study directly measured the personal PM exposure of school-age children and adult women in rural China [18]. To our knowledge, no study has directly measured personal PM exposure of children younger than 5 years of age, who account for most pneumonia deaths [19]; nor have previous studies compared estimates of PM exposure from the above two indirect methods. Finally, due to a lack of measured PM exposure data, it has not been possible to correlate children’s PM exposure with their CO exposure or with household PM concentration. The lack of validated methods for measuring children’s PM exposure from biomass fuels is an obstacle to estimating the dose-response relationship for biomass smoke and in evaluating how much different interventions reduce exposure.
We conducted a study in The Gambia in which we used an indirect method to estimate PM exposure by applying the PM-CO relationship from stationary monitors to personal CO exposure. In addition, to assess the validity of CO as a proxy for PM exposure, we conducted a distinct validation study in which we directly measured personal PM exposure for a small number of children. We examined both the systematic and random error of the indirect estimates of PM exposure based on CO exposure with direct PM measurements collected over the same period.
Methods
The study was approved by The Gambia Government-MRC Joint Ethics Committee (SCC/EC 1062) and was assessed as exempt by the Harvard School of Public Health Office of Human Research Administration.
Overview
Our study had three components: First, we used an indirect method to estimate PM2.5 exposure for 1,266 children. Following a previous study in Guatemala [10–11, 13] and a pilot study in The Gambia [20], we used CO as a proxy for PM exposure, and estimated PM2.5 using the PM2.5-CO relationship from stationary cookhouse monitors. In doing so, this component applied an indirect method that has been deemed feasible for large field studies in an African setting, where child mortality is higher than in other world regions [21]. We extended this indirect approach by including fuel type, season (rainy vs. dry), and study site in the PM2.5-CO relationship. We selected our statistical model based on formal tests among multiple candidate models. Second, we estimated PM2.5 exposure for 76 of these children using continuous cookhouse PM2.5 data and questionnaire data on time-location-activity budgets. We examined the correlation between indirect PM2.5 exposure using CO and indirect PM2.5 exposure using time-location-activity budgets. Finally and importantly, we directly measured PM2.5 exposure for 48 children, and examined the systematic and random error of the indirect estimates based on CO in relation to the direct measurements over the same period. We could not compare indirect estimates using time-location-activity budgets with direct measurements because there were very few usable cookhouse measurements during direct exposure measurement days.
Study area, population, and participants
Our study took place in The Gambia, in the greater Banjul area and the Basse area of the Upper River Region. The study areas consist primarily of periurban and rural locations, but a few urban homes were also included. Study participants were children aged between 2 and 59 months at the time of recruitment into an epidemiologic study of child pneumonia conducted at the Medical Research Council (MRC), The Gambia Unit. Details on the study area and population, recruitment, and the child-care behaviors, cooking fuel and location, and demographic characteristics of study participants are provided elsewhere [12]. At the time of this analysis, 1,303 children had been enrolled. Of these, 37 withdrew from the study as detailed elsewhere [12], and were excluded from all analyses, leaving a sample of 1,266 children.
CO exposure
We used a parsimonious mixed effects model to estimate CO exposure. The model is described in detail elsewhere [12]. In brief, the model used measurement season (rainy vs. dry), and questionnaire and measured CO exposure data to estimate each child’s “usual” CO exposure (see below). The questionnaire data used were type of fuel used for cooking and other PM sources in the child’s home (incense, trash, and insect coil burning). The model had two parts that together accounted for the following features of CO exposure (i) CO exposure of some children may be non-zero but below the limit of detection (LOD) of the CO measurement equipment and (ii) there is within-child exposure variability across days that can be addressed using repeated measurements. The model parameters were estimated using CO exposure data measured between July 2007 and January 2011; 43% of children had up to five repeated measurements to quantify within child exposure variability. Repeated measurements were done over a period of 2–3 months for each child, to reflect the exposure period that is likely to be relevant for pneumonia. We used the model to predict CO exposure using this method for all 1,266 children in the rainy and dry seasons, removing or reducing the within-child variability as above; exposure to a time-varying risk factor calculated using this method is commonly referred to as a “usual exposure” [22]. Season-specific usual CO exposures were predicted by separately setting the season variable to rainy or dry. Annual CO exposure for each child was calculated using a weighted average of rainy and dry season usual exposures, with weights being the approximate duration of each season (5 months rainy and 7 months dry).
Cookhouse CO and PM2.5
We measured 72-hour integrated CO and PM2.5 concentrations in cookhouses of the homes of 321 of the above subjects (Table S1), simultaneously with their personal CO exposure measurements. These homes were randomly selected by the scheduling database among those eligible for CO exposure measurement in each week. In each cookhouse, we placed CO and PM2.5 monitors on a wooden stand approximately 1 m away from the main fire used for cooking. If the sampling pump connected to the PM2.5 sample operated for <90% of the 72-h measurement period, the PM2.5 measurement was not included in analysis (106 households). Data from another 12 households were excluded because the filter or measurement equipment was compromised, leaving 203 households with 219 integrated cookhouse PM2.5 measurements included in the analysis. Of these, 197 households also had a valid cookhouse CO measurement leading to 213 co-located CO and PM2.5 measurements; CO measurements in the other 6 households were excluded because the CO tubes were missing from the cookhouse or were discolored, possibly due to excessive exposure to direct sunlight.
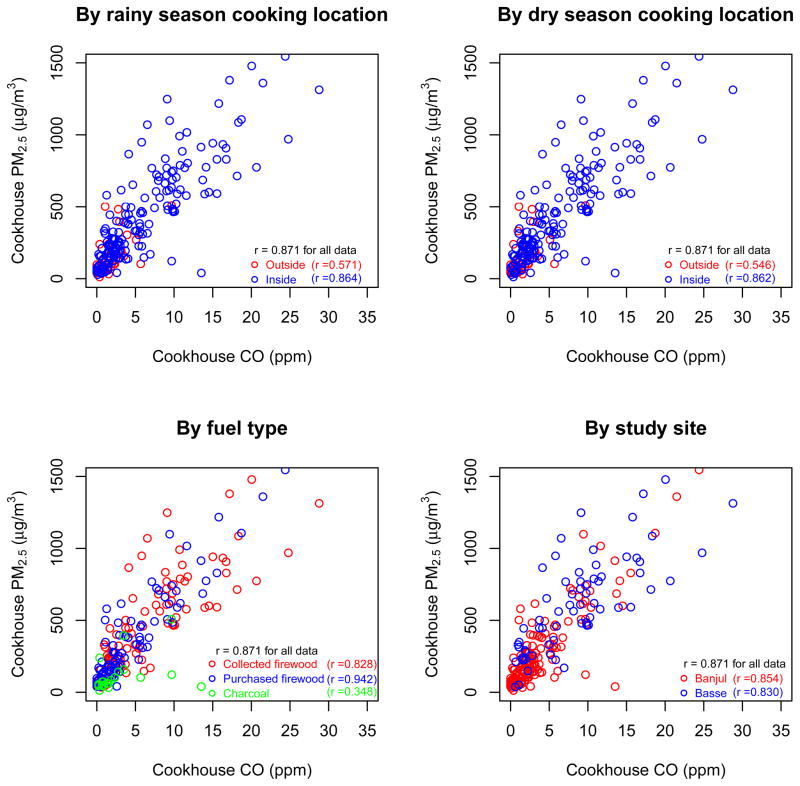
We estimated the relationship between cookhouse integrated PM2.5 and CO using the 197 co-located measurements. To assess whether variables available in questionnaires help improve PM2.5 prediction, we developed a series of regression models with different covariates including fuel used for cooking, cooking location and duration, study site, and measurement season. We also considered the interactions among variables. We applied natural splines with 2–6 degrees of freedom because exploratory analysis showed that the PM2.5-CO relationship was non-linear (Figures 1 and 2). We developed a total of 36 regression models. We used the Akaike information criterion (AIC) and Bayesian information criterion (BIC) to evaluate the models. The AIC and BIC measure the relative goodness of fit of a model; they reward how well the model fits the data but discourage overfitting, with the BIC including a larger penalty term for overfitting than the AIC [23–25]. The model below emerged as the model with the lowest AIC and 6th lowest BIC (Supplementary Figure 1), indicating that this model has good relative fit without being overly complex in terms of number of terms and their interactions. The model with the lowest AIC was chosen because the model will be used for prediction. We used the spline with 4 degrees of freedom because the additional regression coefficient was statistically significant, and could help characterize the non-linear PM2.5-CO relationship.
Figure 1.
Relationship between cookhouse PM2.5 and CO concentrations; sample sizes range between 204 and 213 because a few households were missing data on cooking location or fuel.
Note: Cooking location is shown for dry and rainy seasons because it varied by season in some households.
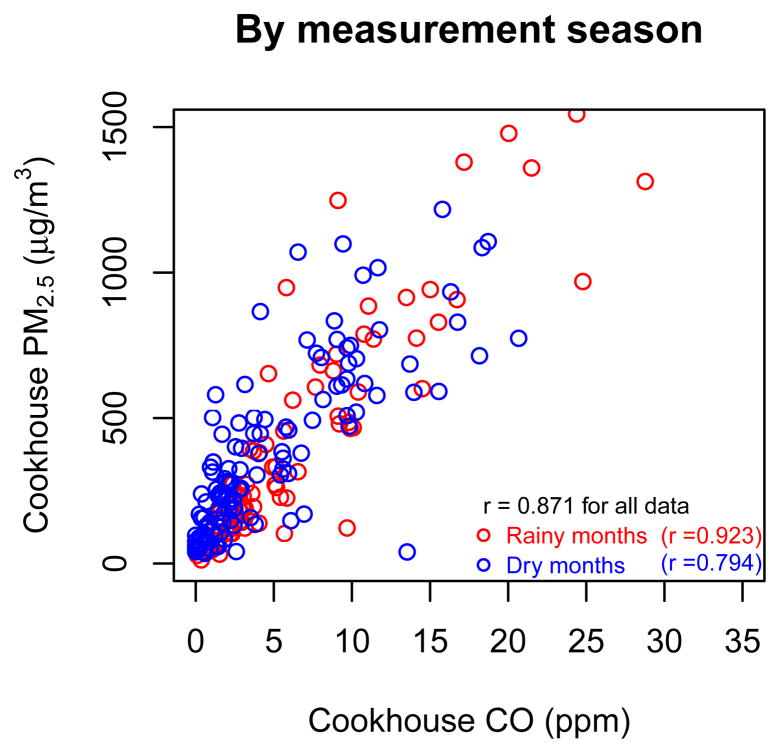
Figure 2.
Annual PM2.5 exposure in relation to annual CO exposure and type of fuel (n = 1,266).
Where β0 is the overall intercept; ns(ln(CO),4) is a natural spline with 4 degrees of freedom applied to the natural log of CO concentration; fuel is type of fuel used most for cooking (purchased firewood, collected firewood, charcoal, other); season represents whether the measurement was done in the rainy or dry season; study site represents whether the measurement was done in Banjul or Basse; and e is random error. Covariate data were from a household questionnaire administered to each child’s mother or primary caregiver by trained MRC fieldworkers. Detailed questionnaire results and characteristics of the study population are provided elsewhere [12].
We also measured PM2.5 continuously in cookhouses of 191 homes, collocated with the integrated monitor (Table S1). After exclusions due to equipment malfunction, we had 124 continuous PM2.5 measurements in 116 homes remaining. Of these, 80 households had usable co-located integrated data.
Annual PM2.5 exposure estimated indirectly using CO as proxy (all children)
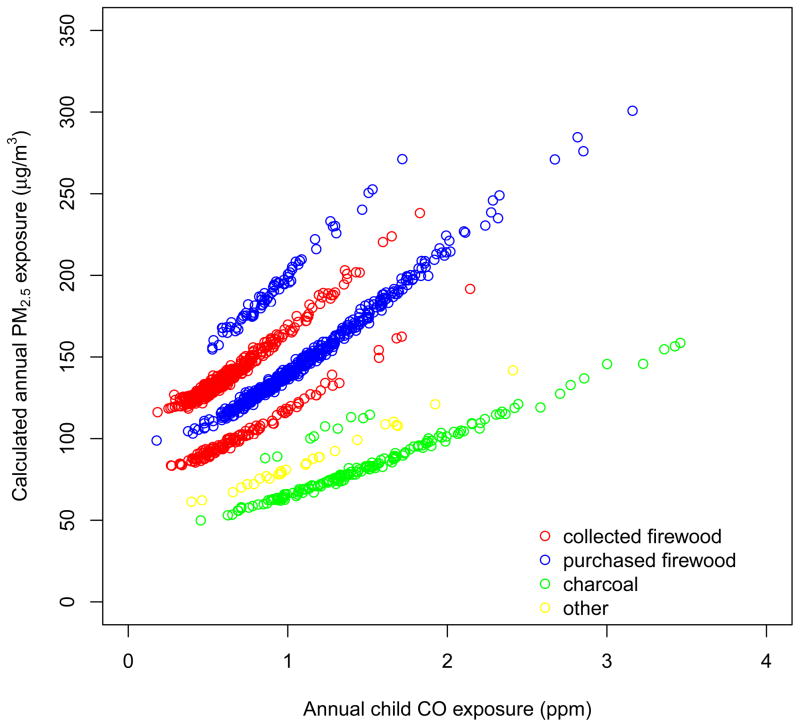
We applied the above PM2.5-CO relationship to the estimates of season-specific usual CO exposure for the 1,266 children in the analysis. Annual PM exposure for each child was calculated using a weighted average of season-specific usual PM exposures. We calculated the uncertainty of each child’s annual PM2.5 exposure accounting for: 1) the uncertainty in the estimated regression coefficients; and 2) uncertainty due to unexplained variation, measured by the residual variance. These uncertainties will be used in subsequent epidemiological analyses.
Direct PM2.5 exposure (validation study)
We directly measured personal exposure to PM2.5 on 48 children between January 2010 and January 2011 (Table S1). These children were aged between 15 and 61 months at the time of measurement, with a mean and median age of 34 months, and standard deviation of 9 months. There were no other criteria for their selection beyond being eligible for measurement at the time of the study and being large enough to comfortably carry the backpack containing measurement equipment. Each child wore a toddler-sized backpack (Figure 3) fitted with a PM2.5 monitor for 48 hours. We could not measure personal exposure on younger children because they were too small to carry the backpack and monitor. Five children were excluded from analysis because of initial equipment failure or because the backpack was removed due to child’s illness or inability to carry the backpack; 12 were excluded because the monitor operated for less than 38.4 hours (80% of the target 48-h period). The remaining 31 children were included in analysis. Of these, 29 had valid simultaneously measured personal CO exposure; CO exposure measurements were initiated 24 h prior to the start of PM exposure measurement, to ensure that there was sufficient color change on the CO tubes.
Figure 3.
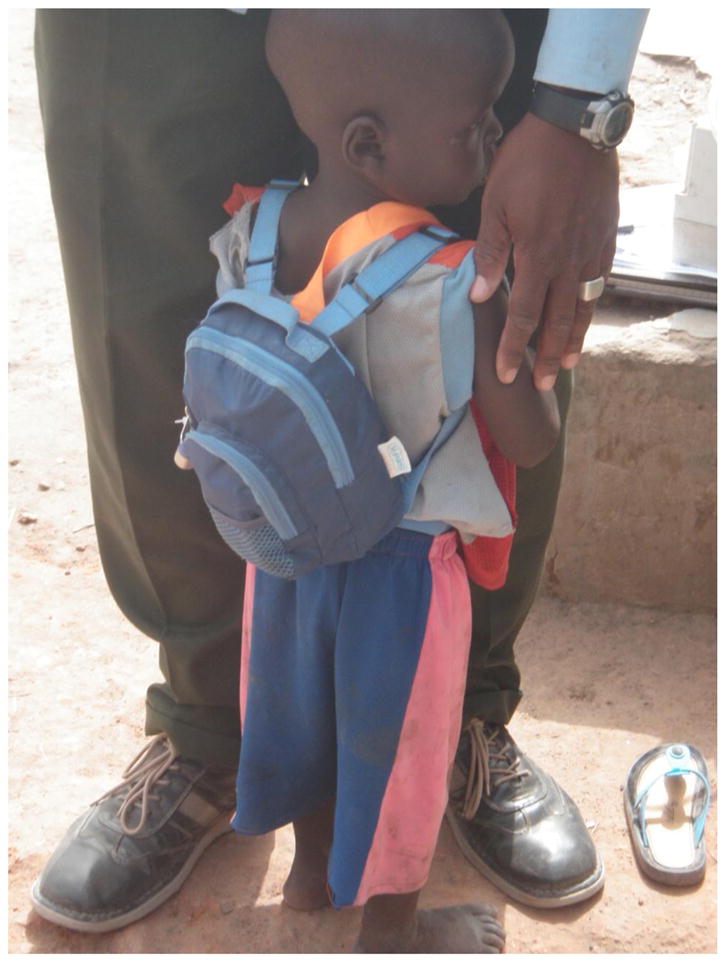
Child wearing backpack fitted with PM2.5 and CO monitors.
Indirect PM2.5 exposure from time-location-activity budgets
We calculated PM2.5 exposure using a time-location-activity budget and cookhouse area measurement of PM2.5 for 76 children from households with cookhouse continuous PM2.5 measurement. Information on time-location-activity budgets was from the questionnaire described above. The questionnaire included questions on stove use and child’s location during different time periods of the day, divided to morning (5:00 am – 10:00 am), mid-day (10:00 am – 5:00 pm), and evening (5:00 pm – 5:00 am). The caregiver was asked to indicate the typical duration the fire was burning (to the nearest whole hour) and the location of the child “most of the time” during each period. Personal PM2.5 exposure for each child was calculated as:
(PM2.5)j: personal exposure to PM2.5 for child j
i =1,2,3: time period of the day, as above
fi,j: fraction of hours the fire is burning in the cookhouse of child j during time period i
li,j: location of child j during time period i
ci,j: mean concentration of PM2.5 in the cookhouse of child j during time period i, corrected against integrated data (see below)
We set li,j = 1 if the stated location of the child was on the caregiver’s back, near the stove (within 1 meter), or not near the stove but around the cooking area (1–4 meters). When the location of the child was away from the stove inside (another room), away from the stove outside, or in a different compound, we set li,j to 0, 0.5, or 1 under three different scenarios.
All analyses were conducted in R version 2.14.0.
Measurement methods
Methods used to measure personal and cookhouse CO and PM2.5 are described in detail in supplementary material.
Results and Discussion
Cookhouse PM2.5 and CO
Mean 72-h cookhouse PM2.5 concentration in the 203 households with 219 measurements was 395 ± 364 μg/m3. This is substantially higher than PM2.5 cookhouse concentrations in China of 107 μg/m3 [18] but lower than the 900 μg/m3 in kitchens with open fires in Guatemala [13]. With somewhat different size fractions, mean PM4 concentrations were 500 μg/m3 in kitchens of wood users in India [26], and ranged from 187 to 719 μg/m3 in different provinces and seasons in China [27]. Mean 72-h cookhouse CO concentration was 6.7 ± 7.3 ppm (356 measurements in 322 households), lower than the 10–11 ppm in Guatemala before stove interventions [28], and similar to those in the non-heating season in China (5.5 ppm) [27]. Both the China and Guatemala studies used a different brand of CO diffusion tube than was used in our study in The Gambia.
25% of cookhouse PM2.5 and CO concentrations in our study were above 585 μg/m3 and 9.4 ppm respectively, while 5% of measurements were above 1,088 μg/m3 and 20.8 ppm respectively. Cookhouse PM2.5 and CO concentrations differed by fuel type, study site, and measurement season (Table 1). Households using collected or purchased firewood had substantially higher cookhouse PM2.5 and CO concentrations, and larger variability, than households using charcoal (mean measured PM2.5 in households using collected firewood, purchased firewood, and charcoal were 476 ± 357 μg/m3, 395 ± 387 μg/m3, and 121 ± 123 μg/m3 respectively) (Table 1), similar to results of a study in Kenya [3]. The reasons for higher PM2.5 in homes using collected (vs. purchased) firewood are not known and may include the type of wood, longer duration of cooking (6.6 ± 2.0 hours in homes with collected firewood vs. 5.2 ± 1.4 hours in homes with purchased firewood) or other cooking behaviors. Pollution was also much higher in households in the Basse region than in the Banjul region; this may be because firewood was the nearly universal fuel in Basse but charcoal was used by 18% of Banjul study households (Table 1). Cookhouse PM2.5 and CO concentrations in the rainy season were slightly higher than concentrations in the dry season (Table 1), possibly due to longer hours of stove use for warmth in the rainy season or differences in fuel moisture.
Table 1.
72-h cookhouse PM2.5 and CO concentrations.
| PM2.5 (μg/m3) | CO (ppm) | |||
|---|---|---|---|---|
| Fuel | Collected firewood | Mean ± std | 476 ± 357 | 8.6 ± 8.1 |
| GM ± GSDa | 341 ± 2.5 | 5.4 ± 2.9 | ||
| n | 98 | 160 | ||
| Purchased firewood | Mean ± std | 395 ± 387 | 5.7 ± 6.6 | |
| GM ± GSDa | 266 ± 2.5 | 3.5 ± 3.0 | ||
| n | 90 | 151 | ||
| Charcoal | Mean ± std | 121 ± 123 | 2.4 ± 3.1 | |
| GM ± GSDa | 85 ± 2.3 | 1.5 ± 2.9 | ||
| n | 21 | 32 | ||
| Study site | Banjul | Mean ± std | 275 ± 265 | 4.3 ± 4.6 |
| GM ± GSDa | 187 ± 2.5 | 2.7 ± 2.9 | ||
| n | 151 | 230 | ||
| Basse | Mean ± std | 660 ± 412 | 11.0 ± 9.2 | |
| GM ± GSDa | 533 ± 2.1 | 7.4 ± 2.7 | ||
| n | 68 | 126 | ||
| Measurement season | Rainy | Mean ± std | 424 ± 444 | 7.6 ± 7.8 |
| GM ± GSDa | 254 ± 2.9 | 4.8 ± 2.8 | ||
| n | 93 | 158 | ||
| Dry | Mean ± std | 373 ± 290 | 5.9 ± 6.9 | |
| GM ± GSDa | 262 ± 2.5 | 3.3 ± 3.4 | ||
| n | 126 | 198 |
GM=geometric mean; GSD=geometric standard deviation. Geometric mean and geometric standard deviation for CO were calculated excluding 8 measurements below the limit of detection of the CO tubes (i.e. excluding CO=0 ppm). We present geometric means because concentration distributions were log-normal. Numbers of valid measurements used in the analysis are reported. See text for total number of measurements.
Minute-by-minute corrected continuous cookhouse PM2.5 show three distinct daily peaks, the largest during the mid-day cooking period and two smaller ones before and after this period; this pattern applied to both rainy and dry months and did not vary systematically over the three days of measurement in each home (Supplementary Figure 2). The time pattern of PM in Banjul households had a similar pattern, whereas Basse households seemed to have two broader peaks in the middle of the day (Supplementary Figure 2). When separated by fuel, households using firewood shared the time pattern of all households, whereas those using charcoal had a single sharp peak in the middle of the day (Supplementary Figure 2).
Integrated cookhouse PM2.5 and CO concentrations had a correlation coefficient of 0.87 (n=213 measurements) (Figure 1). The correlation coefficients in different measurement seasons and at different study sites ranged from 0.79 to 0.92. When data were restricted to the range of CO concentrations that represent children’s personal exposure (0–21 ppm), the correlation was slightly lower at 0.83 (n=208 measurements). However below the 75th percentile of child CO exposure (1.3 ppm), the cookhouse PM2.5-CO correlation dropped to 0.51 (n=41 measurements) (Supplementary Figure 3).
The PM2.5-CO correlation differed by fuel type: 0.83 (n=95) and 0.94 (n=89) for collected and purchased firewood respectively, but only 0.35 (n=20) for charcoal. The PM2.5-CO correlation was lower in homes that cooked outside (under a roof, or in open air; r = 0.55–0.57) than inside (in the main house, or inside a separate cookhouse; r = 0.86) (Figure 1). The correlation coefficient between 22-h cookhouse PM2.5 and CO concentrations in kitchens using open fires in Guatemala was 0.50 (n=9) [11]. In China, where coal is commonly used, the correlation between 24-h PM4 and CO ranged from 0.29 to 0.48 across provinces and measurement locations [29]. The two pollutants also had low correlation in Kenya [3], although measurement methods were different.
There was a non-linear relationship between cookhouse PM2.5 and CO, demonstrated by the significance of the natural spline terms (Table 2). Including covariates in the PM2.5-CO model explained an additional 9% of the PM2.5 variance, even after adjusting for the number of explanatory variables (adjusted R2=0.66 without covariates and adjusted R2=0.75 with covariates) (Table 2). The coefficients of fuel, measurement season, and study site in the model were significant at p = 0.05 (Table 2). The multivariate relationship shows that for any CO concentration, PM concentration was lower for charcoal users than for wood users (Figure 2), and for measurements in the rainy season than those in the dry season. The low PM concentrations for charcoal, as compared to its CO emissions, are consistent with a study in Kenya [3]. Possible reasons for the difference in the PM-CO relationship in Basse compared to Banjul include differences in other sources or in the cooking and fire tending habits and techniques.
Table 2.
Regression coefficients (95% confidence intervals) for association between cookhouse ln(PM2.5) and CO concentrations, with and without covariates.
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | ||
|---|---|---|---|---|---|
| Adjusted R2=0.654; n = 208a | Adjusted R2=0.747; n = 202a,b | ||||
| Constant | 3.830 (3.210 to 4.450) | <0.001 | 4.314 (3.719 to 4.909) | <0.001 | |
| ns (ln CO), 1 | 1.879 (1.298 to 2.459) | <0.001 | 1.529 (0.994 to 2.063) | <0.001 | |
| ns (ln CO), 2 | 2.430 (1.909 to 2.951) | <0.001 | 1.870 (1.378 to 2.361) | <0.001 | |
| ns (ln CO), 3 | 4.030 (2.612. to 5.448) | < 0.001 | 2.876 (1.548 to 4.205) | <0.001 | |
| ns (ln CO), 4 | 3.513 (2.706 to 4.321) | < 0.001 | 3.349 (2.622 to 4.075) | <0.001 | |
| Type of fuel used most for cooking | Collected firewood | 0.0 | NA | ||
| Purchased firewood | 0.174 (0.004 to 0.344) | 0.046 | |||
| Charcoal | −0.593 (−0.860 to −0.326) | <0.001 | |||
| Other | −0.411 (−1.026 to 0.203) | 0.191 | |||
| Measurement season | Dry | 0.0 | NA | ||
| Rainy | −0.245 (−0.388 to −0.102) | 0.001 | |||
| Study site | Banjul | 0.0 | NA | ||
| Basse | 0.352 (0.159 to 0.544) | <0.001 | |||
5 measurements were not included in the regression because measured cookhouse CO=0.
6 measurements were not included in the regression because type of fuel used most for cooking was unknown.
Annual PM2.5 exposure using CO as proxy
As reported in detail elsewhere [12], mean annual (weighted average of rainy and dry seasons) CO exposure for the 1,266 children was 0.96 ± 0.50 ppm. The corresponding annual PM exposure, estimated by applying the cookhouse PM2.5-CO relationship, was 135 ± 38 μg/m3 (Supplementary Figure 4). Indirect exposures ranged from 50 to 410 μg/m3, with 25% of children having exposures above 151 μg/m3 and 5% having exposures above 199 μg/m3 (Supplementary Figure 4). Mean annual exposure was 144 μg/m3 for children in households using firewood (purchased or collected) and 85 μg/m3 for those using charcoal (Figure 2). Children living in Basse had higher exposure than those in Banjul (146 μg/m3 and 127 μg/m3, respectively), possibly due to the nearly exclusive use of firewood in Basse. When rainy and dry seasons were considered separately, there was little difference in exposure (138 vs. 133 μg/m3). Using a similar approach, children in households who used open fires in the highlands of Guatemala had mean exposures of 160–200 μg/m3 [13]. The higher exposures in Guatemala, where CO exposure was also higher [10, 28–29], may be because the fire is burned for longer periods of time for heating and temascal wood-fired sauna, and because children spend more time indoors than in The Gambia which has mild-hot weather (average monthly temperature ranges from 24–29°C).
Indirect PM2.5 exposure using CO as proxy vs. directly-measured exposure
One of the 31 children with direct PM2.5 exposure measurement used charcoal as their main cooking fuel; the remainder used firewood. Most of these children were from Basse (77%); and most measurements were done in the rainy season (68%). Mean directly-measured PM2.5 exposure was 65 ± 41 μg/m3, comparable to summer-time exposure of school-aged children in rural China, the only other study known to directly measure personal PM exposure of children in the developing world [18]. The China study measured 24-hour gravimetric PM2.5 on women (in two seasons) and on children (in one season).
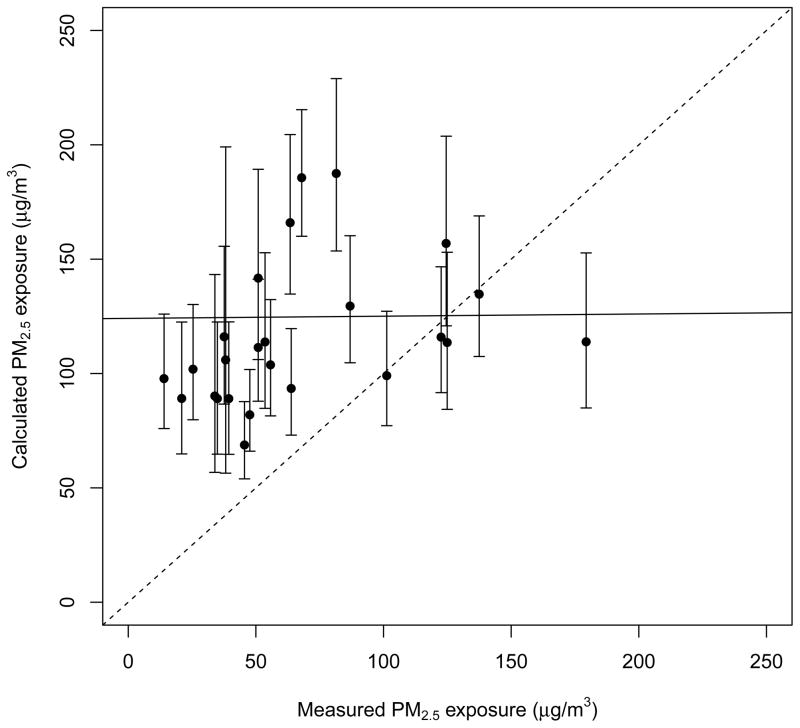
Mean PM2.5 exposure of the same children calculated using their simultaneously-measured CO exposure in the above statistical model (which also included fuel and measurement season) was 125 ± 54 μg/m3, about twice the directly-measured level (Figure 4). Mean difference between direct and indirect methods for 26 children with both sets of data was −58 μg/m3; mean absolute difference was 65 μg/m3. Not only were the indirect estimates biased, but also there was no correlation between the direct and indirect exposures (Pearson r=0.01).
Figure 4.
Relationship between directly-measured PM2.5 exposure and exposure estimated indirectly by applying cookhouse PM2.5-CO relationship to CO exposure over the same period. See Supplementary Figure 8 for the relationship without the single child whose household fuel was charcoal.
This bias and low correlation exists because the PM-CO relationship on children is different from that of the cookhouse, with lower correlation (correlation coefficient = 0.87 for measured cookhouse PM-CO vs. −0.04 for measured personal PM-CO) (Supplementary Figure 5). The difference in cookhouse vs. child PM-CO relationship may occur due to a number of reasons: First, because children may be exposed to sources of these pollutants other than biomass smoke. Second, the PM-CO relationship may vary during the burn cycle with the children near the fire only during specific parts of the burn cycle. Finally, the PMCO relationship may be stronger closer to the source (near the fire/in the cookhouse) but the two pollutants may disperse differently outside the cookhouse or with increasing distance from the source. One of the limitations of our study and the only other study that used CO as a proxy for PM exposure [13] was that we did not have data to characterize the PM-CO relationship for different parts of the day and in different microenvironments where children spend time; there is some evidence that the PM-CO relationship may be location specific [29]. We did nonetheless include fuel, season, and study site in the relationship. Similarly, due to the difficulty in measuring personal PM exposure, the sample size of our validation study was too small to allow for stratifying the PM-CO relationship on children by demographic and environmental variables.
There was a weak inverse correlation between directly-measured child PM2.5 exposure and measured cookhouse PM2.5 (r = −0.2) (Supplementary Figure 6); the correlation between child CO exposure and cookhouse CO both measured over the same time period was −0.03 (n=307 measurements). The weak correlation between cookhouse and child PM2.5 may be because children with direct exposure measurement tended to be older and may therefore have spent more time in other parts of the house, whereas our area measurements and time-location-activity budgets were focused on the cookhouse.
Correlation of two indirect PM2.5 exposures, using time-location-activity budget vs. using CO exposure
58 children had indirect PM2.5 exposures calculated using two methods: by applying the cookhouse PM2.5-CO relationship to measured CO exposure and using time-location-activity budget and continuous cookhouse PM2.5. Correlations between the two indirect child PM2.5 exposures ranged from 0.07 to 0.14 for different scenarios described in Methods (Supplementary Figure 7). The low correlations suggest that these two methods of indirectly estimating exposure do not agree. Possible reasons for the low correlation include an inaccurate indirect estimate of PM exposure using the CO exposure, and the crude time-location-activity budget data. Detailed records of daily time-location-activity budgets and PM concentrations with moderate-high spatial and temporal resolution, as used in previous work [30], may increase the accuracy of the estimate of exposure from this method but may not be feasible in large studies. Validation of children’s exposure estimated from time-location-activity budgets with directly measured exposure could not be done in our study because there were very few usable cookhouse measurements during direct exposure measurement days, however this validation is necessary before relying on time-location-activity methods to estimate exposure in future studies.
We indirectly estimated PM exposure using the cookhouse PM-CO relationship applied to CO exposure for 1,266 children, which to our knowledge is the largest sample size of an exposure assessment study in the developing world. To our knowledge, our study is also one of the two to have directly measured children’s personal PM exposure in homes where biomass fuels are used, and the first for children younger than 5 years of age. Our directly measured mean PM2.5 exposure of 65 μg/m3 is many times the World Health Organization (WHO) Ambient Air Quality Guidelines of 10 μg/m3, and is comparable to ambient concentrations in low-SES neighborhoods in Accra, Ghana [31], and polluted cities in Asia [32] and Delhi [33].
The lack of correlation between direct and indirect exposures and the systematic bias of the latter in our validation study indicates that widespread use of CO as a proxy for PM exposure requires additional research on the relationship between the two pollutants and how this relationship varies in different pollution ranges, by fuel and environmental factors, and by cooking behaviors. Similarly, there is a need for additional research to develop models to better estimate PM or CO exposures on the basis of area concentrations by understanding the role of household microenvironments and the specific sources of these pollutants.
More than 30 years after the first set of biomass smoke exposure studies [34], and 10 years after larger exposure studies of household air pollution levels and exposures [3, 11, 14, 16, 26–30, 35–36], the progress in our ability to accurately measure personal PM exposure in the developing world seems limited, particularly for children. There is tremendous global interest in clean cookstove technologies and projects to implement them [37]. Clearly past studies that had quantified exposure to biomass smoke and its health effects were influential in providing the evidence for these efforts. However, improving measurement technologies and validating current modeling methods are crucial because valid and accurate exposure measurements are needed to evaluate the effectiveness of these interventions in reducing exposure and to conduct epidemiologic studies that quantify their health effects.
Supplementary Material
Table 3.
Annual usual child PM2.5 and CO exposures, estimated as described in Methods.
| PM2.5 (μg/m3) | CO (ppm) | |||
|---|---|---|---|---|
| Fuel | Collected firewood | Mean ± std | 131 ± 23 | 0.7 ± 0.3 |
| GM ± GSDa | 129 ± 1.2 | 0.6 ± 1.4 | ||
| n | 561 | 561 | ||
| Purchased firewood | Mean ± std | 157 ± 37 | 1.1 ± 0.5 | |
| GM ± GSDa | 153 ± 1.2 | 1.0 ± 1.4 | ||
| n | 512 | 512 | ||
| Charcoal | Mean ± std | 85 ± 22 | 1.5 ± 0.6 | |
| GM ± GSDa | 83 ± 1.3 | 1.4 ± 1.4 | ||
| n | 166 | 166 | ||
| Study site | Banjul | Mean ± std | 127 ± 42 | 1.1 ± 0.5 |
| GM ± GSDa | 120 ± 1.4 | 1.0 ± 1.6 | ||
| n | 746 | 746 | ||
| Basse | Mean ± std | 146 ± 27 | 0.7 ± 0.3 | |
| GM ± GSDa | 144 ± 1.2 | 0.6 ± 1.4 | ||
| n | 520 | 520 |
GM=geometric mean; GSD=geometric standard deviation.
Acknowledgments
This work was supported by a grant from the National Institute of Environmental Health Sciences (1R21ES017855-01). Majid Ezzati is supported by a Strategic Award from the UK Medical Research Council. We thank the households who participated in the study for their help and hospitality, our field workers and field supervisors for valuable assistance in data collection, the Biomedical Engineering Department at the MRC Laboratories for technical assistance throughout the study, Jose Vallarino for information on methods and instruments for personal exposure measurement, and Mariel Finucane for advice on presentation of statistical results. We also thank Grant Mackenzie for operational support and Nigel Bruce and Kirk Smith for advice on exposure measurement.
Footnotes
Supporting Information Available
Additional text regarding measurement methods, descriptive statistics for model selection (Figure S1), cookhouse continuous PM2.5 concentrations (Figure S2), children’s measured CO and predicted PM2.5 exposures overlaid on cookhouse PM2.5-CO relationship (Figure S3), distribution of annual PM2.5 exposure calculated from CO (Figure S4), cookhouse vs. measured child PM2.5-CO relationship (Figure S5), directly-measured child PM2.5 exposure vs. cookhouse PM2.5 concentration (Figure S6), PM2.5 exposure calculated from CO exposure vs. PM2.5 exposure from time-location-activity budgets and cookhouse PM2.5 concentration (Figure S7), and directly-measured PM2.5 exposure vs. indirect PM2.5 exposure from CO exposure with only firewood users (Figure S8), flow chart of number of measurements (Figure S9), and number of personal and cookhouse measurements (Table S1) is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Smith KR, Mehta S, Maeusezahl-Feuz M. Indoor air pollution from household solid fuel use. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. World Health Organization; Geneva: 2004. pp. 1435–1493. [Google Scholar]
- 2.Smith KR. Biofuels, Air Pollution, and Health: A Global Review. Plenum Press; New York: 1987. [Google Scholar]
- 3.Ezzati M, Mbinda BM, Kammen DM. Comparison of emissions and residential exposure from traditional and improved biofuel stoves in rural Kenya. Environmental Science and Technology. 2000;34:578–583. [Google Scholar]
- 4.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86(5):390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope CAr, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Air Quality Guidelines: Global Update 2005. WHO Regional Office for Europe; Copenhagen: 2006. [Google Scholar]
- 7.Airborne particles and health: HEI epidemiologic evidence. Health Effects Institute; Cambridge, MA: 2001. [Google Scholar]
- 8.Brook R, Rajagopalan S, III, CP, Brook J, Bhatnagar A, Diez-Rouz A, Holguin F, Hong U, Luepker R, Mittleman M, Peters A, Siscovick D, Smith S, Whitsel L, Kaufman J. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 9.Bruce N, McCracken J, Albalak R, Schei MA, Smith KR, Lopez V, West C. Impact of improved stoves, house construction and child location on levels of indoor air pollution exposure in young Guatemalan children. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S26–33. doi: 10.1038/sj.jea.7500355. [DOI] [PubMed] [Google Scholar]
- 10.McCracken JP, Schwartz J, Bruce N, Mittleman M, Ryan LM, Smith KR. Combining Individual- and Group-Level Exposure Information: Child Carbon Monoxide in the Guatemala Woodstove Randomized Control Trial. Epidemiology. 2009;20(1):127–136. doi: 10.1097/EDE.0b013e31818ef327. [DOI] [PubMed] [Google Scholar]
- 11.Naeher LP, Smith KR, Leaderer BP, Neufield L, Mage DT. Carbon Monoxide as a tracer for assessing exposures to particulate matter in wood and gas cookstove households of highland Guatemala. Environmental Science and Technology. 2001;35:575–581. doi: 10.1021/es991225g. [DOI] [PubMed] [Google Scholar]
- 12.Dionisio KL, Howie SR, Dominici F, Fornace KM, Spengler JD, Donkor S, Chimah O, Oluwalana C, Ideh RC, Ebruke B, Adegbola R, Ezzati M. The exposure of infants and children to carbon monoxide from biomass fuels in The Gambia: A measurement and modeling study. Journal of Exposure Science and Environmental Epidemiology. 2011 doi: 10.1038/jes.2011.47. Accepted manuscript. [DOI] [PubMed] [Google Scholar]
- 13.Northcross A, Chowdhury Z, McCracken J, Canuz E, Smith KR. Estimating personal PM2. 5 exposures using CO measurements in Guatemalan households cooking with wood fuel. Journal of Environmental Monitoring. 2010;12:873–878. doi: 10.1039/b916068j. [DOI] [PubMed] [Google Scholar]
- 14.Balakrishnan K, Sankar S, Parikh J, Padmavathi R, Srividya K, Venugopal V, Prasad S, Pandey VL. Daily average exposures to respirable particulate matter from combustion of biomass fuels in rural households of southern India. Environmental Health Perspectives. 2002;110:1069–1075. doi: 10.1289/ehp.021101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta S, Huq M, Khaliquzzaman M, Pandey K, Wheeler D. Who suffers from indoor air pollution? Health Policy and Planning. 2006;21(6):444–458. doi: 10.1093/heapol/czl027. [DOI] [PubMed] [Google Scholar]
- 16.Ezzati M, Kammen DM. Quantifying the effects of exposure to indoor air pollution from biomass combustion on acute respiratory infections in developing countries. Environ Health Perspect. 2001;109(5):481–8. doi: 10.1289/ehp.01109481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mestl HES, Aunan K, Seip HM, Wang S, Zhao Y, Zhang D. Urban and rural exposure to indoor air pollution from domestic biomass and coal burning across China. Science of the Total Environment. 2007;377:12–26. doi: 10.1016/j.scitotenv.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner J, Schauer JJ, Ezzati M, Lu L, Cheng C, Patz JA, Bautistia LE. Patterns and predictors of personal exposure to indoor air pollution from biomass combustion among women and children in rural China. Indoor Air. 2011 doi: 10.1111/j.1600-0668.2011.00730.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global Burden of Disease and Risk Factors. Oxford University Press; New York: 2006. [PubMed] [Google Scholar]
- 20.Dionisio KL, Howie S, Fornace KM, Chimah O, Adegbola RA, Ezzati M. Measuring the exposure of infants and children to indoor air pollution from biomass fuels in The Gambia. Indoor Air. 2008;18(4):317–27. doi: 10.1111/j.1600-0668.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajaratnam JK, Marcus JR, Flaxman AD, Wang H, Levin-Rector A, Dwyer L, Costa M, Lopez AD, Murray CJ. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970–2010: a systematic analysis of progress towards Millenium Development Goal 4. Lancet. 2010;375(9730):1988–2008. doi: 10.1016/S0140-6736(10)60703-9. [DOI] [PubMed] [Google Scholar]
- 22.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 23.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; New York: 2007. [Google Scholar]
- 24.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19(6):716–723. [Google Scholar]
- 25.Schwarz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–464. [Google Scholar]
- 26.Balakrishnan K, Sambandam S, Ramaswamy P, Mehta S, Smith KR. Exposure assessment for respirable particulates associated with household fuel use in rural districts of Andhra Pradesh, India. J Expo Anal Environ Epidemiol. 2004;14(Suppl 1):S14–25. doi: 10.1038/sj.jea.7500354. [DOI] [PubMed] [Google Scholar]
- 27.Jin Y, Zhou Z, He G, Wei H, Liu J, Liu F, Tang N, Ying B, Liu Y, Hu G, Wang H, Balakrishnan K, Watson K, Baris E, Ezzati M. Geographical, spatial, and temporal distributions of multiple indoor air pollutants in four Chinese provinces. Environ Sci Technol. 2005;39(24):9431–9. doi: 10.1021/es0507517. [DOI] [PubMed] [Google Scholar]
- 28.Smith KR, McCracken JP, Thompson L, Edwards R, Shields KN, Canuz E, Bruce N. Personal child and mother carbon monoxide exposures and kitchen levels: Methods and results from a randomized trial of woodfired chimney cookstoves in Guatemala (RESPIRE) Journal of Exposure Science and Environmental Epidemiology. 2009;20(5):406–416. doi: 10.1038/jes.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He G, Ying B, Liu J, Gao S, Shen S, Balakrishnan K, Jin Y, Liu F, Tang N, Shi K, Baris E, Ezzati M. Patterns of household concentrations of multiple indoor air pollutants in China. Environ Sci Technol. 2005;39(4):991–8. doi: 10.1021/es049731f. [DOI] [PubMed] [Google Scholar]
- 30.Ezzati M, Saleh H, Kammen DM. The contributions of emissions and spatial microenvironments to exposure to indoor air pollution from biomass combustion in Kenya. Environ Health Perspect. 2000;108(9):833–9. doi: 10.1289/ehp.00108833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dionisio KL, Arku RE, Hughes AF, Vallarino J, Carmichael H, Spengler JD, Agyei-Mensah S, Ezzati M. Air Pollution in Accra Neighborhoods: Spatial, Socioeconomic, and Temporal Patterns. Environmental Science and Technology. 2010;44(7):2270–2276. doi: 10.1021/es903276s. [DOI] [PubMed] [Google Scholar]
- 32.Zheng M, Salmon LG, Schauer JJ, Zeng L, Kiang CS, Zhang Y, Cass GR. Seasonal trends in PM2. 5 source contributions in Beijing, China. Atmospheric Environment. 2005;39(22):3967–3976. [Google Scholar]
- 33.Gupta P, Christopher SA, Wang J, Gehrig R, Lee Y, Kumar N. Satellite remote sensing of particulate matter and air quality assessment over global cities. Atmospheric Environment. 2006;40(30):5880–5892. [Google Scholar]
- 34.Anderson HR. Respiratory Abnormalities in Papua New Guinea Children: The Effects of Locality and Domestic Wood Smoke Pollution. International Journal Epidemiology. 1978;7:63–72. doi: 10.1093/ije/7.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Ezzati M, Kammen D. Indoor air pollution from biomass combustion and acute respiratory infections in Kenya: an exposure-response study. Lancet. 2001;358(9282):619–24. doi: 10.1016/s0140-6736(01)05777-4. [DOI] [PubMed] [Google Scholar]
- 36.Naeher L, Leaderer B, Smith K. Particulate Matter and Carbon Monoxide in Highland Guatemala: Indoor and Outdoor Levels from Traditional and Improved Wood Stoves and Gas Stoves. Indoor Air. 2000;10:200–205. doi: 10.1034/j.1600-0668.2000.010003200.x. [DOI] [PubMed] [Google Scholar]
- 37.Roehr B. Environmentalists seek to set research agenda on indoor air pollution. British Medical Journal. 2011:342. doi: 10.1136/bmj.d3062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.