Abstract
Objective
To investigate motor cortical map patterns in children with diplegic and hemiplegic cerebral palsy (CP), and the relationships between motor cortical geometry and motor function in CP.
Methods
Transcranial magnetic stimulation (TMS) was used to map motor cortical representations of the first dorsal interosseus (FDI) and tibialis anterior (TA) muscles in 13 children with CP (age 9–16 years, 6 males.) The Gross Motor Function Measure (GMFM) and Melbourne upper extremity function were used to quantify motor ability.
Results
In the hemiplegic participants (N=7), the affected (right) FDI cortical representation was mapped on the ipsilateral (N=4), contralateral (N=2), or bilateral (N=1) cortex. Participants with diplegia (N=6) showed either bilateral (N=2) or contralateral (N=4) cortical hand maps. The FDI and TA motor map center-of-gravity mediolateral location ranged from 2–8 cm and 3–6 cm from the midline, respectively. Among diplegics, more lateral FDI representation locations were associated with lower Melbourne scores, i.e. worse hand motor function (Spearman’s Rho = −0.841, p=0.036)
Conclusions
Abnormalities in TMS-derived motor maps cut across the clinical classifications of hemiplegic and diplegic CP. The lateralization of the upper and lower extremity motor representation demonstrates reorganization after insults to the affected hemispheres of both diplegic and hemiplegic children.
Significance
The current study is a step towards defining the relationship between changes in motor maps and functional impairments in CP. These results suggest the need for further work to develop improved classification schemes that integrate clinical, radiologic, and neurophysiologic measures in CP.
Keywords: Transcranial magnetic stimulation (TMS), cerebral palsy, motor mapping, motor function
INTRODUCTION
Cerebral palsy (CP) is a group of disorders of the development of movement and posture attributed to non-progressive disturbances that occurred in the developing fetal or infant brain (Bax et al., 2005). Cerebral palsy (CP) occurs in approximately 1/500 live births (Stanley et al., 1992) and presents with a diverse range of symptoms, ranging in severity from generalized motor disabilities to mild spasticity limited to the lower limbs. However, ICD diagnostic classifications and severity measures in CP are currently established clinically, based primarily on type and anatomic distribution of the clinical symptoms (Bax et al., 2005; Wittenberg, 2009). Common types of CP include spastic, dyskinetic, and ataxic forms, and the most common are the diagnostic classifications of spastic hemiplegia and diplegia (Bax et al., 2005). Current classification schemes are unreliable because mixed symptomatic features may be present, leaving some patients with unclassifiable or overlapping disorders (Colver et al., 2003).
Similar to the diverse types of clinical symptoms, CP also presents with diverse neuroanatomical and neurophysiological abnormalities. A better understanding of the neural correlates of CP could help implement improved classification schemes, and provide insights into the neural substrates for rehabilitative or surgical interventions. Transcranial magnetic stimulation (TMS) has the advantage of being a non-invasive, relatively inexpensive, and rapid procedure that can be used to obtain information regarding abnormalities in the central motor representation in CP (Garvey et al., 2008; Lin et al., 2002; Wittenberg, 2009). TMS-derived measures can serve as a useful adjunct to clinical and radiological tools such as MRI (Staudt et al., 2003) and help to understand the associations between neurobiology and motor function in CP.
There has been considerable work on the development of ipsilateral motor pathways in the developing brain of children with CP (Staudt, 2010). Eyre and colleagues did seminal work on the maturation of the corticospinal tract normally and in hemiplegic CP, demonstrating preservation of the uncrossed pathways in CP (Eyre et al., 2001). Three types of hand muscle cortical representation patterns have been shown in unilateral CP: ipsilateral, contralateral, and bilateral (Holmstrom et al., 2010). Overall, better hand function was found in children with the normal contralateral projection pattern, intermediate function with bilateral projection patterns, and worse function with ipsilateral projections only (Holmstrom et al., 2010). However, variability of hand function in individuals with ipsilateral projections suggests that they do not consistently result in worse motor function.
There has been less focus in previous literature on the geometry (i.e., changes in size or location) of the cortical motor maps in CP. Magaeki and colleagues first demonstrated abnormal cortico-motor map geometry in the spastic diplegic form of CP, with a lateral shift of TA maps in CP compared to their normal location on the medial wall (Maegaki et al., 1999). Also, stimulation of the lateralized TA representation could produce both ipsilateral and contralateral MEPs (Maegaki et al., 1999). Although it might be assumed that greater abnormalities in cortical motor map geometry (i.e., greater lateralization of maps or greater overlap between motor maps) are associated with greater clinical impairments, to date there is no conclusive evidence to suggest how the geometry of cortical reorganization after neurological injury relates to motor function or potential for functional recovery. There is intriguing data, however, showing a relationship of crossed versus uncrossed (ipsilateral) maps with the ability to respond to a rehabilitation intervention (Kuhnke et al., 2008). By better understanding the relationship between neuropathology and clinical function in CP, we can individualize neuro-rehabilitative interventions according to the neurological substrate available for recovery, and maximize the efficacy of neuro-rehabilitation in individuals with CP.
The effects of different types of functional motor map geometry prevalent in CP, such as greater proximity between motor maps and lateralization of maps, on clinical impairments need to be systematically tested. For example, it can be hypothesized that greater cortical representation for a certain muscle would lead to better motor function for that muscle (Kleim et al., 2010). It can also be hypothesized that greater proximity between cortical maps of two muscles may relate to decreased ability of those muscles to produce isolated movements, thereby leading to poorer real world motor function.
The purpose of this study, therefore, was two-fold: (1) to investigate the types of cortical reorganization in individuals with CP with both hemiplegic and diplegic clinical classifications, and (2) to investigate the relationship between cortical reorganization patterns and motor function in CP.
METHODS
Thirteen individuals with spastic CP referred from a pediatric orthopedic practice were recruited for this study (Table 1). Subjects were excluded from participation if they (1) had the onset of pathology related to CP after their first birthday, (2) had chorea, athetosis, or ballismus, (3) were <7 years or >16 years of age, (4) were on anticonvulsant medications, (5) had a history of tendon transposition, baclofen intrathecal pumps, or recent botulinum injections, (6) did not have the cognitive ability to provide assent or feedback for data collection. Clinical measures obtained were the Gross Motor Function (GMFM)-66 (Avery et al., 2003; Rosenbaum et al., 2002) and Melbourne assessment of unilateral upper extremity function (Bourke-Taylor, 2003; Randall et al., 2001). All subjects signed informed consent approved by the institutional review board at Wake Forest University.
Table 1.
Participant characteristics.
| Subject | ICD-9 | Age (years) | Gender | WM abnl. | Vent. Enl. | GMFM Score (%) | Melbourne Score (%) |
|---|---|---|---|---|---|---|---|
| CP1 | R-Hemi | 11 | F | L | N | 89.8 | 44 |
| CP2 | R-Hemi | 12 | F | L | N | 90 | 64 |
| CP3 | R-Hemi | 15 | F | L | L | 98 | 88 |
| CP4 | R-Hemi | 16 | F | L (mild) | L | 99.2 | 94 |
| CP6 | R-Hemi | 14 | F | L (mild) | N | 76.4 | 44.26 |
| CP7 | R-Hemi | 11 | F | L | N | 98 | 100 |
| CP8 | R-Hemi | 12 | M | B | B | 99.4 | 100 |
| CP5 | Diplegia | 11 | F | B | B | 83 | 90 |
| CP10 | Diplegia | 10 | M | N | N | 96.4 | 100 |
| CP11 | Diplegia | 12 | F | B | B | 100 | 100 |
| CP12 | Diplegia | 15 | F | B | B | 97.6 | 93 |
| CP13 | Diplegia | 9 | M | L>R (mild) | N | 92.6 | 94 |
| CP14 | Diplegia | 9 | M | R | R > L | 88.4 | 98.36 |
| Average | 12.08 | 92.98 | 85.36 | ||||
| StDev | 2.29 | 7.23 | 20.65 | ||||
Abbreviations: WM Abnl. White Matter Abnormalitiies, Vent. Enl. – Ventricular Enlargement, N – None, B – Bilateral, R – Right, L – Left. White matter abnormalities and ventricular enlargement were rated on MRI images by a neuroradiologist blind to either the clinical classification or TMS results.
TMS was delivered to the motor cortex using a MagStim 200 stimulator with a double 7 cm circular coil (Magstim Ltd, Wales, UK). TMS-evoked motor evoked potentials (MEPs) were recorded using surface EMG sensors attached to bilateral tibialis anterior (TA) and first dorsal interosseus (FDI) muscles. Amplification of 1000X was used for the EMG signals (James Long Co, Canada Lake, NY, USA). A custom-written LabVIEW program (National Instruments, Austin, TX, USA) was used for data acquisition and analysis.
First, hotspots for the FDI and TA were identified. Contralateral and ipsilateral (if present) motor thresholds were identified for each muscle. Next, TMS intensity was set at 120% motor threshold and motor mapping data for these muscles were collected. The subjects wore a closely fitting electroencephalography cap containing an approximate 1-cm grid with the axes centered on the vertex and oriented in the antero-posterior and medio-lateral directions. During mapping, 10 consecutive stimuli were delivered at scalp locations at 2-cm distance intervals. The coil was held with the handle pointing posteriorly and its center tangential to each stimulated point on the scalp. Mapping for a muscle was performed from the hotspot to border locations where <5/10 stimuli generated an MEP (Wittenberg, 2009).
During a separate session, all subjects underwent MRI in a 1.5T GE scanner, with standard clinical sequences, including T1, T2, and FLAIR images. The images were reviewed and rated by a neuroradiologist (JHB) for abnormalities in ventricular size and in the white matter, motor cortex, basal ganglia and cerebellum.
Data Analyses
TMS-derived dependent variables included map volume (size) and location of the map center of gravity (COG) (Wittenberg, 2009). Map volume was defined as the sum of all the normalized MEPs for a muscle. Map COG was calculated as a weighted average of all positions with a response (Wittenberg, 2009): Map COG (shown for x-direction as an example) was calculated as:
Two additional TMS-derived dependent variables were distances between COGs of ipsi- and contra-lateral maps for the same muscle (if present), and distances between FDI and TA map COGs within the same hemisphere (Wittenberg, 2009).
Non-parametric (Spearman’s) correlation analysis was performed to test for the presence of correlations between clinical measures of motor function (Melbourne and GMFM scores) versus each of the 4 TMS-derived variables (map volume, X-distance of the map COG, ipsi- to contra-lateral MEP distances, and FDI-TA distances).
RESULTS
Data were obtained from 13 participants with cerebral palsy (6 males, age 9–16 years, GMFM scores 44–100, Melbourne Score 76.4–100). In one additional participant recruited for the study (CP9), MEPs could not be obtained in response to TMS and therefore data from this participant could not be included. Seven of the 13 participants had ICD9 diagnosis of hemiplegia and six subjects had a diagnosis of diplegia; however, 2 of the diplegics showed asymmetrical functional impairment (left>right) (Table 1). MRI abnormalities generally were consistent with the clinical diagnosis; however, one hemiplegic (CP8) had bilateral abnormalities and two diplegics had asymmetrical white matter involvement. Besides CP1, who had a left middle cerebral artery infarct, cortical abnormalities were rather subtle.
Motor Map Abnormalities
The TMS-derived motor map geometry data for each of the individual subjects is presented in Table 2. Examples of raw MEP data are shown in Figure 1. In the hemiplegic subgroup (N=7), the affected (right) hand (FDI) motor cortical representation was mapped on the ipsilateral (N=4), contralateral (N=2), or bilateral (N=1) motor cortices (Table 2, Figure 2). Participants with diplegia (N=6) showed either bilateral (N=2) or contralateral (N=4) cortical hand maps. The X-coordinate of the FDI COGs from the vertex ranged from 2 to 8 cm.
Table 2.
TMS-derived metrics for the participants included in the study. Patterns of motor mapping and X and Y locations of map COGs (cm) are shown for the left (L) and right (R ) first dorsal interosseus (FDI) and tibialis anterior (TA) muscles. Also shown are the total map volumes for the FDI muscles. FDI-FDI distance (cm) represents the distance between ipsi- and contra-lateral FDI map COG locations. FDI-TA distance (cm) represents the distance between FDI and TA map COG locations.
| Pattern of Mapping of Affected Limb | Left Brain | Right Brain | Map Volume | Distances | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Aff. hand | Total Both hands | FDI-FDI | FDI-TA | ||||||||||||||||||
| L FDI | R FDI | L TA | R TA | L FDI | R FDI | L TA | R TA | ||||||||||||||
| X | Y | X | Y | X | Y | X | Y | X | Y | X | Y | X | Y | X | Y | ||||||
| CP1 | Ipsilateral | 4.2 | 2.6 | 3.7 | 3.0 | 2.1 | 7.4 | 0.6 | |||||||||||||
| CP2 | Ipsilateral | 8.0 | 4.4 | 7.7 | 4.6 | 4.7 | 8.9 | 0.3 | |||||||||||||
| CP3 | Ipsilateral | 3.6 | 1.8 | 3.5 | 1.7 | 7.2 | 16.1 | 0.1 | |||||||||||||
| CP4 | Mixed | −3.7 | 4.3 | 5.8 | 2.1 | 5.7 | 2.2 | 3.1 | 3.0 | 9.8 | 15.3 | 0.1 | 2.9 | ||||||||
| CP6 | Ipsilateral | 5.1 | 5.2 | 5.3 | 5.3 | 5.1 | 10.8 | 0.2 | |||||||||||||
| CP7 | Contralateral | −5.1 | 2.0 | 4.2 | 2.1 | 3.7 | 3.4 | 4.2 | 9.8 | 1.4 | |||||||||||
| CP8 | Contralateral | −6.2 | 3.5 | −5.8 | 3.1 | 5.5 | 3.5 | 5.6 | 3.6 | 4.0 | 8.0 | 0.5 | |||||||||
| CP5 | Contralateral | −3.5 | 2.7 | 6.0 | 2.9 | 4.9 | 7.3 | ||||||||||||||
| CP10 | Contralateral | −2.0 | 1.7 | 3.3 | 2.5 | 2.5 | 7.1 | ||||||||||||||
| CP11 | Contralateral | −3.2 | 2.6 | 3.8 | 2.9 | 2.3 | 6.1 | ||||||||||||||
| CP12 | Mixed | −6.1 | 3.2 | −6.1 | 3.2 | 3.3 | 6.4 | 0.0 | |||||||||||||
| CP13 | Contralateral | −7.1 | 1.7 | 5.8 | 2.2 | 4.3 | 11.7 | ||||||||||||||
| CP14 | Mixed | −5.8 | −1.5 | −5.4 | −1.4 | 3.8 | −2.2 | 7.5 | 11.0 | 0.4 | |||||||||||
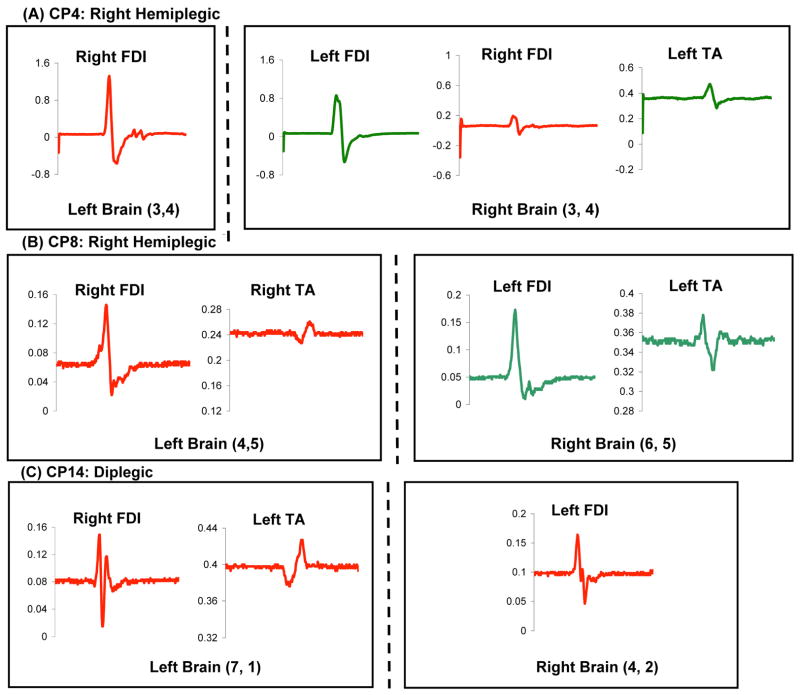
Figure 1.
Examples of raw motor evoked potential (MEP) data (millivolts) for 3 study participants. The site of stimulation (left versus right brain and X,Y coordinates) is specified for each subject. As illustrated in panel A, when bilateral representations were present, there was little variation in latency between the two.
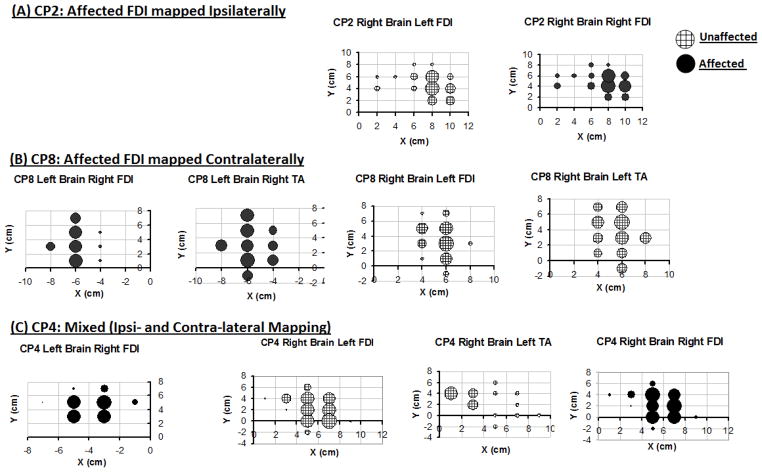
Figure 2.
Examples of cortical motor maps for 3 representative subjects with right hemiplegia. Three patients with the same clinical classification of right hemiplegia showed 3 different corticomotor projection patterns: ipsilateral (A), contralateral (B), and bilateral (C). Note that TA MEPs are evoked at cortical sites >4 cm lateral to the vertex (B and C). Also note the proximity between map locations for FDI and TA (B and C) and for right and left FDI (A and C).
TA map data were obtained from 4 of the 13 subjects (3 hemiplegics, 1 diplegic); TA MEPs were not elicited in the remaining subjects. X- coordinate distances of the TA COGs ranged from 3.1 to 5.8 cm. Proximity between TA and FDI maps was observed, with TA-FDI distances ranging from 0.37 to 2.87 cm.
Six individuals (5 hemiplegics, 1 diplegic) showed maps for both the contra- and ipsilateral FDI in the same region of motor cortex, with distances between the ipsi- and contra-lateral FDI COGs ranging from 0.05 to 0.64 cm (Table 2, Figure 2).
Patterns of Cortical Mapping and Functional Outcomes
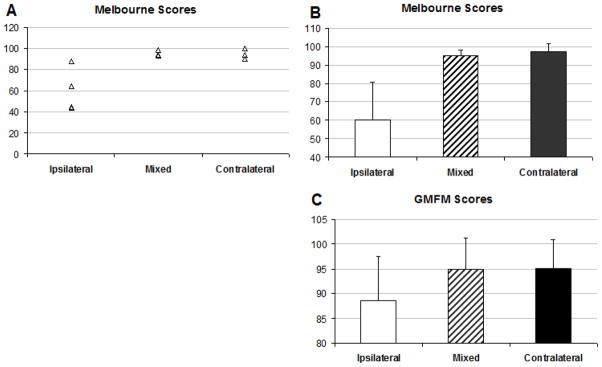
Following Holmstrom (Holmstrom et al., 2010), we organized the subjects based on the type of motor cortical mapping patterns for their affected FDI. Bilateral and contralateral projection patterns were associated with greater Melbourne scores (97.3±4.3 and 95.1±2.8, respectively) compared to ipsilateral patterns (60.1±20.8) (Figure 3A). The subgroup of subjects who showed ipsilateral patterns also demonstrated greater variability in their Melbourne scores compared to the subgroup with contralateral or bilateral patterns (Figure 3B). For GMFM scores, similar findings were observed, although the difference between ipsilateral (88.5±8.9) versus contralateral (95.1±5.8) or bilateral (94.9±6.4) was less marked (Figure 3C).
Figure 3.
Melbourne (individual subject scores shown in A and group means shown in B) and mean GMFM scores (C) for the study participants organized according to the 3 cortical mapping patterns. Error bars represent standard deviations.
Correlations between TMS-derived measures and motor function scores
Across the 13 study participants, there were no significant correlations between FDI COG-vertex distance or FDI-volume versus Melbourne or GMFM scores. However, analysis of correlations within various sub-groups revealed some interesting relationships.
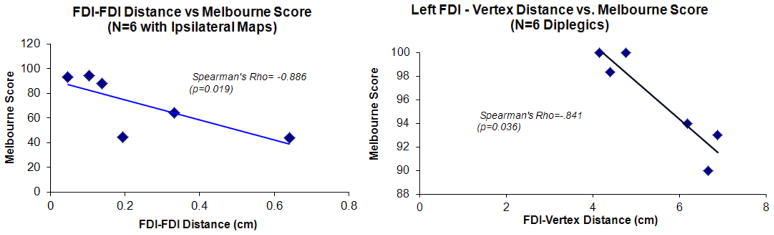
For the sub-group of 6 participants who demonstrated left and right FDI maps on the same side of the brain, greater distances between the ipsi- and contra-lateral FDI COGs were associated with lower Melbourne hand function scores, i.e. worse hand motor function (Spearman’s Rho = −0.886, p=0.019). However, a considerable range of Melbourne hand function scores (44–94) were observed for a relatively small ranges of distance (0.4–6 mm) between the ipsi- and contra-lateral FDI COG locations (Figure 4).
Figure 4.
Scatterplots showing selected results of the correlation analyses between TMS-metrics and Melbourne motor function scores. The Spearman’s rho coefficient and the p-value for the correlation are stated on each plot. On the left panel, FDI-FDI distance refers to the distance between ipsi- and contra-lateral FDI map COG for the 6 individuals who demonstrated ipsi- and contra-lateral FDI maps on the same side of the brain. On the right panel, FDI-vertex distance is the medio-lateral distance of the FDI motor map COG from the midline, i.e., a measure of the lateralization of FDI motor map COG location.
Among the 6 participants with diplegia (N=6), larger FDI-vertex distances were associated with lower Melbourne scores, i.e. worse hand motor function (Spearman’s Rho=-.841, p=0.036) (Figure 4). Also, a trend for a significant negative relationship between the GMFM score and the total hand map volume was detected (Spearman’s rho = −0.77, p=0.072).
For the 7 individuals with hemiplegia and for subjects who demonstrated both FDI and TA maps (N=4), no relationships were found between Melbourne or GMFM and affected TMS metrics.
DISCUSSION
This study used TMS to demonstrate a wider range of primary motor cortical map abnormalities than was previously recognized. Motor cortical map data were obtained from 13 children with clinical classifications of both hemiplegia (N=7) and diplegia (N=6). Compared to normal FDI and TA map COG x-coordinates of 0 and 4 cm, respectively (Maegaki et al., 1999; Wittenberg, 2009), children with CP showed a lateral shift of both FDI and TA maps, with considerable proximity between COG locations for FDI and TA maps. This finding has not been reported previously, to our knowledge. As has been shown before, a subset of individuals tested in our study (N=6) showed both ipsi- and contra-lateral FDI maps. The individuals with CP tested in our study showed 3 types of patterns of motor cortical maps: ipsilateral, contralateral, and bilateral. Also, non-parametric correlation analysis revealed interesting associations between corticomotor functional geometry and motor function. For example, greater lateralization of FDI maps was associated with worse upper extremity motor function. Furthermore, TMS-derived data from the participants in our study revealed three types of atypical cortical motor functional geometry: (1) lateral shift of hand and ankle motor maps; (2) increased proximity between hand and ankle maps; (3) overlapping ipsi- and contra-lateral FDI maps (a consequence of having two motor representations in the same hemisphere.) The same individual often showed more than one type of atypical cortical map geometry. Interestingly, however, these diverse patterns of corticomotor functional map geometry were not well related to the clinical subtypes of hemiplegia or diplegia for these individuals, suggesting that the clinical classification only approximates the degree of motor cortical reorganization that occurs after early brain insults. Similarly, the presence of unilateral or bilateral white matter abnormalities was also not perfectly related to either clinical classification or presence of lateralized motor maps (e.g. CP4 demonstrates how a small white matter lesion can lead to significant map abnormalities during development.).
Lateralization of TA maps is consistent with the report of Magaeki and colleagues who described ipsilateral TA MEPs and a lateral shift of TA maps in diplegic CP with pre-term birth (Maegaki et al., 1999). In our study of children with both diplegic and hemiplegic CP, a lateral shift of both TA and FDI maps was observed, with considerable proximity between COG of hand and ankle maps. It has been suggested that the cortical motor representation within the damaged cortex can shift and/or spread over adjacent (presumably intact) areas, resulting in a reshaping of cortex somatotopy (Rossini et al., 1998; Wittenberg, 2009). The current study provides evidence for the lateral shift of motor maps in individuals with CP with both unilateral- and bi-lateral brain lesions. Correlation analysis in individuals with diplegia provides preliminary support for the hypothesis that greater lateralization of cortical motor maps relates to worse motor function. The lateral shift likely occurs because periventricular lesions (shown by 7 of our subjects, see Table 1) preferentially damage the medial wall’s corticospinal tract projection (Staudt et al., 2000).
In the subset of individuals (N=4) whose TMS data demonstrated both FDI and TA maps in the lateral convexity of one hemisphere, we found preliminary evidence for a negative relationship between TA-vertex versus FDI-TA distances, suggesting that lateral shift of the TA maps is associated with greater proximity between the FDI and TA map COG. Although the current analysis of relative locations of FDI and TA map COGs was is limited by a small sample size, it serves as preliminary work suggesting a need for future studies to investigate the effects of increased proximity between cortical motor representations of muscles on motor function.
Another type of cortical reorganization shown by a subset of individuals tested in our study participants was the presence of both ipsi- and contra-lateral hand maps on the same side of the brain. Ipsilateral MEPs have been reported previously in CP (Carr et al., 1993; Eyre et al., 2007; Holmstrom et al., 2010; Maegaki et al., 1999; Maegaki et al., 1997; Nezu et al., 1999; Wittenberg, 2009). In this study, by mapping both representations, we sought to distinguish the two maps by their geometry. An interesting finding of our study was that in contrast to the hypothesis that greater proximity between ipsi- and contra-lateral map COG locations would relate to worse clinical scores, smaller distance between ipsi- and contra-lateral FDI map COG locations was associated with higher Melbourne scores and therefore, better motor function. Furthermore, and perhaps more importantly, a wide range of Melbourne hand function scores (44–94) were observed for relatively minor ranges of FDI-FDI distances (0.4–6 mm) (Figure 4), suggesting that these maps were almost always comprised of the same neural elements, and geometrical differences did not relate to function.
Another notable finding of our study was the presence of overlapping types of corticomotor functional geometry in individuals with CP with clinical classifications of diplegia and hemiplegia, showing a discrepancy between the TMS-mapping data and the clinical classification for individuals with CP. It appears that, similar to the clinical impairments in CP, which vary in affected body parts and severity, CP presents with a continuum of diverse neurophysiologic abnormalities, as opposed to distinct and non-overlapping neurophysiologic classifications or categories. However, due to the relatively small sample (N=13) of participants in our study, we were unable to detect strong or conclusive evidence for relationships between motor map data and clinical function for the group. Nevertheless, comparison of data collected using identical protocols across small yet relevant sub-groups within our data (e.g. those with diplegia or hemiplegia, 6 participants with ipsi- and contra-lateral hand maps on the same side of the brain, etc.) revealed interesting associations between TMS-derived neurophysiologic measures and motor function. Also, although both the Melbourne and the GMFM are good measures of global upper and lower extremity motor function in CP, respectively, the lack of sensitivity of these clinical measures may have prevented the detection of some relationships between TMS-derived and clinical data. For example, perhaps the proximity between TA and FDI maps would correlate better with direct measures of TA and FDI muscle strength or selective motor control as opposed to global clinical measures used in our study
The TA representation is usually in the medial wall of the posterior frontal lobe, which presents some problems for TMS, due to limited depth of the induced electric field. Yet TMS can successfully stimulate TA in the majority of subjects and has a long history of use for that purpose (Awiszus et al., 1994; Berardelli et al., 1991). While we may have missed some TA representations on the medial wall, lateral convexity TA maps are never seen in an unaffected population. Lateralized foot motor map representations have, however, been found in adults with dysmelia (Stoeckel et al., 2009). In our current study, we were unable to identify TA motor maps in some children. This could be due to a variety of reasons. It is possible that these children did not have lateralization of TA motor maps; their TA cortico-motor representations were located at the midline and deeper locations (i.e. medial wall of the posterior frontal lobe), as is typical in able-bodied children and adults, preventing acquisition of TA motor evoked potentials from TMS pulses delivered using a standard figure of 8 coil. It is also possible that these patients had considerably high TA motor thresholds, due to which we were unable to record TA MEPs using our TMS coil.
This study paves the way for future studies systematically investigating the relationships between neuropathology, impairments, and disability in large, heterogeneous samples of individuals with CP. Our results reveal evidence for a discrepancy between the common clinical classification and the TMS-derived neurophysiologic metrics, suggesting the need for further work to develop improved classification schemes that integrate clinical, radiologic, and neurophysiologic measures. This study also explored the elusive relationship between neurophysiology and functional impairments in CP. A wide range of functional scores was found in study participants who demonstrated the same types of cortical reorganization patterns and individuals with similar motor function often demonstrated different cortical reorganization patterns; this suggests a high degree of variability in clinical impairments is manifested for similar neuropathologic abnormalities. There is a need for large-scale studies that can provide further insights into how TMS-derived cortical motor map metrics relate to motor impairment in CP. Ultimately, such information has the potential to identify predictors for response to clinical neurorehabilitation, to guide clinical decision making, and to devise novel and individualized intervention strategies(Johnston, 2009; Kuhnke et al., 2008; Piron et al., 2005; Wittenberg, 2009; Wittenberg, 2010)
This is a cross-sectional study investigating motor cortical organization patterns in children with CP. Motor cortical representations are likely influenced by complex interactions between genetics, development, and experience. For example, the cortico-motor functional geometry may be influenced by the size, location, and time of onset of the initial neurological lesion (Kulak et al., 2007; Lee et al., 2011; Martinez-Biarge et al., 2011; Okumura et al., 1997; Romei et al., 2007; Trivedi et al., 2010). Furthermore, motor cortical functional geometry in the children with CP included in our study could be influenced by differences in physical activity levels, motor practice, and/or intensity of physical or occupational therapy (Boyd et al., 2010; Johnston, 2009; Redman et al., 2008; Wittenberg, 2010). It is challenging to obtain quantitative data on the extent of motor practice that each child had over a lifetime, and its relative influence on motor geometry. An interesting, feasible, and useful approach for future studies would be to longitudinally track within-subject changes in motor cortical functional geometry with time and/or with an exercise intervention.
In summary, our findings provide information about types of atypical corticomotor functional geometry demonstrated by individuals with both diplegic and hemiplegic CP. TMS, a relatively inexpensive, non-invasive and well-tolerated technique, was used to understand cortical reorganization in CP. Understanding the changes in motor maps can enable the design of specific pharmacologic, rehabilitative, and surgical interventions for CP in the future. The current study is a step towards defining the relationship between pathophysiology and functional impairments in CP. In our study, a wide range of clinical scores was observed for individuals with CP demonstrating the same types of cortical reorganization patterns, suggesting a high degree of variability in clinical impairments manifest in similar neuropathologic abnormalities. Also, individuals with similar motor function or similar clinical classifications demonstrated different cortical organization patterns. There is a need for larger-scale studies that can provide better insights into the neurophysiologic and behavioral mechanisms underlying impairment, disability, and response to interventions in cerebral palsy.
Highlights.
In cerebral palsy patients, for the first time, we demonstrate hand maps lateral to the expected location, and replicate the finding of lateralized ankle maps in diplegia, but in an expanded population.
Three patterns of cortical mapping in a pediatric study group consisting of both diplegics and hemiplegics, previously shown only in hemiplegics
A putative relationship between motor map geometry and motor function
Acknowledgments
The study was funded by USPHS award R21 HD049019, G.F.W, P.I. The authors also thank Dr. Stuart Binder-Macleod, PT, PhD, FAPTA (University of Delaware) for supporting Dr. Kesar’s participation in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avery LM, Russell DJ, Raina PS, Walter SD, Rosenbaum PL. Rasch analysis of the Gross Motor Function Measure: validating the assumptions of the Rasch model to create an interval-level measure. Arch Phys Med Rehabil. 2003;84:697–705. doi: 10.1016/s0003-9993(02)04896-7. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Feistner H. Quantification of D- and I-wave effects evoked by transcranial magnetic brain stimulation on the tibialis anterior motoneuron pool in man. Exp Brain Res. 1994;101:153–8. doi: 10.1007/BF00243225. [DOI] [PubMed] [Google Scholar]
- Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. 2005;47:571–6. doi: 10.1017/s001216220500112x. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Cruccu G, Mercuri B, Manfredi M. Electrical and magnetic transcranial stimulation in patients with corticospinal damage due to stroke or motor neurone disease. Electroencephalogr Clin Neurophysiol. 1991;81:389–96. doi: 10.1016/0168-5597(91)90028-v. [DOI] [PubMed] [Google Scholar]
- Bourke-Taylor H. Melbourne Assessment of Unilateral Upper Limb Function: construct validity and correlation with the Pediatric Evaluation of Disability Inventory. Dev Med Child Neurol. 2003;45:92–6. [PubMed] [Google Scholar]
- Boyd R, Sakzewski L, Ziviani J, Abbott DF, Badawy R, Gilmore R, et al. INCITE: A randomised trial comparing constraint induced movement therapy and bimanual training in children with congenital hemiplegia. BMC Neurol. 2010;10:4. doi: 10.1186/1471-2377-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116 ( Pt 5):1223–47. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- Colver AF, Sethumadhavan T. The term diplegia should be abandoned. Arch Dis Child. 2003;88:286–90. doi: 10.1136/adc.88.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA, Smith M, Dabydeen L, Clowry GJ, Petacchi E, Battini R, et al. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Mall V. Transcranial magnetic stimulation in children. Clin Neurophysiol. 2008;119:973–84. doi: 10.1016/j.clinph.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom L, Vollmer B, Tedroff K, Islam M, Persson JK, Kits A, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52:145–52. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Schwerin S. Brain repair after stroke. Cambridge: 2010. Motor map plasticity: a neural substrate for improving motor function after stroke; pp. 1–10. [Google Scholar]
- Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol. 2008;50:898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- Kulak W, Sobaniec W, Kubas B, Walecki J, Smigielska-Kuzia J, Bockowski L, et al. Spastic cerebral palsy: clinical magnetic resonance imaging correlation of 129 children. J Child Neurol. 2007;22:8–14. doi: 10.1177/0883073807299953. [DOI] [PubMed] [Google Scholar]
- Lee JD, Park HJ, Park ES, Oh MK, Park B, Rha DW, et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain. 2011;134:1199–210. doi: 10.1093/brain/awr021. [DOI] [PubMed] [Google Scholar]
- Lin KL, Pascual-Leone A. Transcranial magnetic stimulation and its applications in children. Chang Gung Med J. 2002;25:424–36. [PubMed] [Google Scholar]
- Maegaki Y, Maeoka Y, Ishii S, Eda I, Ohtagaki A, Kitahara T, et al. Central motor reorganization in cerebral palsy patients with bilateral cerebral lesions. Pediatr Res. 1999;45:559–67. doi: 10.1203/00006450-199904010-00016. [DOI] [PubMed] [Google Scholar]
- Maegaki Y, Maeoka Y, Seki A, Ueno M, Yamamoto T, Takeshita K. Facilitation of ipsilateral motor pathways during recovery from hemiplegia in two adolescent patients. Eur J Paediatr Neurol. 1997;1:79–84. doi: 10.1016/s1090-3798(97)80067-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, et al. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76:2055–61. doi: 10.1212/WNL.0b013e31821f442d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezu A, Kimura S, Takeshita S, Tanaka M. Functional recovery in hemiplegic cerebral palsy: ipsilateral electromyographic responses to focal transcranial magnetic stimulation. Brain Dev. 1999;21:162–5. doi: 10.1016/s0387-7604(98)00094-1. [DOI] [PubMed] [Google Scholar]
- Okumura A, Kato T, Kuno K, Hayakawa F, Watanabe K. MRI findings in patients with spastic cerebral palsy. II: Correlation with type of cerebral palsy. Dev Med Child Neurol. 1997;39:369–72. [PubMed] [Google Scholar]
- Piron L, Piccione F, Tonin P, Dam M. Clinical correlation between motor evoked potentials and gait recovery in poststroke patients. Arch Phys Med Rehabil. 2005;86:1874–8. doi: 10.1016/j.apmr.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Randall M, Carlin JB, Chondros P, Reddihough D. Reliability of the Melbourne assessment of unilateral upper limb function. Dev Med Child Neurol. 2001;43:761–7. doi: 10.1017/s0012162201001396. [DOI] [PubMed] [Google Scholar]
- Redman TA, Gibson N, Finn JC, Bremner AP, Valentine J, Thickbroom GW. Upper limb corticomotor projections and physiological changes that occur with botulinum toxin-A therapy in children with hemiplegic cerebral palsy. Eur J Neurol. 2008;15:787–91. doi: 10.1111/j.1468-1331.2008.02194.x. [DOI] [PubMed] [Google Scholar]
- Romei M, Galli M, Fazzi E, Maraucci I, Schwartz M, Uggetti C, et al. Analysis of the correlation between three methods used in the assessment of children with cerebral palsy. Funct Neurol. 2007;22:17–21. [PubMed] [Google Scholar]
- Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. Jama. 2002;288:1357–63. doi: 10.1001/jama.288.11.1357. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Caltagirone C, Castriota-Scanderbeg A, Cicinelli P, Del Gratta C, Demartin M, et al. Hand motor cortical area reorganization in stroke: a study with fMRI, MEG and TCS maps. Neuroreport. 1998;9:2141–6. doi: 10.1097/00001756-199806220-00043. [DOI] [PubMed] [Google Scholar]
- SCPE. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816–24. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- Stanley FJ, Watson L. Trends in perinatal mortality and cerebral palsy in Western Australia, 1967 to 1985. Bmj. 1992;304:1658–63. doi: 10.1136/bmj.304.6843.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M. Reorganization after pre- and perinatal brain lesions. J Anat. 2010;217:469–74. doi: 10.1111/j.1469-7580.2010.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M, Niemann G, Grodd W, Krageloh-Mann I. The pyramidal tract in congenital hemiparesis: relationship between morphology and function in periventricular lesions. Neuropediatrics. 2000;31:257–64. doi: 10.1055/s-2000-9239. [DOI] [PubMed] [Google Scholar]
- Staudt M, Pavlova M, Bohm S, Grodd W, Krageloh-Mann I. Pyramidal tract damage correlates with motor dysfunction in bilateral periventricular leukomalacia (PVL) Neuropediatrics. 2003;34:182–8. doi: 10.1055/s-2003-42206. [DOI] [PubMed] [Google Scholar]
- Stoeckel MC, Seitz RJ, Buetefisch CM. Congenitally altered motor experience alters somatotopic organization of human primary motor cortex. Proc Natl Acad Sci U S A. 2009;106:2395–400. doi: 10.1073/pnas.0803733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Shah V, Goyel P, Paliwal VK, Rathore RK, et al. Correlation of quantitative sensorimotor tractography with clinical grade of cerebral palsy. Neuroradiology. 2010;52:759–65. doi: 10.1007/s00234-010-0703-8. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF. Motor mapping in cerebral palsy. Dev Med Child Neurol. 2009;51 (Suppl 4):134–9. doi: 10.1111/j.1469-8749.2009.03426.x. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF. Neural plasticity and treatment across the lifespan for motor deficits in cerebral palsy. Dev Med Child Neurol. 2009;51 (Suppl 4):130–3. doi: 10.1111/j.1469-8749.2009.03425.x. [DOI] [PubMed] [Google Scholar]
- Wittenberg GF. Experience, cortical remapping, and recovery in brain disease. Neurobiol Dis. 2010;37:252–8. doi: 10.1016/j.nbd.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]