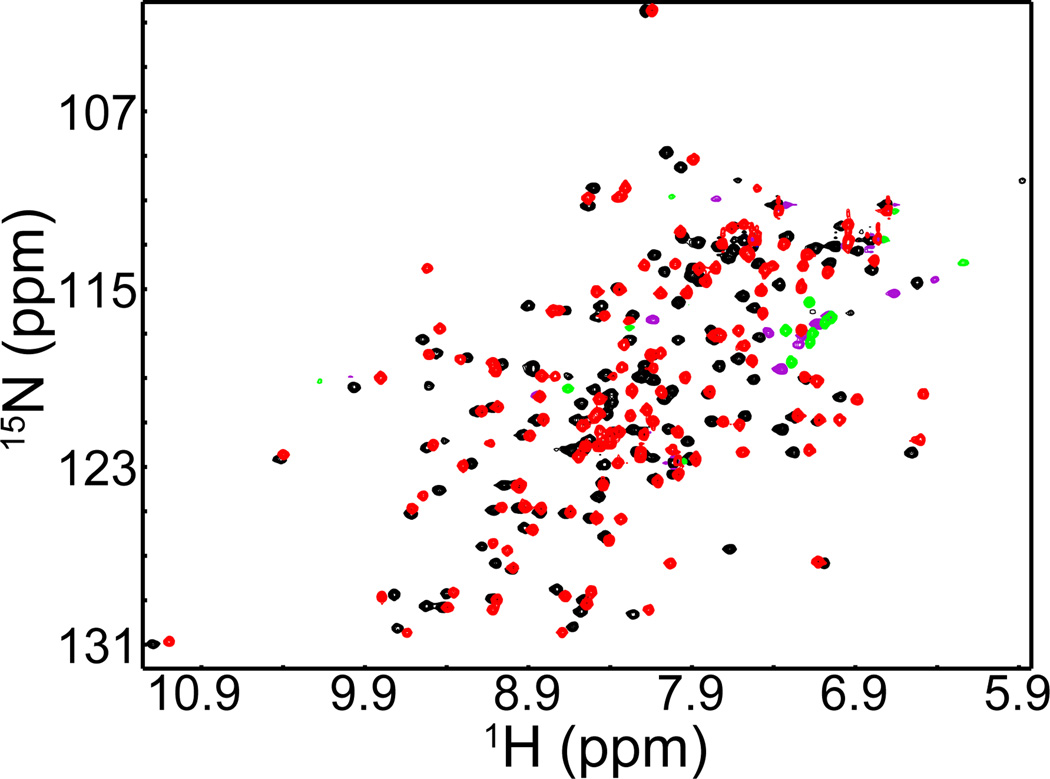
Abstract
Kaiso is a Cys2His2 zinc finger protein that mediates methyl-CpG -dependent and sequence-specific transcriptional repression. As a first step towards elucidating the structural and molecular basis for recognition of these disparate DNA sequences, the minimal binding region of Kaiso was identified and optimal DNA sequences for high-affinity interactions were characterized. Contrary to previous findings, Kaiso requires all three zinc fingers plus adjacent protein regions for DNA recognition. An N-terminal extension contributes to structural stability, while an extended C-terminal region augments DNA binding. Complexes formed between the optimized Kaiso construct and both DNA sequences are suitable for future structural evaluation.
Keywords: Kaiso, methyl-CpG DNA binding protein, zinc finger, methylated DNA
1. Introduction
Methylation of cytosine in the context of CpG dinucleotide sequences is a prevalent and essential epigenetic modification in mammals that signals for genes to be transcriptionally silenced. Significant variances in genomic DNA methylation patterns have been linked to neuro-developmental diseases and cancer [1–3], prompting a need to understand the mechanisms of DNA methylation in altering the transcriptional state of genes. One method by which DNA methylation induces transcriptional repression is through recruitment of specialized transcription factors, termed methyl-CpG DNA binding proteins (MBPs) that preferentially recognize methylated CpG sites (mCpG) [4]. These proteins act as mediators between recognition of the methylation signal and recruitment of chromatin remodeling co-repressor complexes to the target gene [5].
Kaiso is the original member of a family of zinc finger MBPs that elicit both methyl-dependent and sequence-specific transcriptional repression through a highly conserved set of three Cys2His2 zinc fingers. Kaiso recognizes target DNA sequences containing two consecutive symmetrically methylated CpG dinucleotides [6–8] as well as the non-methylated sequence TCCTGCNA, termed the Kaiso binding site (KBS) [9]. There is growing evidence that transcriptional repression by Kaiso is directly implicated in cancer [10–12], though it has not yet been established whether interactions with methylated and KBS sequences are correlated or independent regulatory events.
Here we describe the minimal region of Kaiso required for recognition of both sequence-specific and mCpG containing sequences. In contrast to a previous report [9], we found that Kaiso requires all three zinc fingers plus adjacent N- and C-terminal extensions to form a stable protein-DNA complex and optimal DNA binding interactions. This Kaiso construct forms high affinity 1:1 complexes with both KBS and a mCpG sequence derived from the E-Cadherin (ECad) promoter region [6,13], and can discriminate between mCpG, CpG and TpG sites. Both the KBS and methyl-DNA complexes give high quality NMR spectra, indicating that these constructs are suitable for future structure determination.
2. Materials and Methods
2.1 Preparation of Kaiso constructs
Kaiso constructs were PCR amplified from a human liver cDNA library (Mobitec) and cloned into the pET21d expression vector (Novagen). Uniformly 15N-labeled constructs were expressed in BL21(DE3) [DNAY] Escherichia coli (E. coli) host cells in M9 minimal medium supplemented with 150 µM ZnSO4 during IPTG induction at 15°C for 16 h. Cells were resuspended in lysis buffer (20 mM Tris (pH 8.0), 8 M urea, 200 mM Arg-HCl, 10 mM dithiothreitol (DTT)), incubated on ice for 10 min, and lysed by sonication. Supernatant was loaded onto 100 ml SP Sepharose FF resin (Pharmacia) equilibrated with 20 mM Tris (pH 8.0), 8 M urea, 200 mM Arg-HCl, and 2 mM DTT. Bound protein was eluted with a linear salt gradient to 1 mM NaCl. Kaiso fractions were pooled, diluted 3x in H2O/0.1% TFA, and further purified by reversed-phase HPLC on a 25×100 mm, 15µm, 300Å Delta-Pak C18 column (Waters) pre-equilibrated with 90%A (H2O/0.1% TFA)/10%B (ACN/0.1% TFA). Bound protein was eluted over a variable gradient (2%/min to 80%A/20%B; 1%/min to 70%A/30%B; 0.3%/min to 60%A/40%B; 1%/min to 50%A/50%B) and lyophilized. Purity and isotope incorporation were verified by SDS-PAGE and MALDI-MS.
Lyophilized protein was dissolved in 1.2 ml denaturing buffer (10 mM Tris (pH 7.0), 8 M urea, 10 mM DTT) and diluted to 0.5 M urea in 20 ml refolding buffer (10 mM Tris (pH 7.0), 10 mM DTT) containing 1.1 molar equivalents ZnSO4 per zinc site. Refolded protein was concentrated in an Amicon stirred cell (Millipore) and exchanged into NMR buffer (10 mM Tris (pH 7.0), 1 mM tris(2-carboxyethyl)phosphine (TCEP), 5% D2O, 0.005% NaN3) utilizing a Nap-10 Sephadex G25 column (GE Healthcare Life Sciences). Final protein concentrations were determined by BCA assay (Thermo Scientific).
2.2 Preparation of DNA
DNA oligonucleotides were purchased from IDT Inc., purified on a NAP-10 Sephadex G25 column, and lyophilized. To form duplex DNA, sense and antisense oligonucleotides were resuspended in 10 mM Tris, adjusted to pH 7.0, mixed in stoichiometric amounts, heated to 90°C, and annealed by slow cooling to room temperature. For EMSA, duplex ECad DNA was symmetrically methylated utilizing the M.SssI CpG methyltransferase and S-adenosylmethionine (New England Biolabs). Methyl group incorporation was verified by MALDI-MS.
2.3 Electrophoretic gel mobility shift assays
Duplex DNA sequences were 5′-end labeled with [γ-32P]ATP using T4 polynucleotide kinase (NEB) and purified on a G-25 Sephadex quick spin column (Roche). 32P-labeled DNAs (25 pM) were incubated with increasing concentrations of Kaiso in 15 µl binding buffer (10 mM Tris (pH 7.0), 1 mM TCEP, 100 µg/ml BSA, 10% glycerol). After incubation at room temperature for 30 min, 1.5 µl 0.19% bromophenol blue was added to each reaction and 10 µl of each mixture was electrophoresed in 5% (w/v) polyacrylamide gels in 89 mM Tris-borate (pH 8.3) for 35 min at 100V. Gels were dried and exposed to a storage phosphor screen for 12 hours, scanned on a phosphorimager (Molecular Dynamics), and analyzed for band intensities (ImagQuant). Experiments were repeated in at least triplicate and apparent Kd measurements were obtained by fitting the fraction of bound DNA as a function of protein concentration to the Hill equation using a floating Hill coefficient in Kaleidagraph software (Synergy Software). EMSAs for Kaiso-ZF123(472–604) with KBS and MeECad were performed under identical conditions using the same concentration of DNA (25pM) and protein (0–3nM). The weaker binding Kaiso-ZF123(472–579) was titrated to 45nM.
2.4 NMR spectroscopy
Kaiso/DNA complexes were prepared by diluting the DNA in 7 ml argon-saturated NMR buffer and adding protein solution dropwise while stirring. Samples were concentrated to 450 µl in an Amicon stirred cell to a final concentration of 100–125 µM complex and transferred to an NMR tube. Binding was monitored by observing the DNA imino proton chemical shifts. All NMR spectra were recorded at 298K on Bruker Avance500, DRX600, or DRX800 MHz spectrometers and data were processed using NMRPipe [14] and analyzed with NMRView [15].
3. Results
3.1 ZF1 is Directly Involved in KBS Recognition
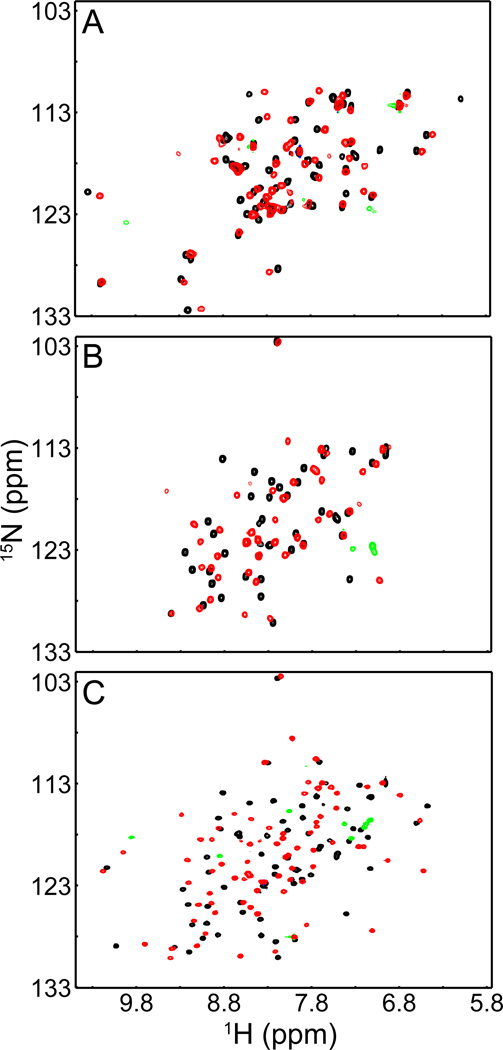
It was originally reported that Kaiso zinc fingers (ZF) 2 and 3 were necessary and sufficient for nucleic acid recognition [9]. However, a Kaiso construct containing only ZF 2 and 3 (Kaiso-ZF23(517–579)) binds only weakly to KBS, resulting in small chemical shift perturbations in the HSQC spectrum (Fig. 1A). Extending the C-terminus of the ZF23 construct to residue 604 or 635 to emulate the construct used by Daniel et al. enhances binding slightly but fails to shift the DNA probe in EMSA experiments and results only in broadening of protein and imino proton resonances rather than the substantial, slow exchange shifts observed for the high affinity ZF123(472–604) complexes. The differences between our results and the published data may also reflect the use of a GST fusion tag by Daniel et al. [9], whereas our constructs are untagged. It is possible that dimerization of the ZF23 construct through the GST in [9] may allow additional interactions with the DNA that enhance the apparent binding affinity.
Fig. 1.
Kaiso requires all three zinc fingers for DNA recognition. 1H-15N HSQC spectra of (A) Kaiso-ZF23(517–579), (B) Kaiso-ZF1(472–517), and (C) Kaiso-ZF12(472–548) free (black) and in complex with KBS (red/green). Substantial chemical shift perturbations in the spectra of the complexes shown in panels (B) and (C) directly implicate ZF1 in the recognition of KBS. Green cross peaks signify aliased arginine side chain resonances that appear upon KBS coordination.
To assess the role of ZF1, constructs containing only ZF1 or ZF 1 and 2 were generated; we discovered that incorporation of a highly conserved region immediately preceding ZF1 was required to form stable protein. Attempts to substantially lengthen or shorten the N-terminal region resulted in poor expression or protein that proved difficult to purify due to high propensity to form aggregates. Expression of constructs beginning at M471 gave high yields of stable protein; however, mass spectrometry measurements showed that the N-terminal methionine is cleaved during E. coli expression.
HSQC analysis of Kaiso-ZF1(472–517) in complex with KBS resulted in a well dispersed spectrum with larger chemical shift perturbations than for Kaiso-ZF23(517–579) (Fig. 1B), although some amides exhibit weak cross peak intensities. Inclusion of the second zinc finger (Kaiso-ZF12(472–548)) also produced a well dispersed HSQC spectrum, with a further increase in chemical shift perturbations and more uniform cross peak intensities (Fig. 1C). These results indicate unequivocally that ZF1 is required for recognition of KBS. Nonetheless, HSQC spectra of Kaiso constructs containing all three zinc fingers showed additional chemical shift changes for ZF3 residues upon binding to KBS. Taken together, the above findings suggest that all three zinc fingers function collectively to recognize cognate DNA sequences.
3.2 Kaiso Requires a C-terminal Extension for High-affinity Binding to KBS
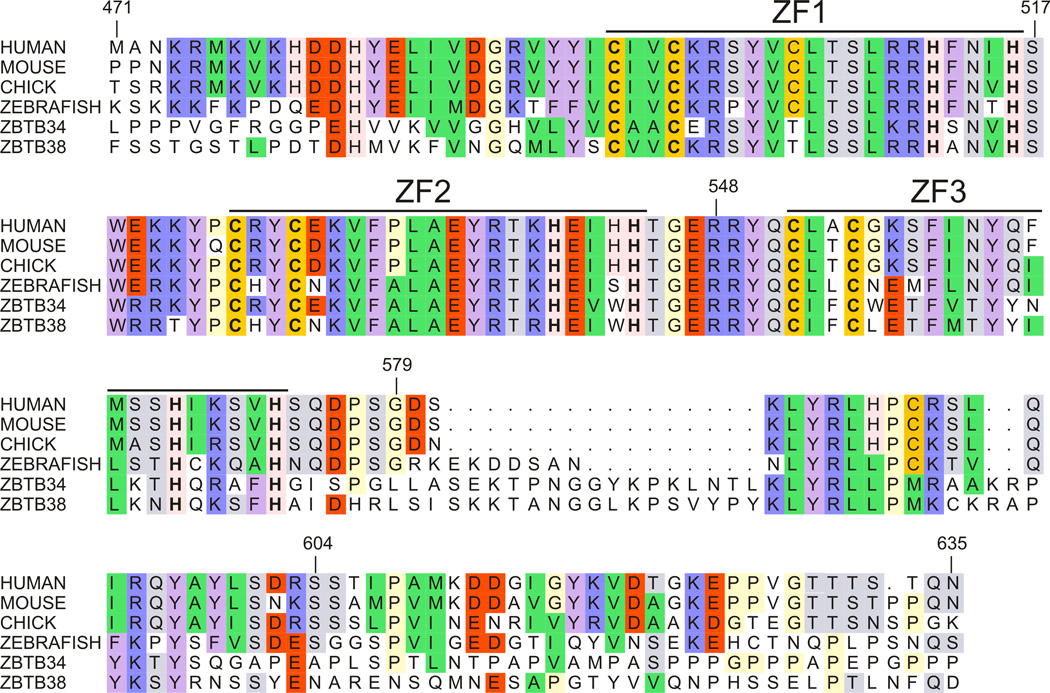
To identify the optimal Kaiso construct necessary to confer both high-affinity binding and specificity, a number of three zinc finger constructs were expressed. Sequence alignment of Kaiso from several species shows a high level of homology not only within the Cys2His2 zinc finger domain but also in flanking regions (Fig. 2). To investigate the role of the conserved region following ZF3, several three-finger constructs were expressed: 1) a construct terminating immediately after ZF3 (Kaiso-ZF123(472–579)); 2) a construct with 31 C-terminal amino acids encompassing the additional highly conserved region (Kaiso-ZF123(472–604)); and 3) a construct more closely resembling the sequence utilized in previous Kaiso/DNA binding studies [9,13,16] (KaisoZF123(472–635)).
Fig. 2.
Sequence alignment shows a high level of homology for the three Cys2His2 zinc fingers and flanking N- and C-terminal regions of Kaiso proteins and the related human Zbtb4 and Zbtb38.
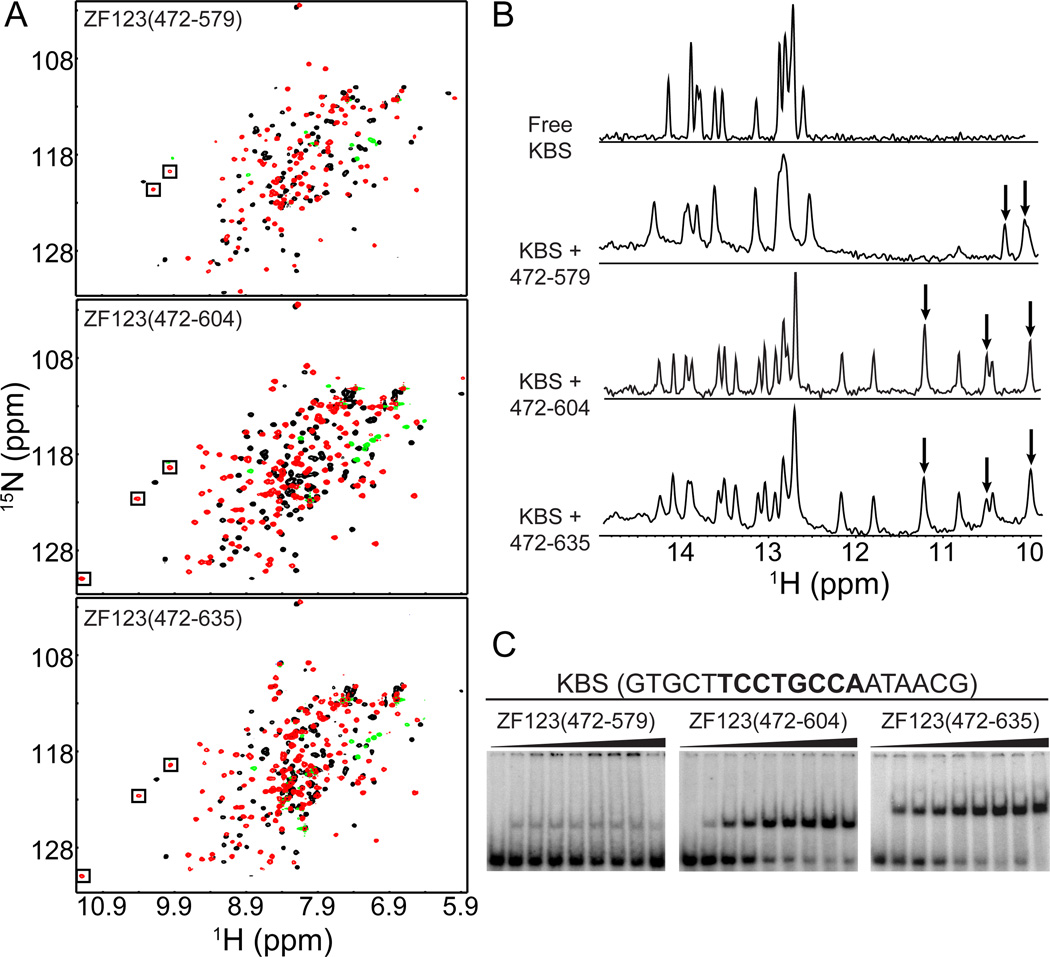
Binding of the three-finger constructs to KBS was monitored by NMR and EMSA. While Kaiso-ZF123(472–579) binds more tightly to KBS than constructs containing only one or two zinc fingers, the affinity is substantially weakened in the absence of a C-terminal tail. Comparison of the HSQC spectra for the various Kaiso/KBS complexes revealed the appearance of several additional peaks between 9.4–11.3 ppm (1H) for constructs containing a C-terminal extension (Fig. 3A). In addition, the imino proton spectra of KBS in complex with Kaiso show that an extended C-terminus induces substantially more DNA chemical shift perturbations, including uniquely shifted resonances between 10.8–12.2 ppm (Fig. 3B). For the two constructs with extended C-termini, Kaiso-ZF123(472–604) and Kaiso-ZF123(472–635), there is little discernible difference in the pattern of induced chemical shift perturbations upon KBS binding, and cross peaks corresponding to extra residues in the C-terminus of Kaiso-ZF123(472–635) lie in the random coil region of the HSQC spectrum. Thus, residues beyond Ser604 do not appear to interact with DNA.
Fig. 3.
Kaiso requires a minimal C-terminal extension to enhance binding to KBS. (A) 1H-15N HSQC spectra of Kaiso-ZF123(472–579), Kaiso-ZF123(472–604) and Kaiso-ZF123(472–635) free (black) and in complex with KBS (red/green). (B) Imino proton region of KBS NMR spectrum free and in complex with the various three zinc finger constructs. Boxes (A) and arrows (B) indicate peaks resulting from Kaiso amide protons. (C) EMSA results for KBS in complex with Kaiso-ZF123(472–579), Kaiso-ZF123(472–604) and Kaiso-ZF123(472–635). Protein was titrated to a final concentration of 45 nM (Kaiso-ZF123(472–579)) or 3 nM (Kaiso-ZF123(472–604) and Kaiso-ZF123(472–635)). Apparent Kd values were determined to be 190 ± 40 and 180 ± 30 pM for Kaiso-ZF123(472–604) and Kaiso-ZF123(472–635), respectively.
EMSA experiments confirmed the NMR results, revealing a large increase in KBS binding affinity in the presence of a C-terminal tail (Fig. 3C). Binding of Kaiso-ZF123(472–579) is weak and non-saturable, while the C-terminal extended constructs bind with sub-nanomolar affinity (apparent Kd values of 190 ± 40 and 180 ± 30 pM for Kaiso-ZF123(472-604) and Kaiso-ZF123(472–635), respectively). Thus, Kaiso absolutely requires a C-terminal extension beyond ZF3 to augment the DNA binding interaction but the 31 residues beyond Ser604 do not contribute any additional affinity enhancement. The N- and C-terminal extensions increase the hydrophobicity of Kaiso resulting in a propensity to form insoluble aggregates under conditions in which the protein concentration is in large excess over DNA. This becomes a significant issue when the protein binds weakly to the DNA, which is the case for ZF1, ZF12, and the three zinc finger constructs without the C-terminal extension, and precludes quantitative Kd measurements for weakly binding constructs.
3.3 Identification of a mCpG DNA Sequence Suitable for Structural Evaluation
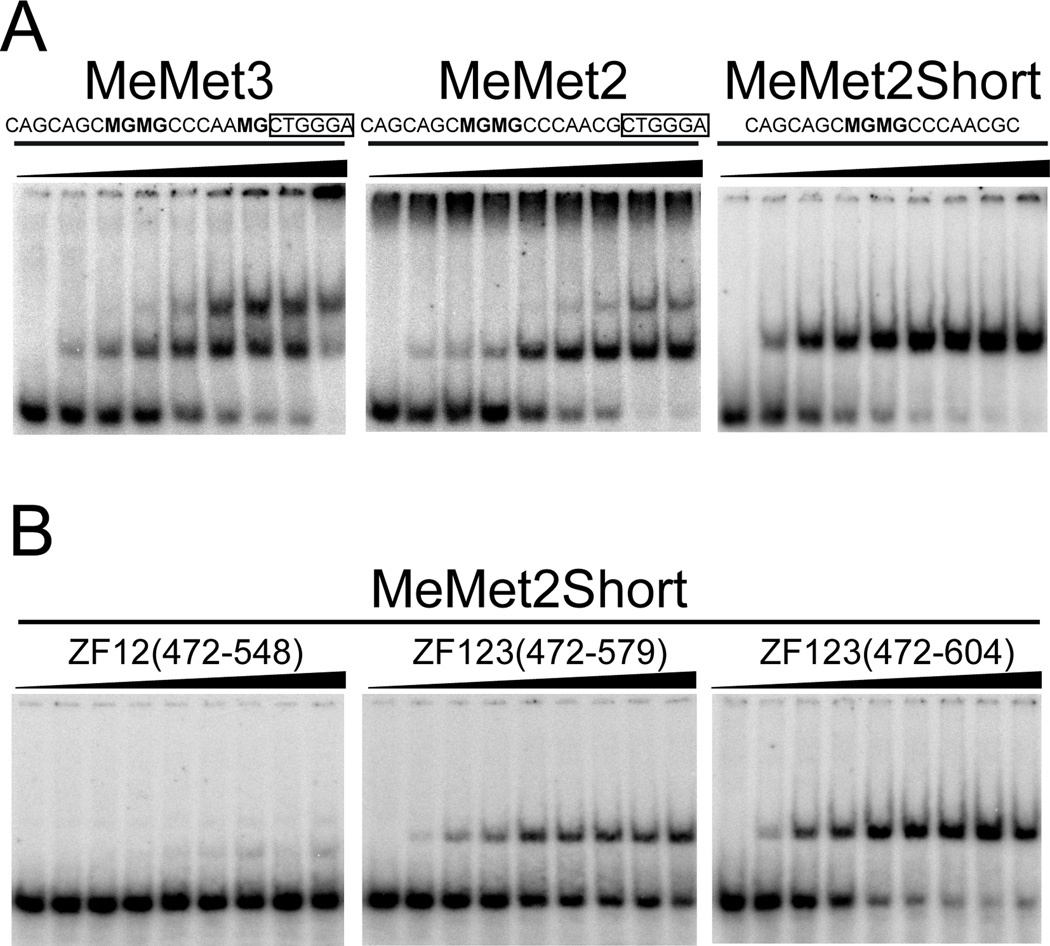
Kaiso binds with high affinity to a methylated DNA probe derived from the metastasin gene with the core sequence MGMGCCCAAMG, where M represents a methylated cytosine [6,7,9]. Initial attempts to monitor interactions between this sequence (designated MeMet3) and Kaiso-ZF123(472–604) resulted in poor quality HSQC spectra characterized by significant line broadening and splitting of cross peaks for several residues. EMSA (Fig. 4A) showed multimeric binding, as was noted previously [9]. This secondary binding was initially attributed to lower affinity interactions at the third mCpG site, which can be omitted without affecting the ability of Kaiso to recognize methylated DNA [6]. A DNA probe methylated only at the consecutive mCpG pairs (designated MeMet2) reduced Kaiso’s affinity for the secondary site relative to MeMet3, but did not abolish it. These findings suggested that Kaiso makes additional DNA interactions, most likely involving a KBS-like sequence on the 3’ side of the methylation site. This was confirmed using a truncated methylated DNA probe lacking the KBS-like sequence (designated MeMet2Short), which formed a 1:1 complex (Fig. 4A). EMSA analysis of the interactions of Kaiso-ZF12(472–548), Kaiso-ZF123(472–579), and Kaiso-ZF123(472–604) with the MeMet2Short DNA probe clearly indicated that, as for KBS binding, all three fingers and an extended C-terminus are required to confer high-affinity binding of methylated DNA (Fig. 4B). However, NMR spectra still exhibited substantial line broadening indicative of persisting nonspecific interactions, which led us to search for alternative mCpG containing sequences that may be more amenable for structure elucidation.
Fig. 4.
Kaiso exhibits multimeric binding to the metastasin methylated DNA target and requires all three zinc fingers plus an extended C-terminus for high-affinity binding. (A) EMSA of Kaiso-ZF123(472–604) in complex with methylated sequences derived from the metastasin gene. Boxes highlight the KBS-like core in the longer DNA sequences. (B) Analysis of Kaiso-ZF12(472–548), Kaiso-ZF123(472–579) and Kaiso-ZF123(472–604) in complex with MeMetShort.
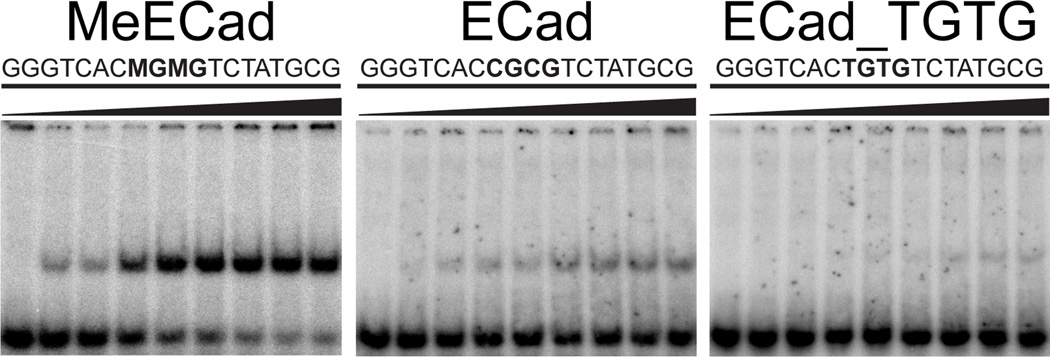
Since Kaiso binds the methylated promoter of E-Cadherin [6,13] in addition to the metastasin gene, we investigated binding to a methylated oligonucleotide containing the proposed E-Cadherin recognition site (designated MeECad). EMSA confirmed that Kaiso-ZF123(472–604) formed a high-affinity 1:1 complex with an apparent Kd of 210 ± 50 pM. This complex produced a high-quality HSQC spectrum suitable for structural analysis and comparable to that observed for KBS (Fig. 5). Finally, we performed EMSA experiments to assess the ability of Kaiso-ZF123(472–604) to discriminate between mCpG, unmethylated CpG, and TpG sites. Kaiso binds preferentially to mCpG sites, although it does bind weakly to CpG and TpG sites (Fig. 6A).
Fig. 5.
Superposition of 1H-15N HSQC spectra of Kaiso-ZF123(472–604) in complex with KBS (black/orange) and MeECad (red/green). The overall similarity of the spectra shows that Kaiso recognizes methylated DNA and KBS through a similar interface. Orange and green resonances indicate folded arginine side chains resonances that appear upon DNA binding.
Fig. 6.
Kaiso preferentially recognizes mCpG sites. (A) EMSA analysis of Kaiso-ZF123(472–604) in complex with MeECad, unmethylated ECad, and a sequence containing substituted TpG sites. The Kaiso concentration was incrementally increased to 3 nM in the titrations. The apparent Kd for the Kaiso-ZF123(472–604):MeECad complex is 210 ± 50 pM.
4. Discussion
Using a combination of NMR spectroscopy and molecular biology, we have shown that Kaiso requires all three zinc fingers plus N- and C-terminal extensions to maintain structural integrity and to mediate high-affinity sequence-specific and methyl-dependent DNA recognition, respectively. The N-terminal extension appears to be required for structural stability of the free protein, similar to the Cys2His2 zinc finger proteins SWI5 and Tramtrack in which residues N-terminal to the core ββα motif adopt an additional β-strand to generate a three-stranded anti-parallel β-sheet [17–19]. In both cases, the additional β-strand does not directly contact DNA but enhances the structural stability of the first zinc finger.
In contrast, the C-terminal extension of Kaiso is required to augment DNA binding. Several other zinc finger proteins utilize sequences beyond the zinc finger modules to enhance binding affinity by extending the region of DNA contacted [20–24]. These extensions are typically arginine/lysine rich, form structured loops upon DNA binding and often make stabilizing contacts in the minor groove. In the case of Kaiso, the C-terminal extension is more hydrophobic than arginine/lysine rich; upon binding to DNA, several cross peaks move from the center of the free Kaiso HSQC spectrum to more dispersed regions (Fig. 3A), indicative of a transition to a more highly structured state. Moreover, chemical shift perturbations observed in the KBS imino spectra when in complex with Kaiso constructs containing a C-terminal tail (Fig. 3B) suggest additional DNA contacts are made by residues outside the zinc finger domain.
It is intriguing that Kaiso appears to require a larger protein domain than the MBD family of MBPs to achieve high-affinity binding with methylated DNA. MBD1 and MeCP2 both utilize a relatively small contact area to bind their respective methylated DNA targets, making limited contacts in the major groove surrounding the mCpG site [25,26]. It has been suggested that these limited contacts allow more facile binding with chromatin by alleviating potential steric interactions with core histones [25]. Nevertheless, recent reports indicate these binding interactions may be more complex than originally thought, suggesting that some MBPs utilize additional protein extensions to modulate DNA binding [27], and that differential recognition of cognate sequences may involve DNA sequence context adjacent to the mCpG site [28–30]. Given the high level of conservation in the DNA binding domain within the Kaiso family [16] (Fig. 2), it is likely that the related zinc finger proteins Zbtb4 and Zbtb38 may also require additional protein regions C-terminal to the zinc fingers for DNA recognition.
It has also been reported that Kaiso binds with higher affinity to specific DNA sites such as KBS than to mCpG DNA targets [9]. However under our conditions, Kaiso binds with equivalent affinity to KBS and MeECad sites. It is interesting to note that, although Kaiso preferentially binds mCpG over CpG and TpG sites, KBS has a TpG site in the core recognition sequence. Furthermore, the Kaiso/KBS and Kaiso/MeECad HSQC spectra are largely superimposable, with significant chemical shift differences observed for only a few residues (Fig. 5), suggesting that the overall structure of both complexes is similar. These observations raise important questions about the physiological role that interaction of Kaiso with quite different DNA sequences plays in normal and disease-state cellular functions. Structure determination of the optimized Kaiso- ZF123(472–604) construct in complex with KBS and MeECad is currently in progress and should provide the necessary insight into the mechanism by which Kaiso is able to recognize these two distinct DNA sequences.
Highlights.
We have identified minimal Kaiso constructs for high-affinity DNA binding
All three Kaiso zinc fingers are required for binding
Extension of the sequence at the N-terminus increases stability of the protein
Extension of the sequence at the C-terminus augments DNA affinity.
Acknowledgements
EMSA experiments and analysis were completed with the helpful advice and resources of Dr. Joel M. Gottesfeld. We thank Euvel Manlapaz, Spencer Tung and Mindy Landes for expert technical assistance; and Gerard Kroon for support with NMR experiments. This work was supported by a grant from the National Institutes of Health (GM36643) and by the Skaggs Institute for Chemical Biology. B. A. Buck-Koehntop was supported by (PF-07-124-01-GMC) from the American Cancer Society.
List of Abbreviations
- MBP
methyl-CpG DNA binding protein
- KBS
Kaiso binding site
- ZF
zinc finger
- NMR
nuclear magnetic resonance
- HSQC
heteronuclear single-quantum coherence
- EMSA
electrophoretic gel mobility shift assays
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat. Rev. Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 3.Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu. Rev. Pharmacol. Toxicol. 2005;45:629–656. doi: 10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- 4.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem. Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Clouaire T, Stancheva I. Methyl-CpG binding proteins: specialized transcriptional repressors or structural components of chromatin? Cell. Mol. Life Sci. 2008;65:1509–1522. doi: 10.1007/s00018-008-7324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prokhortchouk A, Hendrich B, Jørgensen H, Ruzov A, Wilm M, Georgiev G, Bird A, Prokhortchouk E. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes and Development. 2001;15:1613–1618. doi: 10.1101/gad.198501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prokhortchouk AV, Aitkhozhina DS, Sablina AA, Ruzov AS, Prokhortchouk EB. Kaiso, a New Protein of the BTB/POZ Family, Specifically Binds to Methylated DNA Sequences. Russian Journal of Genetics. 2001;37:603–609. [PubMed] [Google Scholar]
- 8.Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J. N-CoR Mediates DNA Methylation-Dependent Repression through a Methyl CpG Binding Protein Kaiso. Molecular Cell. 2003;12:723–734. doi: 10.1016/j.molcel.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Daniel JM, Spring CM, Crawford HC, Reynolds AB, Baig A. The p120ctn binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 2002;30:2911–2919. doi: 10.1093/nar/gkf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly KF, Daniel JM. POZ for effect - POZ-ZF transcription factors in cancer and development. Trends Cell Biol. 2006;16:578–587. doi: 10.1016/j.tcb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Sansom OJ, Maddison K, Clarke AR. Mechanisms of disease: methyl-binding domain proteins as potential therapeutic targets in cancer. Nat. Clin. Pract. Oncol. 2007;4:305–315. doi: 10.1038/ncponc0812. [DOI] [PubMed] [Google Scholar]
- 12.Lopes EC, Valls E, Figueroa ME, Mazur A, Meng FG, Chiosis G, Laird PW, Schreiber-Agus N, Greally JM, Prokhortchouk E, Melnick A. Kaiso Contributes to DNA Methylation-Dependent Silencing of Tumor Suppressor Genes in Colon Cancer Cell Lines. Cancer Res. 2008;68:7258–7263. doi: 10.1158/0008-5472.CAN-08-0344. [DOI] [PubMed] [Google Scholar]
- 13.Zhang BZ, Gu LK, Deng DJ. Methylation specific binding activity of zinc finger protein Kaiso. Zhonghua. Yu. Fang Yi. Xue. Za. Zhi. Chinese Journal Preventive Medicine. 2007;41 Suppl:43–46. [PubMed] [Google Scholar]
- 14.Delaglio F, Grzesiek S, Vuister GW, Guang Z, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BA, Blevins RA. NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 16.Filion GJP, Zhenilo S, Salozhin S, Yamada D, Prokhortchouk E, Defossez PA. A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol. Cell Biol. 2006;26:169–181. doi: 10.1128/MCB.26.1.169-181.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakaseko Y, Neuhaus D, Klug A, Rhodes D. Adjacent zinc-finger motifs in multipe zinc-finger peptides from SWI5 form structurally independent, flexibly linked domains. J. Mol. Biol. 1992;228:619–636. doi: 10.1016/0022-2836(92)90845-b. [DOI] [PubMed] [Google Scholar]
- 18.Dutnall RN, Neuhaus D, Rhodes D. The solution structure of the first zinc finger domain of SW15: A novel structural extension to a common fold. Structure. 1996;4:599–611. doi: 10.1016/s0969-2126(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 19.Fairall L, Schwabe JWR, Chapman L, Finch JT, Rhodes D. The crystal structure of a two zinc-finger peptide reveals an extension to the rules for zinc-finger/DNA recognition. Nature. 1993;366:483–487. doi: 10.1038/366483a0. [DOI] [PubMed] [Google Scholar]
- 20.Schoenmakers E, Alen P, Verrijdt G, Peeters B, Verhoeven G, Rombauts W, Claessens F. Differential DNA binding by the androgen and glucocorticoid receptors involves the second Zn-finger and a C-terminal extension of the DNA-binding domains. Biochem. J. 1999;341:515–521. [PMC free article] [PubMed] [Google Scholar]
- 21.Gearhart MD, Holmbeck SMA, Evans RM, Dyson HJ, Wright PE. Monomeric complex of human orphan estrogen related receptor-2 with DNA: A pseudodimer interface mediates extended half-site recognition. J. Mol. Biol. 2003;327:819–832. doi: 10.1016/s0022-2836(03)00183-9. [DOI] [PubMed] [Google Scholar]
- 22.Melvin VS, Harrell C, Adelman JS, Kraus WL, Churchill M, Edwards DP. The role of the C-terminal extension (CTE) of the estrogen receptor alpha and beta DNA binding domain in DNA binding and interaction with HMGB. J. Biol. Chem. 2004;279:14763–14771. doi: 10.1074/jbc.M313335200. [DOI] [PubMed] [Google Scholar]
- 23.Crane-Robinson C, Dragan AI, Privalov PL. The extended arms of DNA-binding domains: a tale of tails. Trends Biochem. Sci. 2006;31:547–552. doi: 10.1016/j.tibs.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Sabogal A, Lyubimov AY, Corn JE, Berger JM, Rio DC. THAP proteins target specific DNA sites through bipartite recognition of adjacent major and minor grooves. Nat. Struct. Mol. Biol. 2010;17:117–123. doi: 10.1038/nsmb.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohki I, Shimotake N, Fujita N, Jee JG, Ikegami T, Nakao M, Shirakawa M. Solution structure of the methyl-CpG binding domain of human MBD1 in complex with methylated DNA. Cell. 2001;105:487–497. doi: 10.1016/s0092-8674(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 26.Ho KL, McNae IW, Schmiedeberg L, Klose RJ, Bird AP, Walkinshaw MD. MeCP2 Binding to DNA Depends upon Hydration at Methyl-CpG. Molecular Cell. 2008;29:525–531. doi: 10.1016/j.molcel.2007.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh RP, Nikitina T, Horowitz-Scherer RA, Gierasch LM, Uversky VN, Hite K, Hansen JC, Woodcock CL. Unique Physical Properties and Interactions of the Domains of Methylated DNA Binding Protein 2. Biochemistry. 2010;49:4395–4410. doi: 10.1021/bi9019753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klose RJ, Sarraf SA, Schmiedeberg L, McDermott SM, Stancheva I, Bird AP. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol. Cell. 2005;19:667–678. doi: 10.1016/j.molcel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Clouaire T, de Las Heras JI, Merusi C, Stancheva I. Recruitment of MBD1 to target genes requires sequence-specific interaction of the MBD domain with methylated DNA. Nucleic Acids Res. 2010;38:4620–4634. doi: 10.1093/nar/gkq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasai N, Nakao M, Defossez PA. Sequence-specific recognition of methylated DNA by human zinc-finger proteins. Nucleic Acids Res. 2010;38:5015–5022. doi: 10.1093/nar/gkq280. [DOI] [PMC free article] [PubMed] [Google Scholar]