Abstract
Nuclear receptors (NR) impact a myriad of physiological processes including homeostasis, reproduction, development, and metabolism. NRs are regulated by post-translational modifications (PTM) that markedly impact receptor function. Recent studies have identified NR PTMs that are involved in the onset and progression of human diseases, including cancer. The majority of evidence linking NR PTMs with disease has been demonstrated for phosphorylation, acetylation and sumoylation of androgen receptor (AR), estrogen receptor α (ERα), glucocorticoid receptor (GR) and peroxisome proliferator activated receptor γ (PPARγ). Phosphorylation of AR has been associated with hormone refractory prostate cancer and decreased disease-specific survival. AR acetylation and sumoylation increased growth of prostate cancer tumor models. AR phosphorylation reduced the toxicity of the expanded polyglutamine AR in Kennedy’s Disease as a consequence of reduced ligand binding. A comprehensive evaluation of ERα phosphorylation in breast cancer revealed several sites associated with better clinical outcome to tamoxifen therapy, whereas other phosphorylation sites were associated with poorer clinical outcome. ERα acetylation and sumoylation may also have predictive value for breast cancer. GR phosphorylation and acetylation impact GR responsiveness to glucocorticoids that are used as anti-inflammatory drugs. PPARγ phosphorylation can regulate the balance between growth and differentiation in adipose tissue that is linked to obesity and insulin resistance. Sumoylation of PPARγ is linked to repression of inflammatory genes important in patients with inflammatory diseases. NR PTMs provide an additional measure of NR function that can be used as both biomarkers of disease progression, and predictive markers for patient response to NR-directed treatments.
Introduction
Nuclear receptor (NR) function is regulated by post-translational modifications (PTM) including phosphorylation, acetylation, sumoylation, methylation, myristylation, nitration, ADP-ribosylation, and isoprenylation. These PTMs can be further divided into two categories: 1) reversible modifications that function by either addition or removal of functional chemical groups (i.e., phosphate, acetyl) on specific amino acid residues of target proteins [serine (S), tyrosine (Y), threonine (T), lysine (K)]; or 2) modifications involving addition of other proteins or polypeptides (e.g., sumoylation and ubiquitination).
Recently, many investigations have provided direct evidence for NR PTM in the pathophysiological progression of many diseases including cancers, diabetes, and obesity, among others. The majority of evidence linking NR PTMs with disease has been demonstrated for phosphorylation, sumoylation, ubiquitination and acetylation in the androgen receptor (AR), estrogen receptor (ER), glucocorticoid receptor (GR) and the peroxisome proliferator activated receptor γ (PPARγ). This report will be limited to a review of PTMs in ER, AR, GR and PPARγ and association with disease.
Androgen receptor
AR phosphorylation and prostate cancer
Advanced prostate cancer treatment has relied on hormone-deprivation therapy for the past 50 years. Response rates are initially high (70–80%); however, almost all patients relapse and develop hormone-refractory prostate cancer (HRPC), resulting in increased morbidity and death [McCall et al., 2008].
The majority of studies that demonstrate a relationship between AR phosphorylation and prostate cancer development have focused on the PI3K/Akt pathway (Figure 1). Studies in vitro demonstrate that the PI3K/Akt pathway is upregulated in HRPC and can result in phosphorylation of the AR. Akt is activated when phosphorylated at threonine 308 (T308), and subsequently serine 473 (S473), and these phosphorylations may play a similar role in the development of HRPC [Liao et al., 2003]. Additional in vitro studies have demonstrated that Akt can phosphorylate AR at serine residues S210 and S790, resulting in modulation of AR transcriptional activity [Lin et al., 2003; Lin et al., 2001].
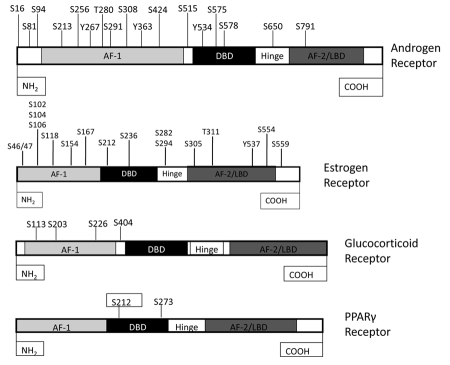
Figure 1. Phosphorylation sites in nuclear receptors.
Nuclear receptor function is regulated in large part by post-translational modification, including phosphorylation. Phosphorylation occurs on serine (S), threonine (T) and tyrosine (Y) residues. AF-1- Activation Function-1; DBD- DNA Binding Domain; AF-2- Activation Function-2; LBD- Ligand Binding Domain.
Studies have shown that pAkt S473 is expressed in PIN (Prostatic Intraepithelial Neoplasia) and invasive prostate cancer with staining intensity positively correlated with PSA levels and Gleason grades [Altomare and Testa, 2005; Ghosh et al., 2003; Majumder and Sellers, 2005]. Increased phospho-Akt at S473 (pAkt S473) and phospho-AR S210 (pAR S210) was associated with decreased disease-specific survival [McCall et al., 2008]. In addition, phosphorylation of Akt at S473 and AR at S210 strongly correlated with HRPC [McCall et al., 2008] and HRPCs expressed significantly higher levels of pAR S210 compared to hormone-sensitive tumors [McCall et al., 2008].
Since upregulation of the PI3K/Akt pathway is associated with phosphorylation of AR during development of HRPC, Akt inhibitors are being developed as targeted therapeutics. Future clinical studies will determine whether activated Akt and/or phosphorylation of AR at S210 may be developed as predictive biomarkers for selecting patients who would respond to Akt inhibitors.
AR phosphorylation in spinal and bulbar muscular atrophy
Spinal and bulbar muscular atrophy (SBMA; also known as Kennedy’s Disease) is a progressive neurodegenerative disease characterized by muscle weakness, muscle atrophy, and fasciculations. SBMA is caused by an abnormally high number of CAG trinucleotide repeats that encode for a stretch of uninterrupted polyglutamine amino acids in the AR known as a polyglutamine tract or polyQ tract [Mukherjee et al., 2009].
Studies in transgenic SBMA mouse models demonstrated that the reduction of serum androgen levels rescues the motor dysfunction and nuclear accumulation of mutant androgen receptors and improves survival of transgenic SBMA mice [Chevalier-Larsen et al., 2004; Katsuno et al., 2003a; Katsuno et al., 2003b]. Therefore, decreasing the effect of ligand on the AR could reduce disease progression. Furthermore, Palazzolo et al. demonstrated that in motor neuron-derived cells, phosphorylation of AR at the Akt consensus sites S215 and S792 reduced ligand-dependent nuclear translocation and toxicity of the expanded polyQ AR, as a consequence of reduced ligand binding [Palazzolo et al., 2007].
Leuprorelin, a luteinizing hormone–releasing hormone agonist that reduces testosterone release from the testis and inhibits nuclear accumulation of mutant AR, rescued motor dysfunction in male transgenic mice carrying full-length mutant human AR with an expanded polyQ tract [Katsuno et al., 2003]. Phase II clinical trials of leuprorelin suggested that androgen deprivation inhibited nuclear accumulation and/or stabilization of mutant AR in the motor neurons of the spinal cord and brainstem [Banno et al., 2009].
It is important to note that the phosphorylation sites in prostate cancer and SBMA are not different. AR may differ in length because of the polyQ and glycine repeats, but the most common size of AR is 919 amino acids. The Akt consensus phosphorylation sites of AR with 919 amino acid residues are S213 and S791 (Figure 1); these same sites were reported as S210 and S790 by McCall et al. [McCall et al., 2008] and S215 and S792 by Palazzolo et al. [Palazzolo et al., 2007], although the sites are not different. The discrepancies in the phosphorylation sites mentioned in prostate cancer and SBMA might be due to the fact that AR differs in their length because of heterogeneity of the polyQ and glycine tracts.
AR sumoylation in prostate cancer and SBMA
Sumoylation, the process of covalently attaching small ubiquitin-like modifiers to proteins, functions to regulate protein stability, protein–protein interactions, transcriptional activity, and subcellular localization [Hay, 2005; Seeler and Dejean, 2003]. The majority of identified SUMO substrates are active in transcription, RNA processing, DNA repair and chromatin remodeling (reviewed in [Karamouzis et al., 2008]). Androgen receptor was the first NR shown to be sumoylated [Poukka et al., 2000].
Sumoylation is mediated by activating, conjugating and ligating enzymes, and is reversed by a family of SUMO-specific proteases (SENPs); one member in particular, SENP1, was recently identified as a gene required for proliferation and survival of normal and prostate cancer cells [Schlabach et al., 2008]. Expression of SENP1 in prostate cancer cells increased the transcription activity of endogenous AR. Silencing of SENP1 attenuated the expression of several AR target genes and inhibited the androgen-stimulated growth of LNCaP cells (androgen-dependent prostate cancer cells) [Bawa-Khalfe et al., 2007; Kaikkonen et al., 2009]. Additionally, SENP1 was overexpressed in human prostate cancer specimens [Cheng et al., 2006]. Transgenic mice overexpressing SENP1 in the prostate gland also developed Prostatic Intraepithelial Neoplasia (PIN) at an early age [Cheng et al., 2006].
Taken together, this suggests that SENP1 plays an important role in not only proliferation of prostate cancer cells, but in onset, as well [Bawa-Khalfe et al., 2007]. These data suggest the SUMO modification pathway as a potential target for prostate cancer therapy.
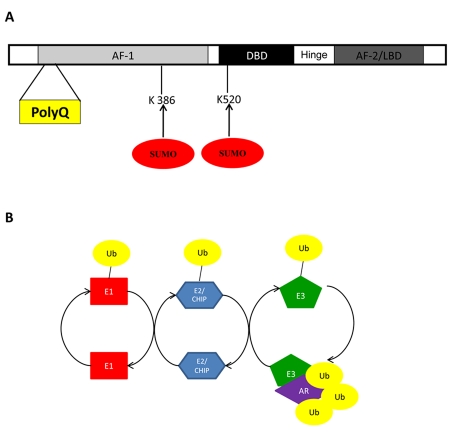
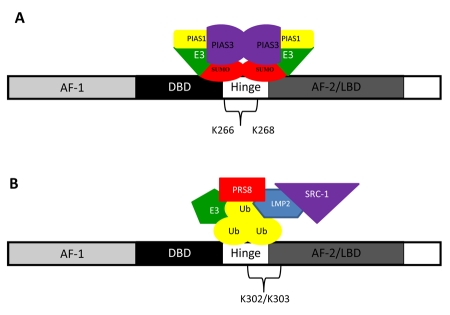
The two sumoylation sites of AR in human are K386 and K520 (Figure 2A). Cell culture studies have shown that sumoylation could reduce the formation of polyQ expanded AR aggregates without affecting the levels of the receptor, and the anti-aggregation effect by sumoylation was not dependent upon AR transcriptional activity [Mukherjee et al., 2009]. Chan et al. [Chan et al., 2002] reported that the Drosophila SBMA model with mutant polyQ AR exhibited a neurodegenerative phenotype.
Figure 2. Sumoylation and ubiquitination of the androgen receptor are involved in spinal and bulbar muscular atrophy.
Spinal and bulbar muscular atrophy is caused by an abnormally high number of CAG trinucleotide repeats that encode for a stretch of uninterrupted polyglutamine amino acids in the AR known as a polyglutamine tract or polyQ tract. (A) Sumoylation of the androgen receptor has been shown to reduce aggregates of polyQ and occurs at lysine residues 386 and 520. (B) Overexpression of carboxyl terminus of Hsp70-interacting protein (CHIP), an ubiquitin E3 ligase, leads to ubiquitination of mutant AR, providing protection from SBMA.
Furthermore, expression of a catalytic-deficient mutant of the SUMO-1 activating enzyme Uba2 further exacerbated the polyQ AR-induced neurodegeneration [Chan et al., 2002; Dorval and Fraser, 2007]. In contrast, in other diseases associated with polyQ expanded regions in proteins such as the protein huntingtin in Huntington's disease, it has been suggested that sumoylation intensifies the polyQ huntingtin-induced neurodegeneration in the Drosophila model [Steffan et al., 2004].
Taken together, sumoylation might have either beneficial or harmful effects, depending on the polyQ protein, and further studies using SBMA double transgenic mice with mutation at the sumoylation site of AR might provide more insight into the role of sumoylation in SBMA.
AR ubiquitination and SBMA
NRs are well known substrates for ubiquitination [Adachi et al., 2007], which is the covalent attachment of a peptide, ubiquitin, to lysine residues of a substrate protein. Similar to sumoylation, ubiquitination requires an activating enzyme (E1), a conjugating enzyme (E2), and an E3 ubiquitin ligase (Figure 2B). Although most NRs are ubiquitinated, discussion here will be limited to a review of ubiquitination of AR, ERα and PPARγ, since the ubiquitination of these NRs were clearly linked to human diseases.
Carboxyl terminus of Hsp70-interacting protein (CHIP) is E2 ubiquitin ligase that interacts with heat shock proteins and chaperones to degrade toxic and misfolded proteins [McDonough and Patterson, 2003]. Double transgenic mice which overexpressed CHIP and mutant AR demonstrated that mutant AR protein was ubiquitinated and degraded by CHIP and the proteosomal system, which resulted in protection of mice from SBMA [Adachi et al., 2007]. Moreover, other neurodegenerative diseases that are caused by polyQ expansion of proteins ataxin-1 (type 1 spinocerebellar ataxia) and Huntingtin were also benefited by ubiquitination and proteosomal degradation [Al-Ramahi et al., 2006; Jana et al., 2005].
Overall, ubiquitination has been shown to play a vital role in protecting neuronal cells against the toxic properties of polyQ expanded proteins.
AR acetylation and prostate cancer
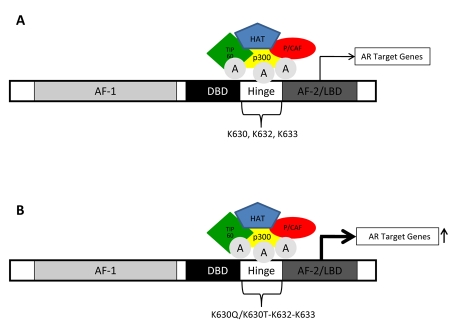
Acetylation of AR is in response to physiological stimuli including DHT (dihydrotestosterone) and bombesin enhanced AR transactivation function at a subset of target promoters [Fu et al., 2004], in particular cell cycle control genes that induce cellular proliferation [Gong et al., 2006; Popov et al., 2007]. Direct acetylation of AR at the highly conserved KXKK/RXKK lysine motif (lysine 630, 632 and 633), is mediated by coactivators p300, P/CAF and TIP60 (Tat-interactive protein) that possess intrinsic histone acetyltransferase (HAT) activity (Figure 3A) [Fu et al., 2003; Fu et al., 2000; Gaughan et al., 2002].
Figure 3. Acetylation of androgen receptor.
(A) Acetylation of AR occurs at lysine residues 630, 632, and 633 and is mediated by interactions with coregulators with intrinsic histone acetyltransferase (HAT) activity, such as coactivators p300, P/CAF and TIP60 (Tat-interactive protein). (B) Acetylation mimicking mutants (K630Q/K630T) exhibited increased cell growth in prostate cancer cell lines.
The proliferation of prostate cancer cell lines stably expressing the AR acetylation mimic mutants [AR lysine 630 to glutamine (AR K630Q/lysine 630 to threonine (AR K630T)] (Figure 3B) was increased in the presence of DHT compared to the wild-type AR. These acetylation-mimicking mutations in prostate cancer cells exhibited increased cell growth in vitro and showed increased tumor growth in vivo, and these tumors were resistant to the AR antagonist, flutamide [Fu et al., 2003].
Although these studies suggest that there is a link between AR acetylation and prostate cancer, specific experiments to test this have not yet been reported.
Estrogen receptor α
Clinical relevance of phosphorylated forms of ERα in human breast cancer
The estrogen receptor α (ERα) status of breast tumors has been used clinically since the late 1970s to identify those patients most likely to gain benefit from endocrine therapies. However, ERα status is an imperfect marker of endocrine therapy response and a current research priority is the search for more precise markers of treatment response and outcome. ERα, similar to other steroid hormone receptors, can be phosphorylated at multiple sites within the protein [Murphy et al., 2011] (Figure 1).
In addition to estrogenic ligands, ERα can be activated in a ligand-independent fashion, often by signals generated at the plasma membrane by growth factor receptors [Lannigan, 2003]. When it was found that ERα could be directly phosphorylated by several kinases activated by membrane growth factor receptor signaling [Kato et al., 1995], the possibility arose of crosstalk between ERα and growth factor receptor signaling, which could underlie ligand-independent activation of ERα signaling and the development of resistance to endocrine therapies [Arpino et al., 2008]. Results from several cell line and xenograft models supported this hypothesis [Arpino et al., 2008].
One way to gain insights into the putative role of a gene in human breast cancer in vivo is frequently obtained by investigating relationships of gene expression in tumor samples to known biomarkers of prognosis and treatment response, and to clinical outcomes such as release free survival (RFS) and overall survival (OS) in retrospectively-collected patient cohorts. When antibodies specific for phosphorylated amino acids within the ERα became available, the opportunity arose to test experimental hypotheses in human breast tumors in vivo.
Antibodies to p-S118 and p-S167 on ERα have been commercially available since 2003. Our laboratory was the first to determine expression of p-S118 - ERα in multiple breast cancer cases stored in the Manitoba Breast Tumor Bank (MBTB) [Skliris et al., 2009]. Subsequently, additional studies were published demonstrating the expression of one or both of these p-ERα sites by IHC in cohorts of breast tumor biopsy samples [Holm et al., 2009; Jiang et al., 2007; Murphy et al., 2004; Skliris et al., 2009].
More recently, detection of other phosphorylated sites on ERα has been published by our laboratory and others [Atsriku et al., 2009; Britton et al., 2008; Williams et al., 2009]. In some of these studies, associations with other histopathological markers, clinical outcome and specific kinases were found [Jiang et al., 2007; Skliris et al., 2010]. Although sometimes contradictory results have been found, some common themes have emerged, especially with regard to p-S118 and p-S167, which have been the major focus to date.
Firstly, and in contrast to what was expected, detection of p-S118- and/or p-S167-ERα in breast tumors is generally associated with features of an intact estrogen responsive signaling pathway [Jiang et al., 2007; Murphy et al., 2004] and therefore more differentiated phenotypes. These data suggested that the detection of either p-S167- and/or p-S118-ERα may be surrogate markers for tumors that are more dependent on ERα signaling for growth, and are therefore more likely to respond to tamoxifen (Tam) therapy.
Indeed, a second emerging theme is that those patients whose tumors express p-S118- and/or p-S167-ERα more often have a better clinical outcome to Tam therapy in retrospective studies [Jiang et al., 2007; Murphy et al., 2004; Yamashita et al., 2005; Yamashita et al., 2008].
More recently, detection of p-S118-ERα in breast tumors was also associated with a better clinical outcome to aromatase inhibitors [Generali et al., 2009]. In our study, we found that measurement of progesterone receptor (PR), either by IHC or LBA (ligand binding assay) [Skliris et al., 2009; Weitsman et al., 2006], was still a stronger predictor of RFS and OS than the p-S118-ERα for patients on tamoxifen. Interestingly, however, we also found that addition of p-S118-ERα to PR further improved the prediction of response to endocrine therapy [Murphy et al., 2004]. Such data support combined use of biologically-relevant markers for the improved prediction of therapy response, at least with respect to endocrine therapy.
Although some studies find a weak positive association of ERBB2/HER2 expression and p-S118 [Jiang et al., 2007; Yamashita et al., 2008], we and others have found no significant differences in the expression of p-S118 and/or p-S167 in ER+ tumors that either overexpress or do not express ERBB2/HER2 [Jiang et al., 2007; Weitsman et al., 2006].
The data published so far therefore do not strongly support the hypotheses that ligand-independent activation of ERα by phosphorylation due to overexpression of growth factor receptors such as HER2 is a mechanism of de novo tamoxifen and possibly aromatase inhibitor resistance in human breast cancer in vivo.
More recently, antibodies to other phosphorylation sites on ERα, i.e., p-S104/106, p-S282, p-S294, p-T311, p-S559, have been validated for IHC of formalin-fixed paraffin-embedded breast tumor tissues [Skliris et al., 2009] and the results obtained support a third emerging theme that multiple phosphorylated forms of ERα can be detected in any one tumor sample [Jiang et al., 2007; Skliris et al., 2009; Yamashita et al., 2008]. The relationship of these other phosphorylation sites to known biomarkers and response to endocrine therapies is currently unclear.
In contrast to the results for p-S118 and/or p-S167, detection of p-S305 in ERα in breast tumors from pre-menopausal women who had received tamoxifen for 2 years was associated with no benefit from tamoxifen [Holm et al., 2009]. Furthermore, experimental studies support a role for phosphorylation at S305 in tamoxifen resistance [Michalides et al., 2004], suggesting that not all types of phosphorylation of ERα predict a good outcome to endocrine therapies.
It is interesting that those phosphorylation sites that are so far associated with better clinical outcome to endocrine therapies are located in the N-terminus of the protein and those identified to be associated with poorer outcome (S305, T311, and S559 [Skliris et al., 2010]), are located in a region at the border of the hinge region and the ligand binding domain.
Interestingly, S305 is adjacent to an acetylation site (K303) that has been implicated in modulating sensitivity to E2 [Fuqua et al., 2000] and regulating the efficiency of S305 as a substrate for PKA [Cui et al., 2004]. Inhibition of acetylation at this site by mutation of K303 to R resulted in reduced sensitivity to tamoxifen [Giordano et al., 2010] and aromatase inhibitors [Barone et al., 2010]. There is precedence for coupling regulation between phosphorylation and acetylation in adjacent areas of proteins and effects on protein function [Kramer et al., 2009].
ERα acetylation and breast cancer
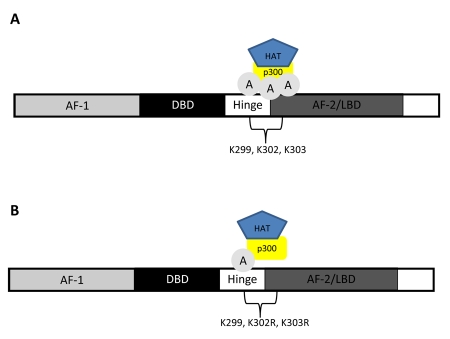
Acetylation of ERα requires the interaction of ERα with coregulators, such as p300/CBP, that have intrinsic histone acetyltransferase (HAT) activity [Wang et al., 2001] and/or recruit HATs. ERα is selectively acetylated on lysine residues 299, 302 and 303 by p300 within the well-conserved hinge/ligand binding domain (Figure 4A) [Wang et al., 2001].
Figure 4. Acetylation of estrogen receptor α.
(A) Acetylation of the estrogen receptor α requires the interaction of the receptor and of coregulators such as p300 that possess intrinsic histone acetyltransferase (HAT) activity. (B) Mutation of lysine residues 302/303 to arginine, rendering these sites incapable of being acetylated, resulted in hypersensitivity to estradiol in breast cancer cell lines.
The mutation of lysine residues 302 or 303 to arginine (rendering these sites incapable of being acetylated) (Figure 4B) resulted in hypersensitivity to estradiol that led to increased estradiol-dependent activation of ERα, suggesting that ERα acetylation normally suppresses ligand sensitivity [Fuqua et al., 2000; Wang et al., 2001]. Additionally, independent clinical studies have identified a Lys-to-Arg substitution at nucleotide 908 (referred to as K303R) in 34% of atypical breast hyperplasia samples [Conway et al., 2005; Fuqua et al., 2000; Herynk et al., 2007]. A K303R mutation enhanced cellular proliferation in response to low concentrations of estradiol, suggesting the ERα K303R mutation provides a “gain of function” mutation in human breast cancer.
Furthermore, Conway et. al. [Conway et al., 2005] reported similar mutations in 5.7% of screened breast tumors, with more frequent occurrences in high grade breast tumors and in mixed lobular/ductal tumors, compared with ductal carcinomas.
In another study, ~50% of breast cancer samples contained K303R mutations [Herynk et al., 2007], and in this cohort of patients, women over the age of 50 had more frequent mutations when compared to lower age groups (54.4% versus 37.5%). Moreover, a higher frequency of mutation was found in lymph node positive compared to negative tumors (70% versus 34.8%) [Herynk et al., 2007].
ERα sumoylation and breast cancer
Sumoylation of ERα occurs through the modification of lysine residue(s) in the AF-1 domain [Choi et al., 2006]. Two ligand-dependent sumoylation sites, lysine 266 and 268 (K266 and K268), have been identified in the ERα hinge region that can result in increased ERα target gene expression in vitro (Figure 5A) [Sentis et al., 2005]. The hinge region of ERα is a specific target of SUMO-E3 ligases (catalyzes the covalent attachment of a SUMO), PIAS1 (Protein inhibitor of Activated STAT), and PIAS3 (Figure 5A) [Karamouzis et al., 2008]. An increase in expression of PIAS3 has been documented in breast cancer [Wang and Banerjee, 2004].
Figure 5. Estrogen receptor α sumoylation and ubiquitination.
(A) Sumoylation of ERα occurs at lysine residues 266 and 268 (K266 and K268). (B) Unstimulated estrogen receptor is ubiquitinated and degraded by carboxyl terminus of Hsp70-interacting protein (CHIP), an ubiquitin E3 ligase. Fulvestrant (ICI 182,780), a pure anti-estrogen, utilizes the ubiquitination mechanism to induce proteasome-dependent degradation of ERα.
Future clinical studies are needed to determine whether ERα sumoylation in breast cancer has prognostic value or serves as a predictor for response to endocrine therapy.
Ubiquitination and ERα
The stability of ERα is affected differentially by agonists, antagonists, and selective estrogen receptor modulators [Reid et al., 2002; Wijayaratne and McDonnell, 2001]. Both ligand-stimulated, as well as unstimulated ERα, have been shown to be ubiquitinated and degraded by the proteasomal system (Figure 5B) [Tateishi et al., 2004].
Studies performed by the Gannon and O'Malley laboratories showed that the proteasomal degradation of transcription factors is a necessary step in the regulation of target gene transcription, possibly by enabling the sequential formation of protein complexes at the promoter region [Lonard et al., 2000; Reid et al., 2002]. Stable, unliganded ERα, which has a half-life of up to 5 days [Nirmala and Thampan, 1995], is ubiquitinated and degraded by carboxyl terminus of Hsp70-interacting protein (CHIP), a ubiquitin E3 ligase [Fan et al., 2005]. Binding of estradiol to ERα dramatically reduced the half-life of ERα to 3-5 h [Nirmala and Thampan, 1995]. As a result of the recruitment of proteins implicated in the proteasome system, ERα is degraded. The proteins that are involved in the ubiquitination are ubiquitin ligases E6-AP, MDM2, SUG1, and the coactivators SRC-1 and SRC-3 (see review by [Le Romancer et al., 2011]).
Fulvestrant (ICI 182,780), a pure anti-estrogen that is currently used in adjuvant therapies for breast cancer, utilizes the ubiquitination mechanism to induce proteasome-dependent degradation of ERα [Lanvin et al., 2007]. Degradation of ERα induced by fulvestrant is independent of its transcriptional activity and new protein synthesis [Marsaud et al., 2003; Wijayaratne and McDonnell, 2001].
Glucocorticoid receptor
Anti-inflammatory effect of GR
Treatment with glucocorticoids is one of the most effective therapies for many chronic inflammatory diseases such as asthma, rheumatoid arthritis, and inflammatory bowel disease [Adcock and Ito, 2000]. Glucocorticoids bind to the cytosolic GR, which then translocates to the nucleus. GR can induce transcription of anti-inflammatory genes, such as secretory leukocyte proteinase inhibitor (SLPI) [Abbinante-Nissen et al., 1995] and mitogen-activated kinase phosphatase-1 (MKP-1) [Lasa et al., 2002], or inhibit transcription of pro-inflammatory genes such as interleukin-6 (IL-6) (Figure 6) [Ray et al., 1994]. GR also reduces inflammatory gene expression induced by NF-κB (Nuclear Factor-κB), AP-1 (Activator Protein-1) or via trans-repression through direct protein interaction with these proteins (reviewed in [De Bosscher et al., 2003]).
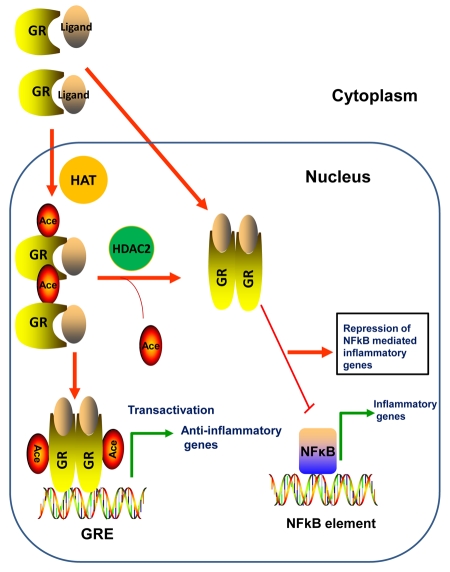
Figure 6. GR regulation of inflammatory genes.
Upon ligand binding, the GR translocates to the nucleus, is acetylated by HATs, and binds to Glucocorticoid Response Elements (GRE) to induce transcription of anti-inflammatory genes. The liganded GR can also functionally repress NF-κB-mediated pro-inflammatory genes.
GR phosphorylation in inflammation and asthma in humans
As previously stated, glucocorticoids are used as primary treatment options for many inflammatory diseases; however, some patients are unresponsive to these treatments due to glucocorticoid resistance. Among the mechanisms for this resistance is decreased glucocorticoid signaling by changes in GR phosphorylation [Bloom, 2004; Li et al., 2004; Tsitoura and Rothman, 2004]. GR is phosphorylated by cyclin-dependent kinases, MAPK and casein kinase II at 5 serine residues, S113, S141, S203, S211 and S226 [Ismaili and Garabedian, 2004]. p38 MAP kinase has been shown to be active in alveolar macrophages of asthmatic patients who showed poor response to glucocorticoids in comparison to patients with a normal response [Bhavsar et al., 2008]. The phosphorylation of GR by p38 MAPK subsequently affects GR function, resulting in reduced responsiveness to glucocorticoid treatment. The serine residue of GR phosphorylated by p38 MAP kinase has yet to be elucidated; it is plausible that S211 or S226 could be phosphorylated [Irusen et al., 2002; Miller et al., 2005; Szatmary et al., 2004]. Use of p38 MAPK inhibitors increased the anti-inflammatory effects of glucocorticoids and reestablished the beneficial effects of glucocorticoids in glucocorticoid-resistant patients suffering from asthma [Irusen et al., 2002]. Studies have also shown that a large proportion of patients with glucocorticoid-resistant asthma showed reduced nuclear translocation of GR and DNA binding in PBMCs (Peripheral Blood Mononuclear Cells) after glucocorticoid exposure, and this could be due to changes in glucocorticoid receptor phosphorylation [Matthews et al., 2004; Szatmary et al., 2004].
Together, these studies suggest GR phosphorylation by p38 MAPK is one of the mechanisms leading to glucocorticoid resistance. Clinical studies are needed to determine if the clinical benefit of p38 MAPK inhibitors in asthmatic patients is related to changes in GR phosphorylation.
GR acetylation increases glucocorticoid insensitivity
Lysine 494 and 495 are the two ligand-dependent acetylation sites identified in GR [Ito et al., 2006]. GR binds to NF-κB and represses inflammatory gene transcription by NF-κB. Acetylated GR loses its affinity for NF-κB and therefore cannot repress inflammatory gene transcription induced by NF-κB (Figure 6). Histone deacetylase 2 (HDAC2) deacetylates GR, thereby enabling the association of GR with NF-κB and attenuation of pro-inflammatory gene transcription. Silencing HDAC2 by RNA interference resulted in reduced corticosteroid-sensitivity in primary alveolar macrophages and human lung epithelial cell lines.
Overexpression of HDAC2 in glucocorticoid-resistant alveolar macrophages from patients with COPD (chronic obstructive pulmonary disease) resulted in restoration of corticosteroid-sensitivity [Ito et al., 2006]. Taken together, acetylated GR could not inhibit NF-κB-dependent gene transcription, due to the reduced binding to NF-κB. It is plausible that hyperacetylation of GR, due to absence of HDAC2, may lead to glucocorticoid insensitivity.
Peroxisome proliferator-activated receptors
Peroxisome proliferator-activated receptors (PPAR) play an important role in the regulation of lipid homeostasis, energy metabolism, inflammatory responses and the induction of apoptosis [Issemann and Green, 1990; Rosen and Spiegelman, 2006]. The PPAR family consists of three subtypes, PPARα, PPARδ and PPARγ, encoded by separate genes, and among these subtypes, PPARγ has been studied extensively. The PPARs form heterodimers with the retinoid-X receptor (RXR), which then binds to response elements (PPRE) in the regulatory regions of target genes [Mangelsdorf and Evans, 1995].
PPAR phosphorylation and insulin resistance and obesity
Adipocyte differentiation is an important component of obesity and other metabolic diseases and this process is inhibited by mitogens and oncogenes. Several growth factors that inhibit fat cell differentiation cause MAPK-mediated phosphorylation of PPARγ and reduce its transcriptional activity. Growth factor-induced phosphorylation of S112 of PPAR-γ repressed its transcriptional and adipogenic functions [van Beekum et al., 2009], whereas expression of PPARγ with a non-phosphorylatable mutation at serine-112 (S112) yielded cells with increased sensitivity to ligand-induced adipogenesis and resistance to inhibition of differentiation by mitogens, as compared to cells expressing wild type PPARγ (Figure 1) [Hu et al., 1996; Iwata et al., 2001; Ristow et al., 1998]. Homozygous PPARγ S112A knock-in mice did not develop insulin resistance and exhibited no change in body weight when fed a high fat diet [Rangwala et al., 2003]. These findings indicate that phosphorylation of PPARγ by growth factors is a major regulator of the balance between cell growth and differentiation in the adipose tissue.
A mutation of proline 115 to glutamine (P115Q) in PPARγ prevents phosphorylation at S112 in PPARγ P115Q [van Beekum et al., 2009], would remove a canonical Ser-Pro phosphorylation motif at S112 and consequently prevent phosphorylation by Ser-Pro directed kinases. It is likely that the P115Q mutation and loss of S112 phosphorylation could lead to an increase in adipogenesis and possibly obesity and insulin resistance in humans. A heterozygous P115Q mutation was reported in four obese individuals in a cohort of 358 [van Beekum et al., 2009]. Three of these obese individuals (BMI 37.9-47.3) were diagnosed with type 2 diabetes. Ristow et al. reported the same mutation associated with obesity without type 2 diabetes [Ristow et al., 1998]. Blüher and Paschke [Bluher and Paschke, 2003] reported an individual with the P115Q mutation who exhibited high fasting insulin levels and profound insulin resistance. However, a German cohort of 85 [Hamer et al., 2002] and 67 [Evans et al., 2000] did not find an association of P115Q with obesity. Similarly, in a French cohort of 626, P115Q was not associated with obesity [Clement et al., 2000].
Additional studies on homozygous and heterozygous S112A and P115Q PPARγ mutations in different genetic backgrounds and under different dietary regimes will help to understand the role of PPARγ phosphorylation in obesity.
Cdk5-mediated phosphorylation of PPARγ may be involved in the pathogenesis of insulin-resistance
Cdk5 has been shown to phosphorylate PPARγ at Serine 273 (S273). Phosphorylation of S273 by Cdk5 did not impact the general transcriptional activity of PPARγ, but did alter the expression of specific genes such as adiponectin (insulin sensitizing hormone) [Choi et al., 2010]. Cdk5-mediated phosphorylation of PPARγ has been linked to obesity in mice induced by high-fat diet [Rangwala et al., 2003]. Anti-diabetic PPARγ ligands such as rosiglitazone and MRL24 directly inhibited S273 phosphorylation by Cdk5 without affecting S112 phosphorylation, resulting in restoration of a non-diabetic pattern of gene expression [Choi et al., 2010]. Moreover, inhibition of S273 phosphorylation by rosiglitazone in humans was associated with the anti-diabetic effects of the drug [Choi et al., 2010]. Further studies relating PPARγ phosphorylation to diabetes may offer the opportunity for development of improved next-generation anti-diabetic drugs.
Sumoylation of PPARγ results in transrepression of inflammatory genes
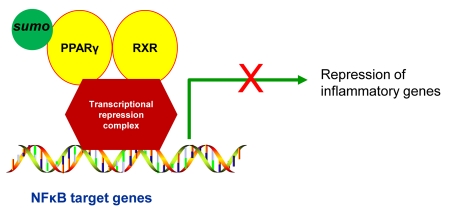
PPARγ has been demonstrated to have an anti-inflammatory effect and PPARγ ligands have been used in clinical trials for patients with inflammatory diseases [Giaginis et al., 2009; Spears et al., 2006]. The mechanism for the anti-inflammatory effect of PPARγ ligands may include sumoylation of the receptor (Figure 7). Expressed in macrophages, PPARγ functions to negatively regulate macrophage activation by repressing a subset of AP1 and NF-κB-dependent genes, ultimately repressing macrophage inflammatory genes [Jennewein et al., 2008; Pascual et al., 2005]. One mechanism regulating PPARγ inhibition of inflammatory genes is sumoylation of PPARγ at its consensus sumoylation sites, lysines 77 and 365 [Pascual et al., 2005]. The initial step in the sumoylation pathway involves ligand-dependent sumoylation of the PPARγ ligand-binding domain, that targets PPARγ for binding to nuclear receptor corepressor (NCoR)/histone deacetylase-3 (HDAC3) complexes at inflammatory gene promoters [Pascual et al., 2005]. This in turn, prevents recruitment of the ubiquitinylation/19S proteasome machinery that normally mediates the signal-dependent removal of corepressor complexes required for gene activation [Pascual et al., 2005]. As a result, NCoR complexes are not cleared from the promoter and target genes are maintained in a repressed state [Pascual et al., 2005]. Recent studies have demonstrated that PPARγ inhibits inflammatory gene expression in activated macrophages by the NCoR/sumoylation-dependent pathway [Ghisletti et al., 2007; Jennewein et al., 2008; Pascual et al., 2007].
Figure 7. Sumoylation of PPARγ and transcriptional repression of inflammatory genes.
Sumoylation of PPARγ promotes interaction with a transcriptional repression complex at NF-κB gene promoters preventing release and turnover of the repression complex, thereby maintaining repression of inflammatory genes.
Ubiquitination and PPARγ
The PPAR-γ protein has a short half-life of 2 hours [Christianson et al., 2008; Waite et al., 2001] and was found to be polyubiquitinated and degraded by the proteasome [Floyd and Stephens, 2002; Hauser et al., 2000]. Ubiquitination of PPAR-γ and degradation are strongly linked to ligand binding and activation, as TZDs (e.g., troglitazone, rosiglitazone) accelerated these processes [Floyd and Stephens, 2002; Hauser et al., 2000]. The exposure of adipocytes to the cytokine interferon-γ elicited by infiltrating interferon-γ-producing lymphocytes into adipose tissue [Kintscher et al., 2008], was also found to increase ubiquitination and degradation of PPAR-γ [Floyd and Stephens, 2002], indicating that ubiquitination can also be regulated by external stimuli. Studies by [Kubota et al., 1999; Miles et al., 2000] have shown that reduced PPARγ expression in mice (PPARγ+/-) is associated with resistance to weight gain along with protection from the insulin resistance that typically accompanies weight gain. In addition, genetic evidence indicates that decreased PPARγ activity may protect against insulin resistance in humans [Deeb et al., 1998]. Conversely, PPARγ is required for the formation of fat cells, and a lack of adipose cells is associated with insulin resistance and hyperglycemia [Willson et al., 2001]. All of these studies indicate that a careful balance between PPARγ expression and activity levels must be maintained to avoid development of diseases such as type II diabetes and obesity. The ubiquitin-proteasome pathway plays an important role in the regulation of PPARγ levels in adipocytes [Hauser et al., 2000].
Interactions of PTM in human disease
A complex network of signaling pathways does not rely only on phosphorylation or acetylation or any single PTM, rather they are likely controlled by the coordinated actions of phosphorylation, acetylation and a myriad of combinations of PTMs. One or more PTMs, or the same PTM at different residues, can occur on a protein. Multiple PTMs may occur simultaneously or sequentially that would be necessary for the distinct outcome of signaling pathways. Although the implications of PTMs such as phosphorylation and acetylation have been relatively well documented, interactions among these PTMs remain fertile ground for future investigations. Both positive and negative crosstalk occurs among NRs. In positive crosstalk, the first PTM serves as a signal for the addition or removal of another PTM, or for the recognition by a protein that induces a second modification. Phosphorylation-dependent ubiquitination and phosphorylation-dependent sumoylation are good examples. It is intriguing to note that phosphorylation of NRs has been linked to ubiquitination and protein turnover. A direct link between ERα phosphorylation and ubiquitination has been demonstrated by Alarid and colleagues [Valley et al., 2005]. Phosphorylation of ERα at S118 plays a role in estradiol-mediated ERα degradation by regulating recruitment of factors mediating ubiquitination [Valley et al., 2005]. Phosphorylation of ERα by P21 activated Kinase (PAK1) at ERα S305 influences the activation status of ER S118 and the S305-associated ERα transactivation activity requires a functional S118. Transgenic mice expressing active PAK1 exhibited both activated ERα-305 and ERα-S118 phosphorylation (for a review of crosstalk of ER PTMs, see [Le Romancer et al., 2011]). Phosphorylation at AR S210 and S790 by Akt has been shown to promote AR ubiquitination and its subsequent proteosomal degradation [Lin et al., 2002]. Mutation at phosphorylation sites of GR (Serine 122, 150, 212, 220, 234, 315, 412, and threonine 159 to alanine) inhibited ligand-dependent GR degradation and consequently, GR-mediated transcriptional activity [Wallace and Cidlowski, 2001; Webster et al., 1997], which indicated yet another NR ubiquitination that is phosphorylation-dependent. A hypo-phosphorylated form of PPARγ that increased transcriptional activity was degraded faster compared to a phosphorylated form (S112), indicating that in this NR, phosphorylation inhibited ubiquitination and protein degradation [Floyd and Stephens, 2002]. Additionally, phosphorylation of PPARγ at S112 stimulated sumoylation at K107 and this was shown to reduce the ability of PPARγ to activate the adipogenic gene expression pathway.
In negative crosstalk, there is direct competition for modification of a single amino acid residue in a protein, or one modification masks the recognition site for the second PTM. In ERα, lysine residue K302 can be modified by ubiquitination, sumoylation, acetylation, or methylation. Since lysine is the target for ubiquitin enzymes, acetylation and methylation status might affect the protein turnover. ERα phosphorylation at S305 has been shown to inhibit acetylation of ERα at K303 [Cui et al., 2004]. A phosphomimicking mutation at S305 to E305 blocked K303 acetylation and produced an enhanced transcriptional response. However, all these interactions among different PTMs have yet to be analyzed in clinical studies.
It is important to note the caveats associated with evaluation of PTMs in nuclear receptors. PTMs are both dynamic and reversible processes that can be rapidly altered in response to changes in the cellular and environmental conditions that occur following tissue excision, including time to preserve or fix harvested tissue samples from patient biopsies or resection samples. It should be noted that the quality of the tissue and labile modifications, most notably phosphorylation, can be dramatically affected by pre-analytic variables (i.e., ischemic time, hypoxia, temperature or fixation time, exposure of patients to anesthetics and other drugs [Pinhel et al., 2010; Siddiqui and Rimm, 2010]). Alterations in phosphorylation and sumoylation status can occur as a result of ischemic and hypoxic conditions due to the change in the activities of endogenous phosphatases, kinases and sumo ligases and proteases during sample collection [Ahmed and Gardiner, 2011; Cimarosti et al., 2008]. Developing common tissue procurement guidelines to collect and store the clinical samples for the analysis of protein PTMs will avoid the alteration in the PTMs by other factors. All these caveats should be accommodated for when evaluating and interpreting results of PTMs in NRs from clinical samples.
Summary and future perspectives
This review has summarized basic and clinical research studies that link specific NR PTMs to a wide range of human diseases. In many cases, perturbation of a PTM can be associated with a change in receptor transcriptional function that underlies a pathological consequence in a tissue or organ system. Ongoing clinical studies will likely identify additional NR PTMs that are associated with disease progression and resistance to standard of care therapies that target NRs. Additionally, the development of more stringent in vivo models will also aid in better understanding of the mechanisms by which NR PTMs impact physiological processes and disease. To date, much of the in vivo research on NR PTMs has been conducted using transgenic mouse models. While these models have provided valuable insight into the functioning of PTMs, the creation of mouse models in which specific PTMs are altered in the endogenous NR will be crucial. Examination of the effects of PTMs on gene expression and chromatin interaction is another important avenue of research. As previously mentioned, the vast majority of NR PTMs ultimately affect transcriptional activity and determining the effect of PTMs on systemic gene expression will not only provide insight into the global effects of aberrant PTMs associated with disease, but can also indicate potential gene targets for development of targeted therapeutics. Finally, research examining the role of PTMs of NR in disease must also evaluate the actual addition of PTMs and determining what role PTM turnover plays in disease pathophysiological progression. Current research has focused on PTMs that result in the addition of specific phosphate groups, ubiquitin tags, sumo proteins, etc. However, there appears to be a dearth of information regarding PTM turnover. As has been elegantly demonstrated for ERα phosphorylation in breast cancer, future studies will firmly establish NR PTMs as valuable surrogate measures of NR function, that may also be used as biomarkers for disease progression, as well as predictive markers for patient response to NR-directed therapies.
Abbreviations
- AR
androgen receptor
- CHIP
carboxyl terminus of Hsp70-interacting protein
- COPD
chronic obstructive pulmonary disease
- DHT
dihydrotestosterone
- E1
activating enzyme
- E2
conjugating enzyme
- E3
ubiquitin ligase
- ER
estrogen receptor
- GR
glucocorticoid receptor
- HAT
histone acetyltransferase
- HDAC2
histone deacetylase 2
- HDAC3
histone deacetylase-3
- HRPC
hormone-refractory prostate cancer
- MKP-1
mitogen-activated kinase phosphatase-1
- NCoR
nuclear receptor corepressor
- NR
nuclear receptor
- OS
overall survival
- PAK1
p21-activated kinase
- PBMCs
peripheral blood mononuclear cells
- PIAS1/3
protein inhibitor of activated STAT 1/3
- PIN
prostatic intraepithelial neoplasia
- PPARγ
peroxisome proliferator activated receptor-γ
- PPRE
PPARγ response element
- PR
progesterone receptor
- PTM
post-translational modification
- RFS
relapse free survival
- SBMA
spinal and bulbar muscular atrophy
- SENPs
SUMO-specific proteases
- SLP1
secretory leukocyte proteinase inhibitor
- Tam
tamoxifen
References
- Abbinante-Nissen J. M., Simpson L. G., Leikauf G. D. Corticosteroids increase secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am J Physiol. 1995;268:L601–6. doi: 10.1152/ajplung.1995.268.4.L601. [DOI] [PubMed] [Google Scholar]
- Adachi H., Waza M., Tokui K., Katsuno M., Minamiyama M., Tanaka F., Doyu M., Sobue G. CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J Neurosci. 2007;27:5115–26. doi: 10.1523/JNEUROSCI.1242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock I. M., Ito K. Molecular mechanisms of corticosteroid actions. Monaldi Arch Chest Dis. 2000;55:256–66. [PubMed] [Google Scholar]
- Ahmed M. M., Gardiner K. J. Preserving protein profiles in tissue samples: differing outcomes with and without heat stabilization. J Neurosci Methods. 2011;196:99–106. doi: 10.1016/j.jneumeth.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ramahi I., Lam Y. C., Chen H. K., de Gouyon B., Zhang M., Perez A. M., Branco J., de Haro M., Patterson C., Zoghbi H. Y., Botas J. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J Biol Chem. 2006;281:26714–24. doi: 10.1074/jbc.M601603200. [DOI] [PubMed] [Google Scholar]
- Altomare D. A., Testa J. R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–64. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Arpino G., Wiechmann L., Osborne C. K., Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:217–33. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsriku C., Britton D. J., Held J. M., Schilling B., Scott G. K., Gibson B. W., Benz C. C., Baldwin M. A. Systematic mapping of posttranslational modifications in human estrogen receptor-alpha with emphasis on novel phosphorylation sites. Mol Cell Proteomics. 2009;8:467–80. doi: 10.1074/mcp.M800282-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno H., Katsuno M., Suzuki K., Takeuchi Y., Kawashima M., Suga N., Takamori M., Ito M., Nakamura T., Matsuo K., Yamada S., Oki Y., Adachi H., Minamiyama M., Waza M., Atsuta N., Watanabe H., Fujimoto Y., Nakashima T., Tanaka F., Doyu M., Sobue G. Phase 2 trial of leuprorelin in patients with spinal and bulbar muscular atrophy. Ann Neurol. 2009;65:140–50. doi: 10.1002/ana.21540. [DOI] [PubMed] [Google Scholar]
- Barone I., Iacopetta D., Covington K. R., Cui Y., Tsimelzon A., Beyer A., Ando S., Fuqua S. A. Phosphorylation of the mutant K303R estrogen receptor alpha at serine 305 affects aromatase inhibitor sensitivity. Oncogene. 2010;29:2404–14. doi: 10.1038/onc.2009.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa-Khalfe T., Cheng J., Wang Z., Yeh E. T. Induction of the SUMO-specific protease 1 transcription by the androgen receptor in prostate cancer cells. J Biol Chem. 2007;282:37341–9. doi: 10.1074/jbc.M706978200. [DOI] [PubMed] [Google Scholar]
- Bhavsar P., Hew M., Khorasani N., Torrego A., Barnes P. J., Adcock I., Chung K. F. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–90. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- Bloom J. W. Mitogen-activated protein kinase pathways: therapeutic targets in steroid resistance? J Allergy Clin Immunol. 2004;114:1055–8. doi: 10.1016/j.jaci.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bluher M., Paschke R. Analysis of the relationship between PPAR-gamma 2 gene variants and severe insulin resistance in obese patients with impaired glucose tolerance. Exp Clin Endocrinol Diabetes. 2003;111:85–90. doi: 10.1055/s-2003-39235. [DOI] [PubMed] [Google Scholar]
- Britton D. J., Scott G. K., Schilling B., Atsriku C., Held J. M., Gibson B. W., Benz C. C., Baldwin M. A. A novel serine phosphorylation site detected in the N-terminal domain of estrogen receptor isolated from human breast cancer cells. J Am Soc Mass Spectrom. 2008;19:729–40. doi: 10.1016/j.jasms.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. Y., Warrick J. M., Andriola I., Merry D., Bonini N. M. Genetic modulation of polyglutamine toxicity by protein conjugation pathways in Drosophila. Hum Mol Genet. 2002;11:2895–904. doi: 10.1093/hmg/11.23.2895. [DOI] [PubMed] [Google Scholar]
- Cheng J., Bawa T., Lee P., Gong L., Yeh E. T. Role of desumoylation in the development of prostate cancer. Neoplasia. 2006;8:667–76. doi: 10.1593/neo.06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Larsen E. S., O'Brien C. J., Wang H., Jenkins S. C., Holder L., Lieberman A. P., Merry D. E. Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci. 2004;24:4778–86. doi: 10.1523/JNEUROSCI.0808-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. H., Banks A. S., Estall J. L., Kajimura S., Bostrom P., Laznik D., Ruas J. L., Chalmers M. J., Kamenecka T. M., Bluher M., Griffin P. R., Spiegelman B. M. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–6. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. J., Chung S. S., ρ E. J., Lee H. W., Lee M. H., Choi H. S., Seol J. H., Baek S. H., Bang O. S., Chung C. H. Negative modulation of RXRalpha transcriptional activity by small ubiquitin-related modifier (SUMO) modification and its reversal by SUMO-specific protease SUSP1. J Biol Chem. 2006;281:30669–77. doi: 10.1074/jbc.M604033200. [DOI] [PubMed] [Google Scholar]
- Christianson J. L., Nicoloro S., Straubhaar J., Czech M. P. Stearoyl-CoA desaturase 2 is required for peroxisome proliferator-activated receptor gamma expression and adipogenesis in cultured 3T3-L1 cells. J Biol Chem. 2008;283:2906–16. doi: 10.1074/jbc.M705656200. [DOI] [PubMed] [Google Scholar]
- Cimarosti H., Lindberg C., Bomholt S. F., Ronn L. C., Henley J. M. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54:280–9. doi: 10.1016/j.neuropharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Clement K., Hercberg S., Passinge B., Galan P., Varroud-Vial M., Shuldiner A. R., Beamer B. A., Charpentier G., Guy-Grand B., Froguel P., Vaisse C. The Pro115Gln and Pro12Ala PPAR gamma gene mutations in obesity and type 2 diabetes. Int J Obes Relat Metab Disord. 2000;24:391–3. doi: 10.1038/sj.ijo.0801191. [DOI] [PubMed] [Google Scholar]
- Conway K., Parrish E., Edmiston S. N., Tolbert D., Tse C. K., Geradts J., Livasy C. A., Singh H., Newman B., Millikan R. C. The estrogen receptor-alpha A908G (K303R) mutation occurs at a low frequency in invasive breast tumors: results from a population-based study. Breast Cancer Res. 2005;7:R871–80. doi: 10.1186/bcr1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Zhang M., Pestell R., Curran E. M., Welshons W. V., Fuqua S. A. Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64:9199–208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- De Bosscher K., Vanden Berghe W., Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- Deeb S. S., Fajas L., Nemoto M., Pihlajamaki J., Mykkanen L., Kuusisto J., Laakso M., Fujimoto W., Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet. 1998;20:284–7. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- Dorval V., Fraser P. E. SUMO on the road to neurodegeneration. Biochim Biophys Acta. 2007;1773:694–706. doi: 10.1016/j.bbamcr.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Evans D., Mann W. A., de Heer J., Michel U., Wendt D., Kortner B., Wolf A., Beisiegel U. Variation in the gene for human peroxisome proliferator activated receptor gamma (PPARgamma) does not play a major role in the development of morbid obesity. Int J Obes Relat Metab Disord. 2000;24:647–51. doi: 10.1038/sj.ijo.0801214. [DOI] [PubMed] [Google Scholar]
- Fan M., Park A., Nephew K. P. CHIP (carboxyl terminus of Hsc70-interacting protein) promotes basal and geldanamycin-induced degradation of estrogen receptor-alpha. Mol Endocrinol. 2005;19:2901–14. doi: 10.1210/me.2005-0111. [DOI] [PubMed] [Google Scholar]
- Floyd Z. E., Stephens J. M. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–8. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- Fu M., Rao M., Wang C., Sakamaki T., Wang J., Di Vizio D., Zhang X., Albanese C., Balk S., Chang C., Fan S., Rosen E., Palvimo J. J., Janne O. A., Muratoglu S., Avantaggiati M. L., Pestell R. G. Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol Cell Biol. 2003;23:8563–75. doi: 10.1128/MCB.23.23.8563-8575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M., Wang C., Reutens A. T., Wang J., Angeletti R. H., Siconolfi-Baez L., Ogryzko V., Avantaggiati M. L., Pestell R. G. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem. 2000;275:20853–60. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Wiltschke C., Zhang Q. X., Borg A., Castles C. G., Friedrichs W. E., Hopp T., Hilsenbeck S., Mohsin S., O'Connell P., Allred D. C. A hypersensitive estrogen receptor-alpha mutation in premalignant breast lesions. Cancer Res. 2000;60:4026–9. [PubMed] [Google Scholar]
- Fu M., Rao M., Wu K., Wang C., Zhang X., Hessien M., Yeung Y. G., Gioeli D., Weber M. J., Pestell R. G. The androgen receptor acetylation site regulates cAMP and AKT but not ERK-induced activity. J Biol Chem. 2004;279:29436–49. doi: 10.1074/jbc.M313466200. [DOI] [PubMed] [Google Scholar]
- Gaughan L., Logan I. R., Cook S., Neal D. E., Robson C. N. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277:25904–13. doi: 10.1074/jbc.M203423200. [DOI] [PubMed] [Google Scholar]
- Generali D., Buffa F. M., Berruti A., Brizzi M. P., Campo L., Bonardi S., Bersiga A., Allevi G., Milani M., Aguggini S., Papotti M., Dogliotti L., Bottini A., Harris A. L., Fox S. B. Phosphorylated ERalpha, HIF-1alpha, and MAPK signaling as predictors of primary endocrine treatment response and resistance in patients with breast cancer. J Clin Oncol. 2009;27:227–34. doi: 10.1200/JCO.2007.13.7083. [DOI] [PubMed] [Google Scholar]
- Ghisletti S., Huang W., Ogawa S., Pascual G., Lin M. E., Willson T. M., Rosenfeld M. G., Glass C. K. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. M., Malik S., Bedolla R., Kreisberg J. I. Akt in prostate cancer: possible role in androgen-independence. Curr Drug Metab. 2003;4:487–96. doi: 10.2174/1389200033489226. [DOI] [PubMed] [Google Scholar]
- Giaginis C., Giagini A., Theocharis S. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligands as potential therapeutic agents to treat arthritis. Pharmacol Res. 2009;60:160–9. doi: 10.1016/j.phrs.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Giordano C., Cui Y., Barone I., Ando S., Mancini M. A., Berno V., Fuqua S. A. Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor alpha and its phosphorylation at serine 305. Breast Cancer Res Treat. 2010;119:71–85. doi: 10.1007/s10549-009-0334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Zhu J., Goodman O. B., Jr., Pestell R. G., Schlegel P. N., Nanus D. M., Shen R. Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene. 2006;25:2011–21. doi: 10.1038/sj.onc.1209231. [DOI] [PubMed] [Google Scholar]
- Hamer O. W., Forstner D., Ottinger I., Ristow M., Bollheimer L. C., Scholmerich J., Palitzsch K. D. The Pro115Gln polymorphism within the PPAR gamma2 gene has no epidemiological impact on morbid obesity. Exp Clin Endocrinol Diabetes. 2002;110:230–4. doi: 10.1055/s-2002-33072. [DOI] [PubMed] [Google Scholar]
- Hauser S., Adelmant G., Sarraf P., Wright H. M., Mueller E., Spiegelman B. M. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J Biol Chem. 2000;275:18527–33. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- Hay R. T. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Herynk M. H., Parra I., Cui Y., Beyer A., Wu M. F., Hilsenbeck S. G., Fuqua S. A. Association between the estrogen receptor alpha A908G mutation and outcomes in invasive breast cancer. Clin Cancer Res. 2007;13:3235–43. doi: 10.1158/1078-0432.CCR-06-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Kok M., Michalides R., Fles R., Koornstra R. H., Wesseling J., Hauptmann M., Neefjes J., Peterse J. L., Stal O., Landberg G., Linn S. C. Phosphorylation of the oestrogen receptor alpha at serine 305 and prediction of tamoxifen resistance in breast cancer. J Pathol. 2009;217:372–9. doi: 10.1002/path.2455. [DOI] [PubMed] [Google Scholar]
- Hu E., Kim J. B., Sarraf P., Spiegelman B. M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–3. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Irusen E., Matthews J. G., Takahashi A., Barnes P. J., Chung K. F., Adcock I. M. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109:649–57. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- Ismaili N., Garabedian M. J. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- Issemann I., Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–50. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Ito K., Yamamura S., Essilfie-Quaye S., Cosio B., Ito M., Barnes P. J., Adcock I. M. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203:7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Haruta T., Usui I., Takata Y., Takano A., Uno T., Kawahara J., Ueno E., Sasaoka T., Ishibashi O., Kobayashi M. Pioglitazone ameliorates tumor necrosis factor-alpha-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator--activated receptor-gamma. Diabetes. 2001;50:1083–92. doi: 10.2337/diabetes.50.5.1083. [DOI] [PubMed] [Google Scholar]
- Jana N. R., Dikshit P., Goswami A., Kotliarova S., Murata S., Tanaka K., Nukina N. Co-chaperone CHIP associates with expanded polyglutamine protein and promotes their degradation by proteasomes. J Biol Chem. 2005;280:11635–40. doi: 10.1074/jbc.M412042200. [DOI] [PubMed] [Google Scholar]
- Jennewein C., Kuhn A. M., Schmidt M. V., Meilladec-Jullig V., von Knethen A., Gonzalez F. J., Brune B. Sumoylation of peroxisome proliferator-activated receptor gamma by apoptotic cells prevents lipopolysaccharide-induced NCoR removal from kappaB binding sites mediating transrepression of proinflammatory cytokines. J Immunol. 2008;181:5646–52. doi: 10.4049/jimmunol.181.8.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Sarwar N., Peston D., Kulinskaya E., Shousha S., Coombes R. C., Ali S. Phosphorylation of estrogen receptor-alpha at Ser167 is indicative of longer disease-free and overall survival in breast cancer patients. Clin Cancer Res. 2007;13:5769–76. doi: 10.1158/1078-0432.CCR-07-0822. [DOI] [PubMed] [Google Scholar]
- Kaikkonen S., Jaaskelainen T., Karvonen U., Rytinki M. M., Makkonen H., Gioeli D., Paschal B. M., Palvimo J. J. SUMO-specific protease 1 (SENP1) reverses the hormone-augmented SUMOylation of androgen receptor and modulates gene responses in prostate cancer cells. Mol Endocrinol. 2009;23:292–307. doi: 10.1210/me.2008-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamouzis M. V., Konstantinopoulos P. A., Badra F. A., Papavassiliou A. G. SUMO and estrogen receptors in breast cancer. Breast Cancer Res Treat. 2008;107:195–210. doi: 10.1007/s10549-007-9552-5. [DOI] [PubMed] [Google Scholar]
- Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H., Metzger D., Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Katsuno M., Adachi H., Doyu M., Minamiyama M., Sang C., Kobayashi Y., Inukai A., Sobue G. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med. 2003a;9:768–73. doi: 10.1038/nm878. [DOI] [PubMed] [Google Scholar]
- Katsuno M., Adachi H., Inukai A., Sobue G. Transgenic mouse models of spinal and bulbar muscular atrophy (SBMA) Cytogenet Genome Res. 2003b;100:243–51. doi: 10.1159/000072860. [DOI] [PubMed] [Google Scholar]
- Kintscher U., Hartge M., Hess K., Foryst-Ludwig A., Clemenz M., Wabitsch M., Fischer-Posovszky P., Barth T. F., Dragun D., Skurk T., Hauner H., Bluher M., Unger T., Wolf A. M., Knippschild U., Hombach V., Marx N. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–10. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- Kramer O. H., Knauer S. K., Greiner G., Jandt E., Reichardt S., Guhrs K. H., Stauber R. H., Bohmer F. D., Heinzel T. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev. 2009;23:223–35. doi: 10.1101/gad.479209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota N., Terauchi Y., Miki H., Tamemoto H., Yamauchi T., Komeda K., Satoh S., Nakano R., Ishii C., Sugiyama T., Eto K., Tsubamoto Y., Okuno A., Murakami K., Sekihara H., Hasegawa G., Naito M., Toyoshima Y., Tanaka S., Shiota K., Kitamura T., Fujita T., Ezaki O., Aizawa S., Kadowaki T. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Lannigan D. A. Estrogen receptor phosphorylation. Steroids. 2003;68:1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Lanvin O., Bianco S., Kersual N., Chalbos D., Vanacker J. M. Potentiation of ICI182,780 (Fulvestrant)-induced estrogen receptor-alpha degradation by the estrogen receptor-related receptor-alpha inverse agonist XCT790. J Biol Chem. 2007;282:28328–34. doi: 10.1074/jbc.M704295200. [DOI] [PubMed] [Google Scholar]
- Lasa M., Abraham S. M., Boucheron C., Saklatvala J., Clark A. R. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22:7802–11. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Romancer M., Poulard C., Cohen P., Sentis S., Renoir J. M., Corbo L. Cracking the estrogen receptor's posttranslational code in breast tumors. Endocr Rev. 2011;32:597–622. doi: 10.1210/er.2010-0016. [DOI] [PubMed] [Google Scholar]
- Liao Y., Grobholz R., Abel U., Trojan L., Michel M. S., Angel P., Mayer D. Increase of AKT/PKB expression correlates with gleason pattern in human prostate cancer. Int J Cancer. 2003;107:676–80. doi: 10.1002/ijc.11471. [DOI] [PubMed] [Google Scholar]
- Lin H. K., Yeh S., Kang H. Y., Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci U S A. 2001;98:7200–5. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. K., Wang L., Hu Y. C., Altuwaijri S., Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. Embo J. 2002;21:4037–48. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. K., Hu Y. C., Yang L., Altuwaijri S., Chen Y. T., Kang H. Y., Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278:50902–7. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- Li L. B., Goleva E., Hall C. F., Ou L. S., Leung D. Y. Superantigen-induced corticosteroid resistance of human T cells occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK-ERK) pathway. J Allergy Clin Immunol. 2004;114:1059–69. doi: 10.1016/j.jaci.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Lonard D. M., Nawaz Z., Smith C. L., O'Malley B. W. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol Cell. 2000;5:939–48. doi: 10.1016/s1097-2765(00)80259-2. [DOI] [PubMed] [Google Scholar]
- Majumder P. K., Sellers W. R. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24:7465–74. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Evans R. M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–50. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Marsaud V., Gougelet A., Maillard S., Renoir J. M. Various phosphorylation pathways, depending on agonist and antagonist binding to endogenous estrogen receptor alpha (ERalpha), differentially affect ERalpha extractability, proteasome-mediated stability, and transcriptional activity in human breast cancer cells. Mol Endocrinol. 2003;17:2013–27. doi: 10.1210/me.2002-0269. [DOI] [PubMed] [Google Scholar]
- Matthews J. G., Ito K., Barnes P. J., Adcock I. M. Defective glucocorticoid receptor nuclear translocation and altered histone acetylation patterns in glucocorticoid-resistant patients. J Allergy Clin Immunol. 2004;113:1100–8. doi: 10.1016/j.jaci.2004.03.018. [DOI] [PubMed] [Google Scholar]
- McCall P., Gemmell L. K., Mukherjee R., Bartlett J. M., Edwards J. Phosphorylation of the androgen receptor is associated with reduced survival in hormone-refractory prostate cancer patients. Br J Cancer. 2008;98:1094–101. doi: 10.1038/sj.bjc.6604152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough H., Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8:303–8. doi: 10.1379/1466-1268(2003)008<0303:calbtc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Griekspoor A., Balkenende A., Verwoerd D., Janssen L., Jalink K., Floore A., Velds A., van't Veer L., Neefjes J. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5:597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Miles P. D., Barak Y., He W., Evans R. M., Olefsky J. M. Improved insulin-sensitivity in mice heterozygous for PPAR-gamma deficiency. J Clin Invest. 2000;105:287–92. doi: 10.1172/JCI8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. L., Webb M. S., Copik A. J., Wang Y., Johnson B. H., Kumar R., Thompson E. B. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol. 2005;19:1569–83. doi: 10.1210/me.2004-0528. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Thomas M., Dadgar N., Lieberman A. P., Iniguez-Lluhi J. A. Small ubiquitin-like modifier (SUMO) modification of the androgen receptor attenuates polyglutamine-mediated aggregation. J Biol Chem. 2009;284:21296–306. doi: 10.1074/jbc.M109.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L. C., Seekallu S. V., Watson P. H. Clinical significance of estrogen receptor phosphorylation. Endocr Relat Cancer. 2011;18:R1–14. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Niu Y., Snell L., Watson P. Phospho-serine-118 estrogen receptor-alpha expression is associated with better disease outcome in women treated with tamoxifen. Clin Cancer Res. 2004;10:5902–6. doi: 10.1158/1078-0432.CCR-04-0191. [DOI] [PubMed] [Google Scholar]
- Nirmala P. B., Thampan R. V. Ubiquitination of the rat uterine estrogen receptor: dependence on estradiol. Biochem Biophys Res Commun. 1995;213:24–31. doi: 10.1006/bbrc.1995.2093. [DOI] [PubMed] [Google Scholar]
- Palazzolo I., Burnett B. G., Young J. E., Brenne P. L., La Spada A. R., Fischbeck K. H., Howell B. W., Pennuto M. Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum Mol Genet. 2007;16:1593–603. doi: 10.1093/hmg/ddm109. [DOI] [PubMed] [Google Scholar]
- Pascual G., Fong A. L., Ogawa S., Gamliel A., Li A. C., Perissi V., Rose D. W., Willson T. M., Rosenfeld M. G., Glass C. K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–63. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G., Sullivan A. L., Ogawa S., Gamliel A., Perissi V., Rosenfeld M. G., Glass C. K. Anti-inflammatory and antidiabetic roles of PPARgamma. Novartis Found Symp. 2007;286:183–96; discussion 196-203. doi: 10.1002/9780470985571.ch16. [DOI] [PubMed] [Google Scholar]
- Pinhel I. F., Macneill F. A., Hills M. J., Salter J., Detre S., A'Hern R., Nerurkar A., Osin P., Smith I. E., Dowsett M. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 2010;12:R76. doi: 10.1186/bcr2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov V. M., Wang C., Shirley L. A., Rosenberg A., Li S., Nevalainen M., Fu M., Pestell R. G. The functional significance of nuclear receptor acetylation. Steroids. 2007;72:221–30. doi: 10.1016/j.steroids.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poukka H., Karvonen U., Janne O. A., Palvimo J. J. Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1) Proc Natl Acad Sci U S A. 2000;97:14145–50. doi: 10.1073/pnas.97.26.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala S. M., Rhoades B., Shapiro J. S., Rich A. S., Kim J. K., Shulman G. I., Kaestner K. H., Lazar M. A. Genetic modulation of PPARgamma phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–63. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- Ray A., Prefontaine K. E., Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994;269:12940–6. [PubMed] [Google Scholar]
- Reid G., Denger S., Kos M., Gannon F. Human estrogen receptor-alpha: regulation by synthesis, modification and degradation. Cell Mol Life Sci. 2002;59:821–31. doi: 10.1007/s00018-002-8470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristow M., Muller-Wieland D., Pfeiffer A., Krone W., Kahn C. R. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–9. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- Rosen E. D., Spiegelman B. M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlabach M. R., Luo J., Solimini N. L., Hu G., Xu Q., Li M. Z., Zhao Z., Smogorzewska A., Sowa M. E., Ang X. L., Westbrook T. F., Liang A. C., Chang K., Hackett J. A., Harper J. W., Hannon G. J., Elledge S. J. Cancer proliferation gene discovery through functional genomics. Science. 2008;319:620–4. doi: 10.1126/science.1149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler J. S., Dejean A. Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol. 2003;4:690–9. doi: 10.1038/nrm1200. [DOI] [PubMed] [Google Scholar]
- Sentis S., Le Romancer M., Bianchin C., Rostan M. C., Corbo L. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Mol Endocrinol. 2005;19:2671–84. doi: 10.1210/me.2005-0042. [DOI] [PubMed] [Google Scholar]
- Siddiqui S., Rimm D. L. Pre-analytic variables and phospho-specific antibodies: the Achilles heel of immunohistochemistry. Breast Cancer Res. 2010;12:113. doi: 10.1186/bcr2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skliris G. P., Nugent Z. J., Rowan B. G., Penner C. R., Watson P. H., Murphy L. C. A phosphorylation code for oestrogen receptor-alpha predicts clinical outcome to endocrine therapy in breast cancer. Endocr Relat Cancer. 2010;17:589–97. doi: 10.1677/ERC-10-0030. [DOI] [PubMed] [Google Scholar]
- Skliris G. P., Rowan B. G., Al-Dhaheri M., Williams C., Troup S., Begic S., Parisien M., Watson P. H., Murphy L. C. Immunohistochemical validation of multiple phospho-specific epitopes for estrogen receptor alpha (ERalpha) in tissue microarrays of ERalpha positive human breast carcinomas. Breast Cancer Res Treat. 2009;118:443–53. doi: 10.1007/s10549-008-0267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears M., McSharry C., Thomson N. C. Peroxisome proliferator-activated receptor-gamma agonists as potential anti-inflammatory agents in asthma and chronic obstructive pulmonary disease. Clin Exp Allergy. 2006;36:1494–504. doi: 10.1111/j.1365-2222.2006.02604.x. [DOI] [PubMed] [Google Scholar]
- Steffan J. S., Agrawal N., Pallos J., Rockabrand E., Trotman L. C., Slepko N., Illes K., Lukacsovich T., Zhu Y. Z., Cattaneo E., Pandolfi P. P., Thompson L. M., Marsh J. L. SUMO modification of Huntingtin and Huntington's disease pathology. Science. 2004;304:100–4. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- Szatmary Z., Garabedian M. J., Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem. 2004;279:43708–15. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- Tateishi Y., Kawabe Y., Chiba T., Murata S., Ichikawa K., Murayama A., Tanaka K., Baba T., Kato S., Yanagisawa J. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. Embo J. 2004;23:4813–23. doi: 10.1038/sj.emboj.7600472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitoura D. C., Rothman P. B. Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J Clin Invest. 2004;113:619–27. doi: 10.1172/JCI18975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley C. C., Metivier R., Solodin N. M., Fowler A. M., Mashek M. T., Hill L., Alarid E. T. Differential regulation of estrogen-inducible proteolysis and transcription by the estrogen receptor alpha N terminus. Mol Cell Biol. 2005;25:5417–28. doi: 10.1128/MCB.25.13.5417-5428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beekum O., Fleskens V., Kalkhoven E. Posttranslational modifications of PPAR-gamma: fine-tuning the metabolic master regulator. Obesity (Silver Spring) 2009;17:213–9. doi: 10.1038/oby.2008.473. [DOI] [PubMed] [Google Scholar]
- Waite K. J., Floyd Z. E., Arbour-Reily P., Stephens J. M. Interferon-gamma-induced regulation of peroxisome proliferator-activated receptor gamma and STATs in adipocytes. J Biol Chem. 2001;276:7062–8. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- Wallace A. D., Cidlowski J. A. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J Biol Chem. 2001;276:42714–21. doi: 10.1074/jbc.M106033200. [DOI] [PubMed] [Google Scholar]
- Wang L., Banerjee S. Differential PIAS3 expression in human malignancy. Oncol Rep. 2004;11:1319–24. [PubMed] [Google Scholar]
- Wang C., Fu M., Angeletti R. H., Siconolfi-Baez L., Reutens A. T., Albanese C., Lisanti M. P., Katzenellenbogen B. S., Kato S., Hopp T., Fuqua S. A., Lopez G. N., Kushner P. J., Pestell R. G. Direct acetylation of the estrogen receptor alpha hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem. 2001;276:18375–83. doi: 10.1074/jbc.M100800200. [DOI] [PubMed] [Google Scholar]
- Webster J. C., Jewell C. M., Bodwell J. E., Munck A., Sar M., Cidlowski J. A. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem. 1997;272:9287–93. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- Weitsman G. E., Li L., Skliris G. P., Davie J. R., Ung K., Niu Y., Curtis-Snell L., Tomes L., Watson P. H., Murphy L. C. Estrogen receptor-alpha phosphorylated at Ser118 is present at the promoters of estrogen-regulated genes and is not altered due to HER-2 overexpression. Cancer Res. 2006;66:10162–70. doi: 10.1158/0008-5472.CAN-05-4111. [DOI] [PubMed] [Google Scholar]
- Wijayaratne A. L., McDonnell D. P. The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J Biol Chem. 2001;276:35684–92. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- Williams C. C., Basu A., El-Gharbawy A., Carrier L. M., Smith C. L., Rowan B. G. Identification of four novel phosphorylation sites in estrogen receptor alpha: impact on receptor-dependent gene expression and phosphorylation by protein kinase CK2. BMC Biochem. 2009;10:36. doi: 10.1186/1471-2091-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson T. M., Lambert M. H., Kliewer S. A. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–67. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Nishio M., Toyama T., Sugiura H., Kondo N., Kobayashi S., Fujii Y., Iwase H. Low phosphorylation of estrogen receptor alpha (ERalpha) serine 118 and high phosphorylation of ERalpha serine 167 improve survival in ER-positive breast cancer. Endocr Relat Cancer. 2008;15:755–63. doi: 10.1677/ERC-08-0078. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Nishio M., Kobayashi S., Ando Y., Sugiura H., Zhang Z., Hamaguchi M., Mita K., Fujii Y., Iwase H. Phosphorylation of estrogen receptor alpha serine 167 is predictive of response to endocrine therapy and increases postrelapse survival in metastatic breast cancer. Breast Cancer Res. 2005;7:R753–64. doi: 10.1186/bcr1285. [DOI] [PMC free article] [PubMed] [Google Scholar]