Abstract
Objective
To investigate cigarette smoking in cutaneous lupus erythematosus (CLE).
Design
Prospective longitudinal cohort study.
Setting
Urban cutaneous autoimmune disease clinic.
Participants
218 volunteers presenting between 1/5/2007 and 7/30/2010 with CLE.
Main Outcome Measures
Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) scores for disease severity and response to treatment. Skindex 29-3 to assess patient quality of life (QoL).
Results
Lupus current smokers had higher median CLASI scores (9.5) than never (7.0) and past (6.0) smokers (p = 0.017). Current smokers had higher median scores on all Skindex 29-3 subsets. Current smokers on hydroxychloroquine had higher quinacrine use than non-smokers (p = 0.041). 2–7 months post-enrollment, current smokers (median CLASI change -3) treated with only antimalarials improved more than never (+1) and past (0) smokers (p = 0.02). ≥ 8 months post-enrollment, current smokers (CLASI change +3.5) treated with antimalarials plus at least one additional immunomodulator improved less than never (−1.5) and past (0) smokers (p = 0.04).
Conclusions
Current smokers with lupus had worse disease, worse quality of life, and were more often treated with hydroxychloroquine plus quinacrine than non-smokers. Never and past smokers showed greater improvement when treated with antimalarials plus at least one additional immunomodulator. Current smokers had greater improvement when treated with only antimalarials.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect nearly every organ system. Between 59% and 85% of SLE patients develop dermatologic involvement, including cutaneous lupus erythematosus (CLE), though patients can frequently have CLE in the absence of SLE.1 The specific manifestations of CLE are diverse, and can be grouped into three major subtypes: Acute CLE (ACLE), subacute CLE (SCLE), and chronic CLE (CCLE).1 Additionally, patients with SLE can develop lupus related skin disease other than CLE, referred to here as SLE with non-specific skin. A number of recent publications have looked at the impact of tobacco, specifically cigarette smoking, on CLE.2, 3 In this paper, we report the results of an ongoing prospective database study that looked at differences between lupus subjects who were never, past, or current smokers.
Methods
Patients
Patients with lupus presenting to the outpatient medical dermatology clinic at the University of Pennsylvania were enrolled in an ongoing database study on the prevalence and severity of lupus. All patients older than 18 years with clinical, histological, and/or serological evidence of lupus were invited to participate in the study. The study protocol was approved by the Institutional Review Board (IRB) at the University of Pennsylvania.
Databases
Data were analyzed for all subjects enrolled in the lupus database from initiation of the study on 1/5/2007 through 7/30/2010. 218 total subjects were enrolled during this time. 15 of these subjects were not included in analyses because they had not yet had their first study visit (consented only). 5 subjects were excluded because they either gave conflicting information about their smoking habits (3) or did not complete smoking related questions (2). 1 subject was excluded because of a problem with the consent form, leaving a total of 197 lupus subjects included in the analyses. To measure severity of lupus specific skin disease, a validated scoring system, the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) was used.4
CLE-only and SLE + skin smoking analysis
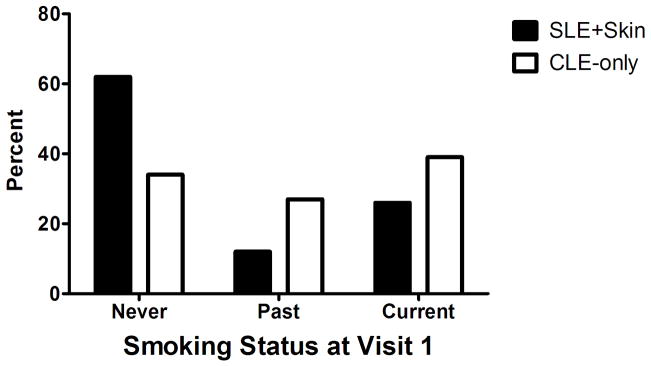
Subjects in the lupus database were assessed for differences in smoking status by CLE subtype (ACLE, SCLE, and CCLE) using a Fisher’s exact test. All lupus subjects were then split into two groups, SLE with skin involvement (SLE + skin), which included subjects with SLE and either a specific CLE subtype or lupus non-specific skin disease, and CLE-only, which consisted of lupus subjects not meeting criteria for SLE. Data for these groups were then analyzed for differences in smoking status by chi-square tests. Results of this analysis are reported as proportions (Figure 1).
Figure 1.
Differences in smoking status between lupus subjects with SLE + skin relative to subjects with CLE-only (p = 0.006).
CLE disease severity and quality of life analysis
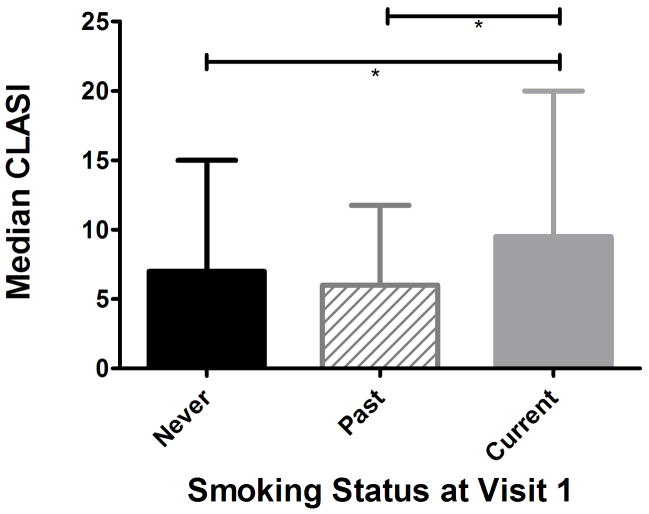
CLASI scores were used to assess for differences in disease severity among CLE subjects with active skin disease based on smoking status. Of the 197 subjects with lupus, 7 subjects did not have CLASI scores recorded at visit one, and were subsequently excluded from this analysis. Additionally, 41 subjects did not have active skin disease at enrollment (CLASI score of 0) and were also excluded. To test whether these 41 subjects differed by smoking status, a Fisher’s exact test was run. To compare differences in smoking status of the 149 CLE subjects with active disease at enrollment, Kruskal-Wallis and Dunn’s multiple comparison tests were run. Results are reported as median with interquartile range (Figure 2).
Figure 2.
Median CLASI scores for subjects in the lupus database at enrollment. Both never and past smokers differed significantly from current (p = 0.017, post-hoc Dunn’s test indicated *p < 0.05 for both never versus current and past versus current; bars represent interquartile range).
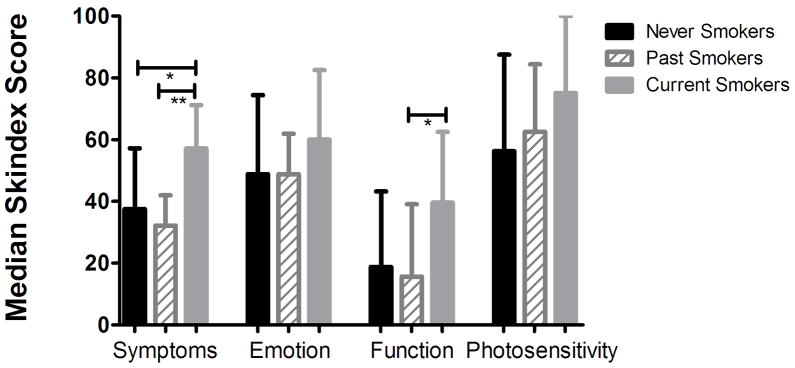
We then analyzed subject responses to the modified Skindex 29–3 questionnaire, a commonly used tool to measure patient quality of life in dermatology that can be divided into four subsets: Function, emotion, symptoms, and photosensitivity.5 For analysis, we used the same initial pool of subjects as shown in Figure 2, with the exclusion of 3 never, 4 past, and 1 current smoker because they did not complete the questionnaire at enrollment. Results of Kruskal-Wallis and Dunn’s multiple comparison tests are reported as median with interquartile range (Figure 3).
Figure 3.
Median Skindex 29-3 scores compared by smoking status. Note that current smokers had higher scores in all subjects (*p < 0.05; **p < 0.01; bars represent interquartile range).
CLE treatment response analysis
CLE subjects were divided into two different treatment groups, those treated with only antimalarials during enrollment (AM only), and those who were also treated with an additional immunomodulator(s) (AM + IM). Table 1 describes subject characteristics within these groups, as well as criteria for exclusion from this particular analysis. For this study, immunomodulators included methotrexate, azathioprine, mycophenolate, rituximab, thalidomide, oral corticosteroids, dapsone, cyclophosphamide, and lenalidomide (Table 2).
Table 1.
Treatment response groups:
| Treatment Group | n | Explanation of group |
|---|---|---|
| Antimalarial only (AM-only) | 50 | These subjects received only antimalarial treatment during enrollment. 6 began treatment at visit one, and 44 had been taking an AM prior to enrollment. 17 subjects were never smokers (34%), 14 past (28%), 19 current (38%). |
| Antimalarial plus immunomodulator (AM + IM) | 57 | These subjects received antimalarial treatment as well as at least one additional immunomodulator at some point during enrollment. 29 subjects in this group were never smokers (51%), 9 past (16%), and 19 current (33%). |
| Visit 1 only, or no CLASI score recorded | 69 | 60 subjects had only one visit and so were excluded from analysis. Additionally, 9 subjects did not have CLASI scores recorded within the timeframe utilized for data analysis and so were excluded. |
| Smoking status change | 8 | These subjects had a change in smoking status during enrollment, and so were excluded from treatment response analyses. |
| No antimalarial treatment | 13 | These subjects did not receive treatment with antimalarials while enrolled, and were excluded from analysis. 3 subjects were never smokers (23%), 6 past (46%), and 4 current (31%). |
Table 2.
Number and type of immunomodulators used for AM + IM group
| Medication | Never | Past | Current | Total |
|---|---|---|---|---|
| Methotrexate | 7 | 4 | 5 | 16 |
| Azathioprine | 8 | 1 | 5 | 14 |
| Mycophenolate | 8 | 2 | 8 | 18 |
| Rituximab | 1 | 0 | 0 | 1 |
| Thalidomide | 4 | 1 | 4 | 9 |
| Oral corticosteroids | 22 | 4 | 9 | 35 |
| Dapsone | 2 | 1 | 1 | 4 |
| Cyclophosphamide | 1 | 0 | 0 | 1 |
| Lenalidomide | 0 | 0 | 1 | 1 |
Specific antimalarial regimens for each subject included in both the AM-only and AM + IM groups were assessed and compared by smoking status using a chi-square test, and results are reported as proportions (Table 3). Additionally, never and past smokers were combined into one non-smoking group and compared to current smokers using Fisher’s exact test. Subjects with SLE were compared between the AM-only and AM + IM groups using Fisher’s exact test, and results are reported as proportions. Mean CLASI scores were compared between each group at each time-point, and results of one-way analysis of variance with post-hoc Tukey tests are reported.
Table 3.
Number of subjects on specific antimalarial treatment regimens, combined AM-only and AM + IM groups
| Smoking Status | Total HCQ | HCQ also on Qc (%Total)* | Total CQ | CQ also on Qc (% Total) |
|---|---|---|---|---|
| Never | 38 | 15 (39%) | 8 | 7 (88%) |
| Past | 22 | 9 (40%) | 1 | 0 (0%) |
| Current | 29 | 19 (66%) | 9 | 5 (56%) |
P = 0.078; HCQ = Hydroxychloroquine; CQ = Chloroquine; Qc = Quinacrine
To analyze treatment response, the following set of rules was followed: CLASI scores at visit 1 were recorded for each subject. CLASI scores were then recorded at a visit within 2–7 months of visit 1. If a subject had multiple visits in this time frame, the visit closest to 2 months was used. CLASI scores at least 8 months after visit 1 were then recorded. If a subject had multiple visits in this time frame, the visit closest to 12 months was used. Some visits were missing CLASI scores, and in those cases the next closest visit with a recorded CLASI score was used. Per patient CLASI activity change scores between visits were calculated, and Kruskal-Wallis tests were used to assess differences between smoking groups for each follow-up visit (visit 1 to the 2–7 month time-point, and visit 1 to the 8+ month time-point). Results are presented as median with interquartile range (Table 4).
Table 4. CLE treatment response.
Presented as median change in CLASI activity score (interquartile range). Negative change reflects improvement, positive change reflects worsening.
| Group+ | Score++ | Smoking Status | p value | ||
|---|---|---|---|---|---|
| Never | Past | Current | |||
| 1 | A | +1 (−1 to +3) | 0 (−5 to +1) | −3 (−8.25 to 0) | 0.02 |
| 1 | B | 0 (−1.5 to +2.5) | −1.5 (−3.5 to −0.75) | −2.5 (−5 to +2.75) | 0.24 |
| 2 | A | −1 (−6 to 0) | +5 (−3 to +9) | 0 (−3 to +2) | 0.07 |
| 2 | B | −1.5 (−6.5 to +0.75) | 0 (−3 to +5.25) | +3.5 (−0.75 to +8.4) | 0.04 |
Group 1 (AM-only): Current smokers improved more over time than never and past smokers.
Group 2 (AM + IM): Never smokers improved more over time.
Score A: Change in CLASI activity between visit 2 and visit 1.
Score B: Change in CLASI activity between visit 3 and visit 1.
Results
Within the lupus database, subjects with ACLE (n = 11), SCLE (n = 46), and CCLE (n = 116) had no significant difference in smoking status (p = 0.12). The CLE-only group consisted of 34% never smokers, 27% past smokers, and 39% current smokers. Never smokers represented a much higher proportion in the SLE + skin group (62% never; 12% past; 26% current), and differences between the SLE + skin and CLE-only groups were significant (Figure 1) (p = 0.0006).
Among CLE subjects with CLASI scores of 0 recorded at enrollment, there were no differences in smoking status (p = 0.13). Of the 149 subjects with active disease (CLASI > 0) at enrollment, 63 (42%) were never smokers, 36 (24%) were past smokers, and 50 (34%) were current smokers. Current smokers had significantly higher median CLASI scores at enrollment, 7.0 for never, 6.0 for past, and 9.5 for current (Figure 2) [p = 0.017, post-hoc Dunn’s test indicated significant differences between never and current smokers (p < 0.05) and past and current smokers (p < 0.05)].
Results of the quality of life analysis indicated that current smokers trended toward higher scores in all four subsets of the Skindex 29-3 (Figure 3). Differences were significant in the symptom subset between never and current (p < 0.05) and past and current (p < 0.01), as well as in the function subset between past and current (p < 0.05).
Among the 50 CLE subjects in the AM-only group, 17 were never smokers (34%), 14 (28%) were past smokers, and 19 (38%) were current smokers. Of the 57 CLE subjects in the AM + IM group, 29 (51%) were never smokers, 9 (16%) were past smokers, and 19 (33%) were current smokers (Table 1). As illustrated in Table 2, oral corticosteroids were the most commonly used IM, followed by methotrexate, azathioprine, and mycophenolate. It should be noted that subjects with SLE were much more likely to be included in the AM + IM group (67% versus 8% in the AM-only group, p < 0.0001). Additionally, of the 35 subjects on oral corticosteroids, 29 had a diagnosis of SLE (83%). When AM-only and AM + IM groups were combined, current smokers treated with hydroxychloroquine trended toward higher rates of quinacrine use than never and past smokers (p = 0.078) (Table 3). Non-smokers had significantly lower rates of concomitant quinacrine and hydroxychloroquine use as compared to current smokers (p = 0.041). Mean CLASI scores at enrollment for never smokers were 7.8 for AM-only, and 11.1 for AM + IM, for past smokers were 6.6 for AM-only, and 9.7 for AM + IM and for current smokers were 7.8 for AM-only, and 11.8 for AM + IM (p = 0.14, p > 0.05 for all post-hoc comparisons).
Table 4 summarizes the median CLASI change at each follow-up visit for both treatment groups. Of note, current smokers in the AM-only group had greater improvement than never or past smokers at both the 2–7 month and 8+ month follow-up visits (p = 0.02 and 0.24 respectively). In the AM + IM group, never smokers had greater improvement than past or current smokers at both the 2–7 month and 8+ month follow-up visit (p = 0.07 and 0.04 respectively).
Discussion
Previous studies have suggested that smoking is associated with CLE in general7, and DLE8, 9, tumid lupus3 and SCLE10 in particular. While our data indicate that ACLE, SCLE, and CCLE groups were not statistically different from each other in terms of smoking status, the fact that subjects in the CLE-only group had a higher percentage of current smokers than subjects with SLE + skin is of interest. A similar difference was also noted in a recently published prospective study.2 In this study, the authors found that 53.5% of CLE subjects with > 4 American College of Rheumatology (ACR) criteria for SLE were current smokers, versus 84.6% among CLE subjects with < 4 ACR criteria.2 Additional studies have shown that smoking may be associated with SLE,11, 12 and our data seem to suggest an even greater association with CLE.
The data presented in Figure 2 are in support of several recent papers that have suggested current smokers with CLE tend to have greater disease activity than non-smokers.3, 13 While a pathophysiologic mechanism for this difference has not been determined, several leading hypotheses were nicely summarized by Kreuter et al.3 Briefly, tobacco smoke has been shown to increase inflammatory cytokines, apoptosis, certain autoantibodies, the development of free radicals, microparticles, and is known to be phototoxic.14–20 Many of these same factors are associated with lupus, and they may be working separately or in tandem to increase CLE disease activity in current smokers as compared to non-smokers.
Current smokers with CLE also have worse quality of life than never or past smokers. While not all differences were statistically significant, current smokers had higher mean scores in all Skindex 29–3 subsets. This suggests that the statistically significant increase in CLASI scores among current smokers also has clinical relevance, as these subjects also had worse quality of life.
Subjects in the AM + IM treatment group were more likely to have SLE than in the AM-only group, and thus the additional IMs may have been added for increased systemic lupus activity, and not necessarily for skin activity specifically. However, by using the CLASI, it was possible to assess for cutaneous improvement irrespective of why lupus subjects were taking additional medications. Furthermore, 38% of subjects in the AM-only group were current smokers, compared with 33% in the AM + IM. This difference suggests that current smokers were not any more likely to be treated with additional immunomodulators, despite the fact that they were more often treated with hydroxychloroquine and quinacrine, rather than hydroxychloroquine alone.
Previous studies have shown that current smokers are more likely to have refractory disease than non-smokers,5 and that antimalarials may be less effective in CLE patients who smoke.3, 21–23 In contrast, a small prospective study in France examined 26 CLE patients treated with antimalarials, and found similar rates of smoking among responders and non-responders.24 Our results showed that current smokers did worse when treated with a combination of AMs and IMs, but when treated with AMs alone actually responded better than non-smokers. One possible explanation for this difference centers on the fact that mean CLASI scores at enrollment trended higher in the AM + IM group than AM-only, regardless of smoking status. It is possible that response to antimalarial therapy is worse among current smokers with higher CLE activity, but not necessarily when activity is lower. Further studies are necessary to test this hypothesis. Another possible explanation is that quantity of smoking has an effect on AM response. A weakness of the study presented here is that the amount of cigarettes smoked was not initially collected. Perhaps there is a threshold amount of cigarettes that need to be smoked over a given time period before differences in antimalarial response between smokers and non-smokers is manifested.
The data presented in this paper enhance the current literature by showing in a prospective cohort study that current smokers with lupus have worse disease, worse quality of life, and respond worse to treatment when requiring both antimalarials and immunomodulators. Taken together, these data further strengthen the need for smoking cessation among the cutaneous lupus population.
Acknowledgments
We would like to thank Dr. Lynne Taylor (Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, PA) for help with statistical analysis.
Funding/Support: This study is based upon work supported by the National Institutes of Health, including NIH K24-AR 18 02207 (Werth).
Footnotes
Financial Disclosure: Relevant disclosure: Copyright for CLASI is owned by the University of Pennsylvania. Professional Financial Relationships not relevant to manuscript (Werth): Consultancies include Pfizer, Novartis, Cephalon, Rigel, Medimmune. Grants (Werth) include Celgene and Amgen.
Author Contributions: Mr. Piette and Dr. Werth had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Piette and Werth. Acquisition of data: Piette, Foering, Chang, and Okawa.Analysis and interpretation of data: Piette, Chang, Foering, and Werth. Drafting of the manuscript: Piette. Critical revision of the manuscript for important intellectual content: Piette, Foering, Chang, Okawa, Ten Have, Feng and Werth. Statistical analysis: Piette, Ten Have, Feng. Obtained funding: Werth. Administrative, technical, or material support: Okawa. Study supervision: Piette, Chang, Foering, Okawa, and Werth.
Contributor Information
Evan W. Piette, Email: epiette@mail.med.upenn.edu.
Kristen P. Foering, Email: foeringk@mail.med.upenn.edu.
Aileen Y. Chang, Email: aileench@mail.med.upenn.edu.
Joyce Okawa, Email: Joyce.Okawa@uphs.upenn.edu.
Thomas R. Ten Have, Email: ttenhave@mail.med.upenn.edu.
Rui Feng, Email: ruifeng@mail.med.upenn.edu.
References
- 1.Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6):365–381. doi: 10.2165/11310780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Boeckler P, Cosnes A, Frances C, Hedelin G, Lipsker D. Association of cigarette smoking but not alcohol consumption with cutaneous lupus erythematosus. Arch Dermatol. 2009 Sep;145(9):1012–1016. doi: 10.1001/archdermatol.2009.199. [DOI] [PubMed] [Google Scholar]
- 3.Kreuter A, Gaifullina R, Tigges C, Kirschke J, Altmeyer P, Gambichler T. Lupus erythematosus tumidus: response to antimalarial treatment in 36 patients with emphasis on smoking. Arch Dermatol. 2009 Mar;145(3):244–248. doi: 10.1001/archdermatol.2008.592. [DOI] [PubMed] [Google Scholar]
- 4.Albrecht J, Taylor L, Berlin JA, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005 Nov;125(5):889–894. doi: 10.1111/j.0022-202X.2005.23889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moghadam-Kia S, Chilek K, Gaines E, et al. Cross-sectional analysis of a collaborative Web-based database for lupus erythematosus-associated skin lesions: prospective enrollment of 114 patients. Arch Dermatol. 2009 Mar;145(3):255–260. doi: 10.1001/archdermatol.2008.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [Accessed 11/16/2010]; www.cdc.gov/tobacco.
- 7.Boeckler P, Milea M, Meyer A, et al. The combination of complement deficiency and cigarette smoking as risk factor for cutaneous lupus erythematosus in men; a focus on combined C2/C4 deficiency. Br J Dermatol. 2005 Feb;152(2):265–270. doi: 10.1111/j.1365-2133.2004.06308.x. [DOI] [PubMed] [Google Scholar]
- 8.Miot HA, Bartoli Miot LD, Haddad GR. Association between discoid lupus erythematosus and cigarette smoking. Dermatology. 2005;211(2):118–122. doi: 10.1159/000086440. [DOI] [PubMed] [Google Scholar]
- 9.Gallego H, Crutchfield CE, 3rd, Lewis EJ, Gallego HJ. Report of an association between discoid lupus erythematosus and smoking. Cutis. 1999 Apr;63(4):231–234. [PubMed] [Google Scholar]
- 10.Koskenmies S, Jarvinen TM, Onkamo P, et al. Clinical and laboratory characteristics of Finnish lupus erythematosus patients with cutaneous manifestations. Lupus. 2008;17(4):337–347. doi: 10.1177/0961203307087403. [DOI] [PubMed] [Google Scholar]
- 11.Costenbader KH, Kim DJ, Peerzada J, et al. Cigarette smoking and the risk of systemic lupus erythematosus: a meta-analysis. Arthritis Rheum. 2004 Mar;50(3):849–857. doi: 10.1002/art.20049. [DOI] [PubMed] [Google Scholar]
- 12.Formica MK, Palmer JR, Rosenberg L, McAlindon TE. Smoking, alcohol consumption, and risk of systemic lupus erythematosus in the Black Women's Health Study. J Rheumatol. 2003 Jun;30(6):1222–1226. [PubMed] [Google Scholar]
- 13.Turchin I, Bernatsky S, Clarke AE, St-Pierre Y, Pineau CA. Cigarette smoking and cutaneous damage in systemic lupus erythematosus. J Rheumatol. 2009 Dec;36(12):2691–2693. doi: 10.3899/jrheum.090403. [DOI] [PubMed] [Google Scholar]
- 14.Freemer MM, King TE, Jr, Criswell LA. Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis. 2006 May;65(5):581–584. doi: 10.1136/ard.2005.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Placzek M, Kerkmann U, Bell S, Koepke P, Przybilla B. Tobacco smoke is phototoxic. Br J Dermatol. 2004 May;150(5):991–993. doi: 10.1111/j.1365-2133.2004.05818.x. [DOI] [PubMed] [Google Scholar]
- 16.Bijl M, Horst G, Limburg PC, Kallenberg CG. Effects of smoking on activation markers, Fas expression and apoptosis of peripheral blood lymphocytes. Eur J Clin Invest. 2001 Jun;31(6):550–553. doi: 10.1046/j.1365-2362.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- 17.Pryor WA, Stone K, Zang LY, Bermudez E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem Res Toxicol. 1998 May;11(5):441–448. doi: 10.1021/tx970159y. [DOI] [PubMed] [Google Scholar]
- 18.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002 May 1;89(9):1117–1119. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 19.Sellam J, Proulle V, Jungel A, et al. Increased levels of circulating microparticles in primary Sjogren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2009;11(5):R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Yu D, Williams KJ, Liu ML. Tobacco smoke induces the generation of procoagulant microvesicles from human monocytes/macrophages. Arterioscler Thromb Vasc Biol. 2010 Sep;30(9):1818–1824. doi: 10.1161/ATVBAHA.110.209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jewell ML, McCauliffe DP. Patients with cutaneous lupus erythematosus who smoke are less responsive to antimalarial treatment. J Am Acad Dermatol. 2000 Jun;42(6):983–987. [PubMed] [Google Scholar]
- 22.Hugel R, Schwarz T, Glaser R. Resistance to hydroxychloroquine due to smoking in a patient with lupus erythematosus tumidus. Br J Dermatol. 2007 Nov;157(5):1081–1083. doi: 10.1111/j.1365-2133.2007.08178.x. [DOI] [PubMed] [Google Scholar]
- 23.Rahman P, Gladman DD, Urowitz MB. Smoking interferes with efficacy of antimalarial therapy in cutaneous lupus. J Rheumatol. 1998 Sep;25(9):1716–1719. [PubMed] [Google Scholar]
- 24.Lardet D, Martin S, Truchetet F, Cuny JF, Virion JM, Schmutz JL. Effect of smoking on the effectiveness of antimalarial drugs for cutaneous lesions of patients with lupus: assessment in a prospective study. Rev Med Interne. 2004 Nov;25(11):786–791. doi: 10.1016/j.revmed.2004.07.005. [DOI] [PubMed] [Google Scholar]