Abstract
Mini-chromosome maintenance (Mcm) proteins are part of the replication licensing complex that is loaded onto chromatin during the G1-phase of the cell cycle and required for initiation of DNA replication in the subsequent S-phase. Mcm proteins are typically loaded in excess of the number of locations that are utilized during S-phase. Nonetheless, partial depletion of Mcm proteins leads to cancers and stem cell deficiencies. Mcm2 deficient mice, on a 129Sv genetic background, display a high rate of thymic lymphoblastic lymphoma. Here array comparative genomic hybridization (aCGH) is utilized to characterize the genetic damage accruing in these tumors. The predominant events are deletions averaging less than 0.5 Mb, considerably shorter than observed in prior studies using alternative mouse lymphoma models or human tumors. Such deletions facilitate identification of specific genes and pathways responsible for the tumors. Mutations in many genes that have been implicated in human lymphomas are recapitulated in this mouse model. These features, and the fact that the mutation underlying the accelerated genetic damage does not target a specific gene or pathway a priori, are valuable features of this mouse model for identification of tumor suppressor genes. Genes affected in all tumors include Pten, Tcfe2a, Mbd3 and Setd1b. Notch1 and additional genes are affected in subsets of tumors. The high frequency of relatively short deletions is consistent with elevated recombination between nearby stalled replication forks in Mcm2 deficient mice.
Keywords: DNA replication, licensing factor, dormant origins, lymphoblastic lymphoma, Mcm2
Introduction
Minichromosome maintenance protein (Mcm) deficiency or hypomorphic function in mice has been shown to result in high rates of cancer where the specific cancer type shows a strong dependence on the genetic background on which the deficiency occurs (Shima et al., 2007; Pruitt et al., 2007; Kunnev et al., 2010; Chuang et al., 2010). Mcm deficiency has been linked to increased rates of genetic damage in several studies. The Mcm4Chaos3 mutation was identified on the basis of a chromosomal instability phenotype (Shima et al., 2007). Further, abnormal karyotypes and elevated levels of chromosome breaks occur in cultured cells in which Mcm expression has been suppressed (Ge et al., 2007; Ibarra et al., 2008; Orr et al., 2010) and elevated rates of LOH are observed in clonal neural stem cells derived from Mcm2 deficient mice (Kunnev et al., 2010).
Mcm proteins are a family of replication licensing proteins where Mcm’s 2–7 form a hexameric complex that is recruited to the chromatin early in the G1-phase of the cell cycle. The complex is thought to function, during the subsequent S-phase, as the replicative helicase. This function is important in both the initial unwinding of DNA at origins of replication and for fork extension (e.g. Blow and Dutta, 2005). However, in cells in culture reduced Mcm concentration does not affect the rate of fork elongation but does influence the ability of cells to initiate replication at dormant (lower efficiency) origins following treatment with agents that induce fork stalling (Ge et al., 2007; Ibarra et al., 2008; Kunnev et al., 2010). Hence, elevated levels of genetic damage in cells in culture and increased cancer rates in Mcm deficient mice may result from inefficient prereplicative complex assembly or origin usage.
In this study a strain of mice (Mcm2IRES-CreERT2, referred to as Mcm2def in this report) in which Mcm2 is expressed at approximately one third of wild type levels (Pruitt et al., 2007) is used to define the sites of genetic damage in the tumors that arise in these mice by aCGH.
Results
aCGH analysis of thymic tumors arising in Mcm2 deficient mice
Mcm2def mice develop thymic tumors with 100% penetrance when carried on a 129/Sv genetic background (Pruitt et al., 2007; Kunnev et al., 2010). The disease is frequently disseminated affecting multiple lymph nodes, the spleen and other organs. That the tumors arising in these mice are from the T-cell lineage was confirmed by staining for CD3 (T-cell) and B220 (B-cell) markers (Supplemental Figure 1). The majority of cells present in these tumors and at disseminated sites (not shown) are CD3 positive and B220 negative consistent with T-lymphoblastic lymphoma.
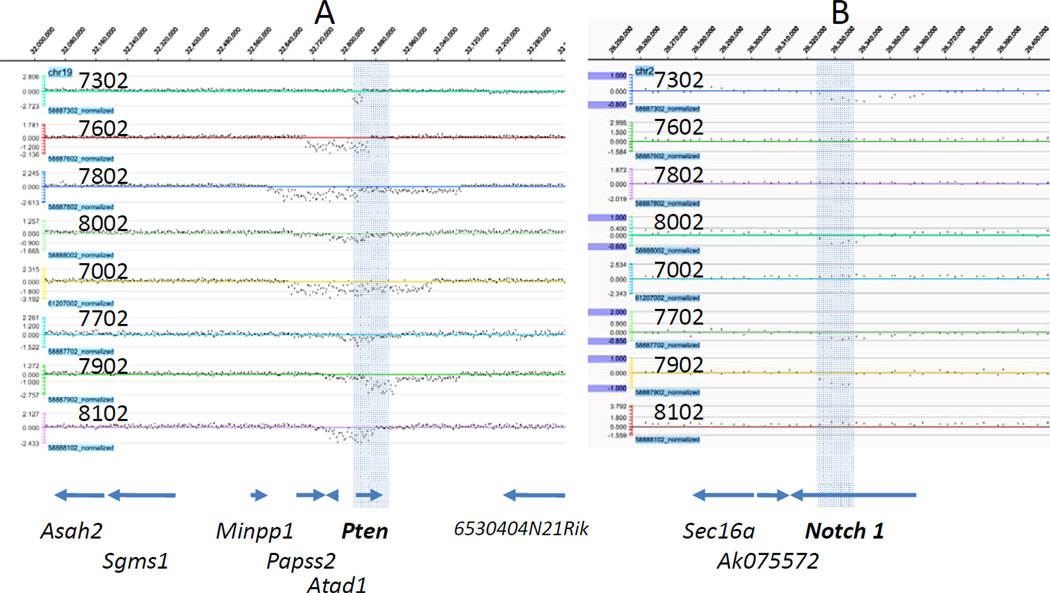
Nimblegen 720K whole-genome tiling arrays were used to characterize copy number changes across the genome in 8 tumors arising in homozygous Mcm2def mice on 129/Sv (6) or 129/Sv;C57Bl/6 F1 (2) genetic backgrounds. Additionally, DNA from paired non-tumorous tissue (tail) was examined for mice from which three of the tumors on the 129/Sv genetic background were derived. All 129/Sv derived samples were assessed relative to the same reference DNA from a wt 129/Sv littermate and 129/Sv;C57Bl/6 samples were similarly assessed relative to DNA from a single wt litter mate from an F1 cross of heterozygous Mcm2 deficient transgenic mice on the different strains. Analysis of non-tumorous tissue identified two short regions of copy number loss in a subset of mice (within the wdr5 and nlgn1 genes). The Mcm2 deficiency transgene is carried on the 129/Sv and other backgrounds as heterozygotes and it is unclear if the transgene contributes to heterogeneity within the strains. Nonetheless the large majority of CNVs seen in tumors were not found in non-tumorous tissue suggesting that they were somatically derived. A summary of all haploid and diploid copy number variations (CNVs) for each tumor are shown mapped to individual chromosomes in Supplemental Figure 2. Higher resolution data for two regions, the Pten gene on chr 19 and the notch1 gene on chr2, are shown in Figure 1.
Figure 1.
aCGH for Pten and notch 1 containing regions of Chr19 and Chr2. Array CGH signal tracks (log2 ratios) for each of eight tumors as indicated by the tumor number are shown for the Pten region of Chr19 (Panel A) and the notch1 region of Chr2 (Panel B). In each case the positions of genes as defined by the UCSC genome browser gene track from the mm9 sequence build is aligned below the aCGH tracks. The shaded region in A denotes the location of Pten across all tracks and the shaded region in B denotes the location of exons 5–22 of notch1 across all tracks.
There are several general features of the CNVs occurring in these tumors that differ from those observed in prior studies in which CNVs were characterized from tumors arising in genetically engineered mice carrying other tumor promoting mutations (e.g. Maser et al., 2007; De Keersmaecker et al., 2010). First, the number of genetic changes resulting in deletions (avg. 20.6 per tumor) far exceeds those resulting in amplifications (avg. 2.2 per tumor); whereas in prior studies CNVs are typically distributed evenly between deletions and amplifications. Second, the intervals covered by the deletions found here are substantially smaller than those observed in prior studies in which the average size ranged between several and tens of Mb’s. In contrast, the average deletion in thymic lymphomas arising on Mcm2 deficient mice is ~464 kb. Finally, there is a high degree of overlap between the CNVs occurring in different specific tumors. These features of the genomic changes occurring in tumors arising in Mcm2 deficient mice allow identification of genetic lesions contributing to tumorigenesis at high resolution using a minimal number of animals.
From the eight tumors analyzed, 21 minimal common deleted regions (MCDR) averaging 68.5 kb and 5 minimal common amplified regions (MCAR) averaging 0.40 Mb were identified (Figure 2). [MCRs were also identified in the TCR-α (Chr14), TCR-β (Chr6), and TCR-γ (Chr13) genes as is expected due to normal biological function and these MCRs are not included in the totals).] The size of the common regions is sufficiently small that in 12 of 21 MCDRs, and 4 of 5 MCARs, a single gene could be localized to the common intervals. Further, the pattern of deletions over 5 of the remaining MCDRs is consistent with the presence of two genes within the interval that contribute independently to selection for tumorigenic growth and, in these cases, 9 specific genes can be uniquely localized. Only 6 MCDRs of 21 contain more than a single candidate gene where one of these intervals includes only Cdkn2a and Cdkn2b. In total, 25 specific genes in unique-gene MCRs and 27 candidate genes distributed over 6 multi-gene MCRs were identified. These 52 genes were assessed for involvement in human cancers using the Catalogue of Somatic Mutations in Cancer (cosmic) and 70% of the MCRs, and 50% of the total genes, were shown to have previously identified mutations in human cancers. These observations provide insight into both the mechanism by which Mcm2 deficiency induces genetic damage and the genes and pathways contributing to T-lymphoblastic lymphomas.
Figure 2.
Chart of minimal common region intervals and candidate genes. The minimum common intervals of CNVs determined by aCGH are shown where the tumors exhibiting the CNVs are marked. CNV within MCRs 6 and 11 (marked with asterisks) were also found in a paired non-tumorous tissue from the same animals and may result from genetic heterogeneity within the strain.
Confirmation of deletions by sequencing fusion junctions
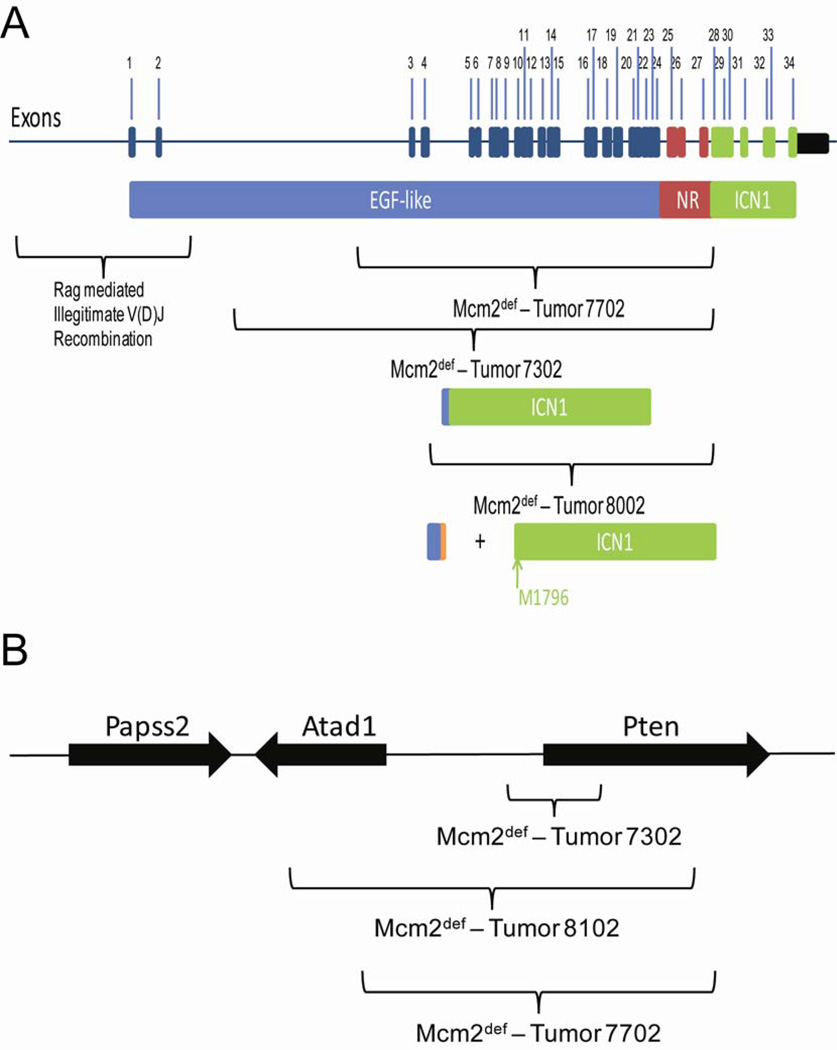
To confirm that chromosomal deletions identified by aCGH are present in tumors, oligonucleotide primers spanning predicted deleted regions from the notch1 and Pten loci were used to generate amplicons containing breakpoint junctions from tumor DNAs by PCR. In the case of notch1, amplicons containing junction fragments were obtained and sequenced for 3 tumors. The location of 5’ and 3’ junctions for the deletions identified in each of these tumors is shown on a map of the notch1 gene in Figure 3, panel A. Tumors 7302 and 7702 each carry deletions resulting in in-frame fusions between exons 2 and 28. The predicted proteins resulting from these fusions carry a truncated and likely non-functional EGF-like domain fused to a near complete notch1 intracellular domain (ICN1) and are likely to result in constitutively activate notch signaling. The deletion in tumor 8002 occurs between a location within exon 4 and 5’ to exon 28 resulting in a prematurely truncated reading frame. Translation initiating at the normal start site would encode a polypeptide consisting of only EGF-like domains. However, prior studies have demonstrated the presence of a cryptic promoter within exon 29 (Jeannet et al. 2010) and transcripts initiating at this promoter are predicted to initiate translation at methionine 1796, which would again result in an activated notch1 intracellular domain. The locations at which notch1 deletions occur in tumors arising on Mcm2 deficient mice are not consistent with the illegitimate V(D)J recombination events (as indicated in Figure 3, panel A) that are frequently observed in other mouse models of T-ALL.
Figure 3.
Locations of deletion breakpoints at notch1 and Pten loci defined by sequencing breakpoint junctions. Panel A shows the notch1 locus where exons of the uninterrupted gene are indicated in the top portion and colored to indicate the different domains of the protein that are encoded by each exon. Blue denotes the EGF-like repeats, red denotes the negative regulatory (NR) region, and green denotes the notch1 intracellular domain (ICN1). Sites at which rag mediated illegitimate recombination occur are shown as marked on the map. The regions spanned by deletions in tumors arising in Mcm2 deficient mice are also marked where the predicted domains encoded by the resulting transcripts are indicated. In the case of tumors 7702 and 7302 in frame fusions between exons 2 and 28 are expected to result in a protein missing the majority of the EGF repeats and the NR region but containing the ICN1 domain. In the case of tumor 8002, the deletion results in a predicted truncation of the protein encoded by transcripts initiating at the normal promoter which may enhance expression from a previously identified internal promoter in exon 29 and expression of the ICN1 domain from methionine 1796 as has been observed in a prior study (Jeannet et al., 2010). Panel B shows the locations of deletions defined by sequencing amplicons containing breakpoint junctions from the Pten locus. In this case individual exons are not marked.
Junctions resulting from Pten deletions were also defined for several tumors (Figure 3, panel B). In each of these cases, the deletions are expected to result in loss of Pten function. Comparison of the sequences present at the 5’ and 3’ breakpoints of notch1 and Pten deletions is shown in Figure 4. There is no evidence for micro-homologies between the 5’ and 3’ breakpoints. In one Pten deletion a 61nt non-templated insertion is present. On average the sequences flanking the breakpoints are ~57% AT in composition and homo-polymeric A or T runs are found in several of the sequences.
Figure 4.
Sequences flanking breakpoints for deletions at the notch1 and Pten loci. 50 nt of the normal 5’ and 3’ flanking sequence relative to the deletion breakpoints (downward arrows) are shown for 3 notch1 and 3 Pten deletions where both the 5’ and 3’ breakpoints are shown for each tumor as indicated. A and T residues are colored red. The sequence of a non-templated 61 nt insertion found at the Pten breakpoint from tumor 7702 is also given.
Genes and pathways implicated in T-LL’s arising on Mcm2 deficient mice
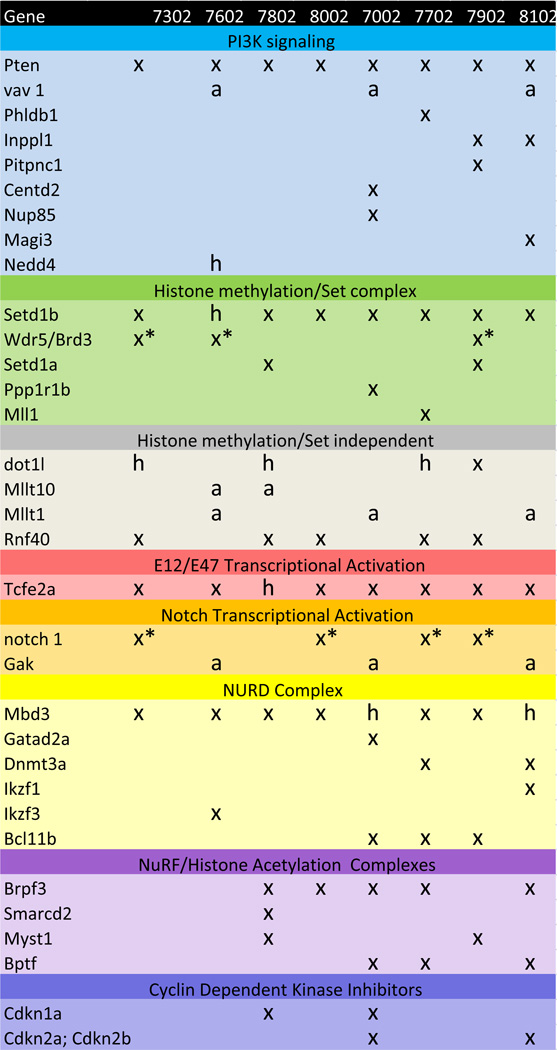
The most frequently occurring CNVs are deletions on Chr19 that overlap the Pten gene. All of the tumors assessed contain a diploid deletion overlapping Pten and the MCDR defined by these CNVs includes only this gene (Figure 1, panel A). Pten is a plasma-membrane lipid phosphatase that antagonizes the PI3K (phosphoinositide 3 kinase)-AKT pathway (reviewed in Buckler et al., 2008). Despite the bi-allelic loss of the Pten gene in all of the tumors assessed, subsets of tumors exhibit additional mutations in a large number of genes that either modulate PI3K activity or are PI3K targets. These include bi-allellic deletion of the PI3K inhibitors Inppl1 and Pitpnc1(Ship2) and the amplification of the PI3K target Vav1 (Figure 5).
Figure 5.
Abbreviated chart of pathways implicated by genes present in MCR deletions and amplifications and complementing non-recurrent CNVs. X, bi-allelic deletion; h, mono-allelic deletion; a, amplification; x*, short internal deletions affecting exons 5–23 in notch1 and intron 1 in Wdr5. In the case of Wdr5, the CNV was also present in paired non-tumorous tissue and may represent heterogeneity within the strain. A complete chart including chromosomal locations, gene function and additional MCRs that do not fall into the molecular pathways indicated here is included in Supplemental Figures.
A second frequent set of mutations affects Tcfe2a that encodes the E-box binding proteins E12 and E47. Seven of the eight tumor samples exhibit bi-allelic loss of Tcfe2a and the eighth (7802) shows loss of one allele. E2A activity is known to modulate the cell cycle during lymphoid cell maturation where Cdkn1a (p21cip1) is a key target (Yang et al., 2008). Two tumors, including 7802, exhibit bi-allelic loss of Cdkna1 and this loss may complement the mono-allelic loss of E2A in this tumor (Figure 5).
Mbd3 is closely linked to Tcfe2a on Chr10 and many of the same deletion events that result in loss of Mbd3 also affect Tcfe2a. Nonetheless, in the case of tumor 7802, which shows only mono-allelic loss of Tcfe2a, bi-allelic loss of Mbd3 has occurred. This observation suggests that loss of Mbd3 can, independently, provide a selective advantage in T-LLs. Consistent with this interpretation, both tumor 7002 and tumor 8102, in which bi-allelic loss of Tcfe2a and mono-allelic loss of Mbd3 is observed, show loss of additional genes related to Mbd3 function. Mbd3 is a component of the Mi2-NuRD complex which functions in chromatin remodeling through histone-deacetylase activity. Mi2-NuRD has been shown to play a role both in maintaining hematopoietic stem cell pools and in normal lineage progression (Ramírez and Hagman, 2009). In addition to Mbd proteins and histone deacetylases, the Mi2-NuRD complex is comprised of a number of additional proteins including Gatad2a (p66 alpha; Brackertz et al., 2002). In lymphocytes, the zinc finger DNA-binding factors Ikaros (Ikzf1), Aiolos (Ikzf3) and/or Helios (Ikzf2) are also associated with the complex and may function to impart DNA sequence specificity to Mi-2/NuRD complexes (Ramírez and Hagman, 2009). Tumor 7002 shows bi-allelic loss of Gatad2a, which is specific to this tumor (Figure 5). Similarly, tumor 8102 shows bi-allelic loss of Ikzf1, which again is specific to this tumor. Dnmt3a has also been reported to associate with the Mi-2/NuRD complex and tumor 8102 shows bi-allelic loss of this gene as well. Despite the bi-allelic loss of Mbd3 function, Dnmt3a is also lost in tumor 7702 and Ikzf3 is lost in tumor 7602, demonstrating redundancy in targeting of this pathway similar to the situation for PI3K signaling.
A third gene that undergoes bi-allelic loss in at least seven of the eight tumors analyzed is the histone H3-Lys4 methyltransferase Setd1b. Mono-allelic loss of this gene is also found in the remaining tumor (7602). Additional related family members include Setd1a, MLL and the interacting protein Wdr5. CNVs in these genes are found in a subset of the tumors in which Setd1b is also lost. Setd1 complexes are localized to euchromatic regions of the genome, but different Setd1 family members are localized to non-overlapping domains suggesting that their functions are non-redundant. An additional cluster of mutations that may affect processes related to the Setd1 complex mutants occurs in components of the BAF and NURF complexes that link histone methylation to chromatin remodeling. Brpf3 function is compromised in each of the five tumors that fail to show an alteration in Wdr5 (which as discussed above may be compromised in the germ line of a subset of the mice in this line). Additional chromatin remodeling complex components (Smard2, Myst1, Mllt1, and Bptf) are also compromised in smaller subsets of tumors (Figure 5).
Bi-allelic deletions in Rnf40 (Bre1) that are also likely to affect chromatin structure and gene activity are found in 5 of the 8 tumors (Figure 5). Rnf40 is recruited to active genes and is the E3 ligase for H2B ubiquitylation (Kim et al., 2009). H2B ubiquitylation has been shown to directly stimulate both hDOT1L-mediated H3K79 methylation and hSET1 complex-mediated H3K4 di- and tri-methylation and may play a role in maintaining an open chromatin configuration in active genes following RNA polymerase passage.
The notch signaling pathway is well established to contribute to T-cell lymphomas and activated forms of notch 1 are found in more than 50% of human tumors. CNVs are found in notch 1 in four of the eight thymic lymphomas assayed here (Figure 3, Panel B, and Figure 5). As discussed above, in each of 3 cases confirmed by sequencing an internal deletion consistent with the generation of activating notch mutations has occurred. In three of the four remaining tumors which do not carry a CNV in notch 1, amplification of cyclin G-associated kinase (Gak) occurs (Figure 5). Gak is an auxilin related gene that has been shown to enhance notch signaling in zebrafish (Bai et al., 2010) and may play a role is elevating notch signaling in tumors in the present study. Cdkn1a is repressed by notch and this repression has been shown to mediate much of the effect of notch on the cell cycle. Tumor 7802, in which both notch and Gak genes are unaffected, carries a bi-alleleic deletion of Cdkn1a.
Several additional recurrent CNVs that are not shown in Figure 5 have also been identified and are provided in Supplementary Figure 3. These include the gene encoding the centromere associated protein Cenp-o, on Chr12, the cGMP activated phosphodiesterase Pde2a on Chr7, Wasf2 (Wave2) on Chr4 that mediates signals from tyrosine kinase receptors and small GTPases to the actin cytoskeleton, septin 9, which plays a role in filament assembly and potentially affects multiple cellular processes including cell division and chromosome segregation, and reticulon 4 like receptor which is known to function in the nervous system but is also expressed in the thymus. Two additional transcription factors, Zfand3 on Chr17 and Rfxdc2 (Rfx7) on Chr9, are also identified by recurrent bi-allelic deletions. Zfand3 is a zinc-finger protein containing a poly-ubiquitin binding domain and has been implicated in cellular differentiation of ES cells (Nishiyama et al., 2009). Rfxdc2 is a ubiquitously expressed winged-helix class transcription factor of unknown function (Aftab et al., 2008). Finally, recurrent diploid deletions occur in regions of Chr12 (108,379,999-108,459,999) and ChrX (132,813,333-132,946,667) and include locations where there are no known genes but at which predicted genes are located.
Comparison of CNVs in T-LLs arising in Mcm2 deficient mice with CNVs in human T-ALLs
To examine the relationship between the CNVs occurring in T-LLs of Mcm2 deficient mice with those found in prior studies of human T-ALLs, the locations of MCRs for recurrent CNVs from three prior studies (Kuiper et al., 2007; Mullighan et al., 2007; Remke et al., 2009) in which CNVs were examined for either 7, 50 or 73 human (largely childhood) T-ALLs were compared to the locations of genes identified by MCRs of recurrent CNVs in mouse tumors (Table I). As in the mouse tumors studied here, deletions affecting the locus encoding Cdkn2a and Cdkn2b genes and the Pten gene are identified in human T-ALLs. Comparison of the mouse and human data sets has also identified locations on human Chr19 p13.3 and Chr19p13.2 that contain amplifications near to the location of genes that are also affected in mouse tumors. This region of the human genome is syntenic with four chromosomal locations in the mouse from Chr10, Chr17, Chr9 and Chr8 two of which show CNV in the present study (Supplementary Figure 4). The syntenic region of mouse Chr17 includes the region between Mtl1 and Vav1 and shows recurrent amplification in the mouse tumors. The syntenic region of mouse Chr10 spans the region from Mbd3-Sirt6 and includes the Tcf-e2a gene. As discussed above, Mbd3 and Tcf-e2a are sites of highly recurrent deletions in the mouse tumors. Whether amplification of the Mbd3 or Tcf-e2a genes in human cells compromises their functions is at present unclear. Additionally, the region of human Chr19 that is syntenic to mouse Chr9 contains a series of MBD3 related genes, MBD3L2–5. Two related family members, Mbd3l1 and Mbd3l2, are found on Chr9 in the mouse but are not affected by CNVs in mouse tumors. Whether the amplifications observed in humans affects the MBD3L2–5 genes to compromise the same pathway as Mbd3 deletion in the mouse is at present unclear but may be important to investigate in additional studies. Further, these observations illustrate the potential effects that the different organizations of the mouse and human genomes may have on the types of genomic alterations that are necessary to result in tumorigenesis even though similar pathways may be affected.
Table I.
Comparison of MCRs between mouse and human T-LLs
| Chr | Start | End | Size | Genes in Interval |
Gain/Lo ss |
Frequency in %(haploid /diploid) |
Position in Human Genome (hg17) |
Ch Band |
Remke et al. (2009) | Mullighan et al., (2007) | Kuiper et al., (2007) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| chr4 | 1.33E+08 | 1.33E+08 | 0 | Wasf2 | Loss | 25/25 | chr1:27,732,126-27,816,669 | 1p36.11 | |||
| 1.33E+08 | 1.33E+08 | 5710 | shp | Loss | 37/37 | chr1:27,237,976-27,240,567 | |||||
| chr12 | 4219999 | 4219999 | 0 | Cenpo | Loss | 50/50 | chr2:25,016,333-25,042,784 | 2p23.3 | |||
| 3859999 | 3899999 | 40000 | Dnmt3a | Loss | 25/25 | chr2:25,455,846-25,564,774 | |||||
| chr3* | 25415559 | 25456523 | 40964 | Nlgn1 | Loss | 50/50 | chr3:173,116,244-174,001,116 | 3q26.31 | |||
| chr5 | 1.09E+08 | 1.09E+08 | 414882 | Gak | Gain | 37.5 | chr4:843,066-926,174 | 4p16.3 | |||
| chr17 | 30287365 | 30181394 | −105971 | Zfand3 | Loss | 50/50 | chr6:37,787,307-38,122,397 | 6p21.2 | |||
| 30474260 | 30579999 | 105739 | Btbd9 | Loss | 25/25 | chr6:38,136,228-38,607,924 | |||||
| chr17 | 28829446 | 29219999 | 390553 | Mapk14 Mapk13 Brpf3 Pnpla1 4930539E 08Rik Pxt1 Kctd20 Stk38 Sfrs3 AK144811 |
Loss | 50/50 | chr6:35,995,454-36,572,243 | 6p21.31 | |||
| chr4 | 88899999 | 89059999 | 160000 | Cdkn2a; Cdkn2b |
Loss | 25/25 | chr9:21,967,752-22,009,312 | 9p21.3 | 9p21.3(Loss);45/72(63%) | 9p21.3(Loss); 36/50 (72%) | 9p21.3(Loss); 5/7 (71%) |
| chr2* | 27349578 | 27363138 | 13560 | Wdr5 | Loss | 37.5/37.5 | chr9:137,001,210-137,025,093 | 9q34.2 | |||
| chr2 | 26324605 | 26337808 | 13203 | notch 1 | Loss | 50/0 | chr9:139,388,897-139,440,238 | 9q34.3 | |||
| chr2 | 17972355 | 17988715 | 16360 | Mllt10 | Gain | 25 | chr10:21,823,574-22,032,555 | 10p12.31 | |||
| chr19 | 32819999 | 32859999 | 40000 | Pten | Loss | 100/100 | chr10:89,623,195-89,728,531 | 10q23.31 | 10q23.2-23.31;4(Loss)/72(6%) | 10q23.31(Loss); 3/50 (6%) | |
| chr7 | 1.09E+08 | 1.09E+08 | −80001 | Pde2a | Loss | 37.5/37.5 | chr11:72,287,186-72,385,494 | 11q13.4 | |||
| chr5 | 1.24E+08 | 1.24E+08 | 15244 | Setd1b | Loss | 100/87.5 | chr12:122,242,630-122,270,561 | 12q24.31 | |||
| chr12 | 1.09E+08 | 1.09E+08 | 40000 | Bcl11b | Loss | 37.5/37.5 | chr14:99,635,627-99,737,822 | 14q32.2 | |||
| chr9 | 72339999 | 72419999 | 80000 | Rfxdc2 | Loss | 37.5/37.5 | chr15:56,379,479-56,394,449 | 15q21.3 | |||
| chr7 | 1.35E+08 | 1.35E+08 | 116874 | Phkg2 Gm166 Rnf40 |
Loss | 62.5/62.5 | chr16:30,759,737-30,786,536 | 16p11.2 | |||
| chr11 | 75019999 | 75099999 | 80000 | Rtn4rl1 | Loss | 37.5/37.5 | chr17:1,837,973-1,928,178 | 17p13.3 | 17p13.3-11.2(Loss); 2/50 (4%) | ||
| chr11 | 97779999 | 97859999 | 80000 | Plxdc1 | Loss | 25/25 | chr17:37,219,556-37,307,902 | 17q12 | |||
| chr11 | 98179999 | 98259999 | 80000 | Neurod2 Ppp1r1b Stard3 titin-cap Pnmt Perld1 |
Loss | 25/25 | chr17:37,760,022-37,37,844,310 | 17q12 | |||
| chr11 | 1.07E+08 | 1.07E+08 | 39999 | Pecam1 | Loss | 25/25 | chr17:62,399,864-62,401,205 | 17q23.3-q24.2 | 17q23.3(Gain);4/73 (5%) | ||
| 1.07E+08 | 1.07E+08 | 159999 | Smurf2 Kpna2 Bptf |
Loss | 25/25 | chr17:62,540,735-65,900,956 | |||||
| chr11 | 1.17E+08 | 1.17E+08 | 276592 | Septin-9 | Loss | 25/25 | chr17:75,277,492-75,496,676 | 17q25.2-q25.3 | |||
| chr10 | 79899999 | 79939999 | 40000 | Tcfe2a | Loss | 87.5/100 | chr19:1,609,293-1,650,286 | 19p13.3 and 19p13.2 | 19p13.3(Gain);7/73 (10%) 19p13.2(Gain);4/73 (5%) | ||
| Mbd3 | Loss | 100/75 | chr19:1,527,678-1,543,652 | ||||||||
| chr17 | 55952968 | 57447177 | 1494209 | Mllt1 Chaf1a Uhrf1 Dennd1c vav 1 |
Gain | 37.5 | chr19:6,210,393-6,,857,371 | ||||
| chr2 | 1.53E+08 | 1.53E+08 | 9248 | Asxl1 | Gain | 37.5 | chr20:30,946,153-31,027,121 | 20q11.21 | |||
| chrX | 1.66E+08 | 1.66E+08 | 60271 | Mid1 | Gain | 37.5 | chrX:10,413,597-10,851,809 | Xp22.2 |
Also present in a subset of non-tumorous control tissues.
One feature that is apparent from data on human tumors is that, with the exception of the CNVs affecting the CDKN2A and CDKN2B genes, even recurrent CNVs in human tumors affect only a small subset, less than 10%, of tumors (Mullighan et al., 2007; Kuiper et al., 2007; Remke et al., 2009). Since only 8 mouse tumors were assessed in the present study, diploid deletion in even one tumor represents a rate of occurrence of 12.5%, higher than most of the recurrent CNVs identified in human T-ALLs. If these cases are also considered, genes affected in mouse tumors that are also present at sites of CNV in the human data sets include Ikzf1(human chr 7p12.2) and Mll1 (human chr 11q23.3).
Non-recurrent haploid deletions are shorter than average
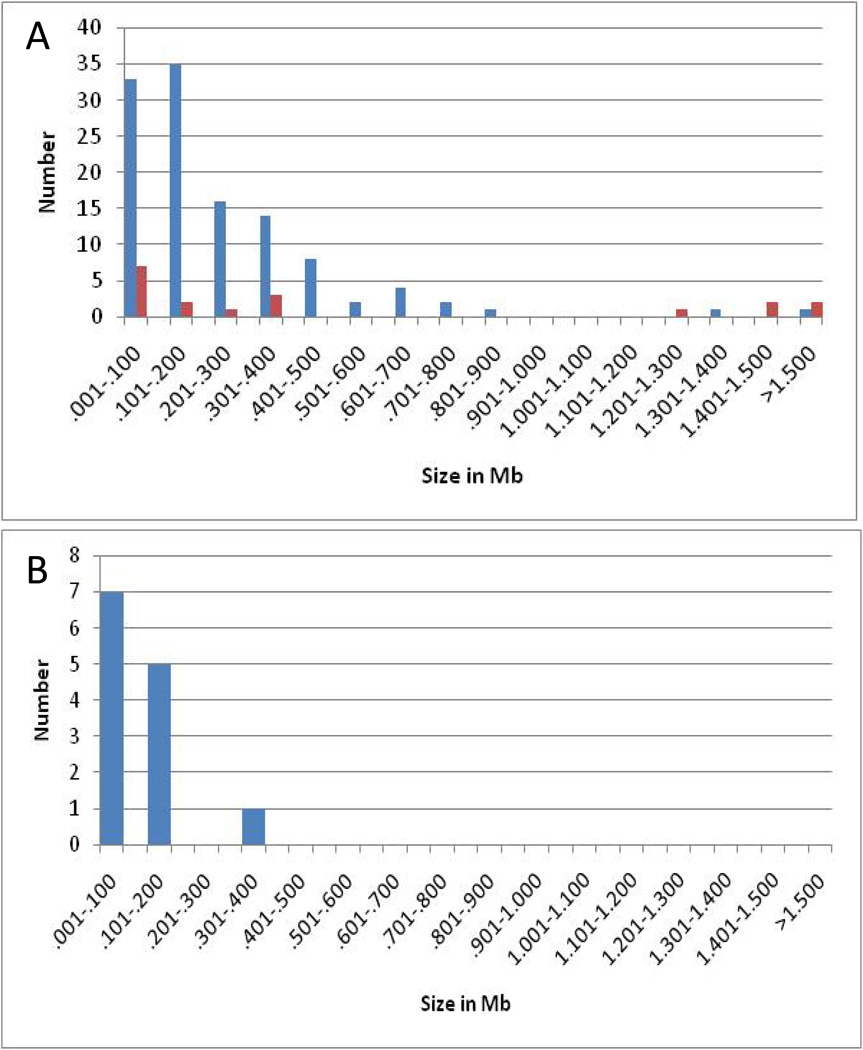
A subset of CNVs found in the mouse tumors described here was non-recurrent and exhibited an average log2 ratio consistent with deletion of one chromosomal copy (<0.4). A few of these events occurred in genes that could be related to pathways implicated in tumorigenesis (e.g. Nedd4) and these were removed from further consideration. A total of 13 non-recurrent, haploid, deletion events that occurred in regions that either contain no known gene or contained a gene(s) unlikely to be related to tumorigenesis were identified and are considered to represent passenger mutations. Since passenger mutations occur in cells prior to selection for genes driving tumorigenesis, this subset of CNVs may most closely reflect the effects of Mcm2 deficiency on genome stability. A comparison of the size distribution of the putative passenger mutations (panel B) with those of all deletions or amplifications (panel A) is shown in Figure 6. This comparison shows that the subset of deletions identified as passenger mutations is even shorter than the average of all deletions (average size 145 kbp compared to 464 kbp).
Figure 6.
Size distributions for different classes of CNVs. The frequency of CNVs of varying sizes is plotted for all deletions (blue) and amplifications (red) in panel A and for a subset of 13 non-recurrent deletions that did not contain genes related to any known oncogenic pathway and are considered to be passenger mutations in panel B.
Discussion
Several studies have demonstrated that reduced Mcm concentrations result in elevated genetic damage (Shima et al., 2007; Pruitt et al., 2007; Ge et al., 2007; Ibarra et al. 2008; Orr et al., 2010; Kunnev et al., 2010). The present study extends this observation by showing that the genetic damage accruing in tumors arising in Mcm2 deficient mice exhibits an unusual pattern of chromosomal damage in which the majority of chromosomal aberrations are deletions and in which the average size of the deletions is substantially shorter than those observed in other mouse cancer models including those that develop a high proportion of T-LLs (e.g. Maser et al., 2007).
One mechanism that could account for both the increased genomic instability and the accumulation of shorter deletion intervals in Mcm2def mice is if deletions are frequently a consequence of recombination between two, nearby, stalled replication forks. Specifically, under conditions of Mcm deficiency both the frequency of initiation at primary origins is expected to be reduced, increasing the likelihood of replication fork stalling, and the ability to overcome double stalled replication forks by reinitiation at an otherwise dormant origin is compromised, potentially resulting in fork collapse (Blow et al., 2011). Replication of different regions of the genome is segmented during S-phase such that active replication origins are clustered and replicated together within replication factories that consist of between 4 and 20 replication forks (i.e. 2 – 10 origins where the average distance between origins is on the order of 50–100 kb). Dormant origin function is tightly linked to this organization through the DNA damage checkpoint kinases ATR and Chk1 (Ge and Blow, 2010; Blow et al., 2011). ATR and Chk1 inhibit the activation of new replication factories but allow dormant origins to fire within already active factories. One consequence of this organization is to focus dormant origin firing to active factories where rescue of stalled replication forks is required. Additionally, partitioning replication into relatively short domains that are physically and temporally discrete minimizes the possibility of interaction between two locations at which replication fork stalling has occurred, reducing the likelihood of recombination. It has been observed that there is a critical minimum threshold of between ~35 and 50% reduction in Mcm concentration at which phenotypic effects including cancer incidence are greatly accelerated (Chuang et al., 2010). The sizes of the deletions observed in tumors of Mcm2 deficient mice, where Mcm2 is expressed at a level just below this threshold, are consistent with recombination events within single replication factories and support the possibility that the threshold at which phenotypic effects of Mcm reduction are observed is a consequence of breaching this physical separation (Figure 7).
Figure 7.
Potential relationship between replicon organization and the genetic lesions produced on replication fork stalling. Replication factories are indicated by green circles, chromosomal DNA is indicated by the line running through the circles, active replicons are indicated by bubbles within the lines and sites of un-recovered stalled replication forks are indicated by the red X’s. It is hypothesized that the frequency of stalled replication forks is increased by Mcm deficiency and that when the increase is sufficient that two or more stalled forks arise within a single replication factory there is an increase in the likelihood that recombination between stalled forks will occur (indicated by dashed white lines).
Analysis of the sequences present at deletion junctions in the notch 1 gene demonstrated that, unlike several other mouse transgenic lines that exhibit T-LL tumors, activating mutations do not result from illegitimate V(D)J recombination. Rather, the notch1 3’ deletion junctions fall within an approximately 200 nt long region of the notch1 gene between exons 27 and 28 that is AT rich and contains homo-polymeric runs of T and A and other short repeating sequences. Sequences present at breakpoints for three deletions in the Pten gene have similar properties. In addition, a short non-templated insertion is present at one junction. These properties are similar to those observed for sequences present at interstitial deletions in human tumors (Abeysinghe et al., 2003).
The remarkably short deletion intervals occurring in T-LLs arising on Mcm2 deficient mice provide a high level of resolution in defining specific genes that contribute to this disease. The genes and pathways identified as contributing to T-LLs in Mcm2 deficient mice share several similarities to those known to contribute to T-cell lymphomas in humans. Activating mutations in notch1 have been found in more than half of human T-cell lymphomas (Weng et al., 2004) similar to the tumors arising in Mcm2def mice. Direct Pten mutations occur in more than 20% of human T-cell lymphomas, whereas hyper-activation of PI3K signaling occurs in more than 85% of cases (Palomero et al., 2007) and CNVs in genes affecting the PI3K signaling pathway are frequently observed in human T-ALLs (Remke et al., 2009). Finally, deletions affecting the locus encoding Cdkn2a and Cdkn2b genes are the most highly recurrent CNVs in human T-ALLs (Williams and Sherr, 2008) and 25% of the Mcm2 deficient mouse tumors are similarly affected.
However, many of the recurrent mutations found in Mcm2 deficient thymic tumors have not been associated with human T-ALLs. Nonetheless, mutations in a number of these genes have been found in other human hematological, and non-hematological, cancers. These include Tcfe2a (LeBrun, 2003), Mll1 (Hess, 2004), Mllt1 (Meyer et al., 2009), Mllt10 (Meyer et al., 2009), Ikzf1 (Collins-Underwood and Mullighan, 2010), Ikzf3 (Rebollo and Schmitt, 2003), Bcl11b (De Keersmaecker et al., 2010), Bcl7a (Zani et al., 1996), Sept9 (Santos et al., 2010), Vav1 (Katzav, 2009), DNMT3a (Ley et al., 2010) and Asxl1 (Tefferi, 2010). The frequent mutation of known tumor suppressor genes in the tumors arising on Mcm2 deficient mice suggest that this data set is likely to contain additional novel tumor suppressor genes. The highly recurrent deletions in Setd1b, Mbd3, Rnf40, and Brpf3, all of which function in aspects of chromatin remodeling, implicate these genes in particular.
The strong tumorigenic effect of reduced Mcm concentrations in mice raises the issue of whether insufficient Mcm activity contributes to human lymphomas or human cancers generally. There is evidence from aCGH for the preferential loss of Mcm proteins 2, 6 and 7 (Supplemental Table II) in a modest subset of human tumors of various types whereas Mcm3 is preferentially amplified. The observation that Mcm3 copy number is increased is consistent with previous studies suggesting that this protein has a negative role in origin licensing. In vivo, reduced Mcm3 levels have been shown to partially rescue the cancer phenotype of mice carrying the Mcm4 mutant allele Chaos3 (Chuang et al., 2010). Finally, CNVs in a subset of human T-ALLs have been observed on human Chr 7q22.1 near the location of the Mcm7 gene (Remke et al., 2009).
Ineffective origin licensing may contribute to genome instability even in the absence of direct Mcm mutation or copy number alteration. The levels of Mcm proteins present in quiescent human T-lymphocytes are substantially lower than in cycling cells (Orr et al., 2010). Further, it has been shown that human T-lymphocytes exiting from quiescence are particularly sensitive to siRNA mediated reduction in Mcm concentration and rapidly acquire genetic damage (Orr et al., 2010). The present studies support that this process is accelerated in the Mcm2 deficient mouse model.
Materials and Methods
Mice
The mice used in this study are the transgenic line Mcm2IRES-CreERT2 carried on a 129/Sv genetic background (Pruitt et al. 2007) and a congenic line in which the Mcm2IRES-CreERT2 transgene was crossed into a C57Bl/6 genetic background through 6 generations. In each case the transgene is carried in heterozygotes. Thymic tumors arising on Mcm2IRES-CreERT2 homozygous mice on the 129/Sv genetic background (6) or from the F1 generation of a cross between the 129 and C57Bl/6 congenic lines at between 2 and 4 months of age were resected and DNA was isolated for aCGH analysis using a Roche DNA Isolation Kit (11 814 770 001) as described by the manufacturer.
Array CGH
aCGH was performed on 720k Roche NimbleGen oligonucleotide arrays using DNAs isolated from the tumors described above and control DNAs derived from tails of mice of the same genetic backgrounds and from the same colonies as mice from which tumors were derived. Tumors 7002, 7302, 7402, 7602, 7902 and 8102 were derived from mice on a 129/Sv genetic background and tumors 7802 and 8002 were derived from F1 129/Sv;C57Bl/6 mice. Data were analyzed using NimbleScan v2.6 and Signal Map 1.9.0.05 software.
Sequencing tumor deletion junctions
Amplicons bridging tumor deletion junctions as predicted by analysis of aCGH data were generated by PCR using Crimson LongAmp Taq DNA Polymerase (New England BioLabs) according to manufacturer’s instructions, excised from 0.8% agarose gels, purified using the GeneJET Gel Extraction Kit (Fermentas) and sequenced.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH-NCI, the Ellison Medical Foundation, and NYSTEM to SCP. Cost of animal maintenance and flow cytometry was supported in part by an NCI-CCS grant to RPCI.
Footnotes
No conflict of interest is declared.
References
- Abeysinghe SS, Chuzhanova N, Krawczak M, Ball EV, Cooper DN. Translocation and gross deletion breakpoints in human inherited disease and cancer I: Nucleotide composition and recombination-associated motifs. Human Mutation. 2003;22:229. doi: 10.1002/humu.10254. [DOI] [PubMed] [Google Scholar]
- Aftab S, Semenec L, Chu JS, Chen N. Identification and characterization of novel human tissue-specific RFX transcription factors. BMC Evol Biol. 2008;8:226. doi: 10.1186/1471-2148-8-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai T, Seebald JL, Kim KE, Ding HM, Szeto DP, Chang HC. Disruption of zebrafish cyclin G-associated kinase (GAK) function impairs the expression of Notch-dependent genes during neurogenesis and causes defects in neuronal development. BMC Dev Biol. 2010;10:7. doi: 10.1186/1471-213X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Dutta A. Preventing re-replication of chromosomal DNA. Nat Rev. 2005;6:476–486. doi: 10.1038/nrm1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Ge XQ, Jackson DA. How dormant origins promote complete genome replication. Trends Biochem Sci. 2011 doi: 10.1016/j.tibs.2011.05.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackertz M, Boeke J, Zhang R, Renkawitz R. Two highly related p66 proteins comprise a new family of potent transcriptional repressors interacting with MBD2 and MBD3. J Biol Chem. 2002;277:40958–40966. doi: 10.1074/jbc.M207467200. [DOI] [PubMed] [Google Scholar]
- Buckler JL, Liu X, Turka LA. Regulation of T-cell responses by PTEN. Immunol Rev. 2008;224:239–248. doi: 10.1111/j.1600-065X.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CH, Wallace MD, Abratte C, Southard T, Schimenti JC. Incremental genetic perturbations to MCM2-7 expression and subcellular distribution reveal exquisite sensitivity of mice to DNA replication stress. PLoS Genet. 2010;6:e1001110. doi: 10.1371/journal.pgen.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins-Underwood JR, Mullighan CG. Genomic profiling of high-risk acute lymphoblastic leukemia. Leukemia. 2010;24:1676–1685. doi: 10.1038/leu.2010.177. [DOI] [PubMed] [Google Scholar]
- De Keersmaecker K, Real PJ, Gatta GD, Palomero T, Sulis ML, Tosello V, et al. The TLX1 oncogene drives aneuploidy in T cell transformation. Nat Med. 2010;16:1321–1327. doi: 10.1038/nm.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XQ, Jackson DA, Blow J. Dormant origins licensed by excess Mcm2-7 are required for human cells to survive replicative stress. Genes Dev. 2007;21:3331–3341. doi: 10.1101/gad.457807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge XQ, Blow JJ. Chk1 inhibits replication factory activation but allows dormant origin firing in existing factories. J Cell Biol. 2010;191(7):1285–1297. doi: 10.1083/jcb.201007074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JL. Mechanisms of transformation by MLL. Crit Rev Eukaryot Gene Exp. 2004;14:235–254. doi: 10.1615/critreveukaryotgeneexpr.v14.i4.10. [DOI] [PubMed] [Google Scholar]
- Ibarra A, Schwob E, Méndez J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc Natl Acad Sci USA. 2008;105:8956–8961. doi: 10.1073/pnas.0803978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannet R, Mastio J, Macias-Garcia A, Oravecz A, Ashworth T, Geimer Le Lay AS, Jost B, Le Gras S, Ghysdael J, Gridley T, Honjo T, Radtke F, Aster JC, Chan S, Kastner P. Oncogenic activation of the Notch1 gene by deletion of its promoter in Ikaros-deficient T-ALL. Blood. 2010;116:5443–5454. doi: 10.1182/blood-2010-05-286658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzav S. Vav1: a hematopoietic signal transduction molecule involved in human malignancies. Int J Biochem Cell Biol. 2009;41:1245–1248. doi: 10.1016/j.biocel.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Kim J, Guermah M, McGinty RK, Lee JS, Tang Z, Milne TA, et al. RAD6-Mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137:459–471. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper RP, Schoenmakers EF, van Reijmersdal SV, Hehir-Kwa JY, van Kessel AG, van Leeuwen FN, Hoogerbrugge PM. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21(6):1258–1266. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- Kunnev D, Rusiniak ME, Kudla A, Freeland A, Cady GK, Pruitt SC. DNA damage response and tumorigenesis in Mcm2-deficient mice. Oncogene. 2010;29:3630–3638. doi: 10.1038/onc.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrun DP. E2A basic helix-loop-helix transcription factors in human leukemia. Front Biosci. 2003;8:s206–s222. doi: 10.2741/1030. [DOI] [PubMed] [Google Scholar]
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, Harris CC, Lichti CF, Townsend RR, Fulton RS, Dooling DJ, Koboldt DC, Schmidt H, Zhang Q, Osborne JR, Lin L, O'Laughlin M, McMichael JF, Delehaunty KD, McGrath SD, Fulton LA, Magrini VJ, Vickery TL, Hundal J, Cook LL, Conyers JJ, Swift GW, Reed JP, Alldredge PA, Wylie T, Walker J, Kalicki J, Watson MA, Heath S, Shannon WD, Varghese N, Nagarajan R, Westervelt P, Tomasson MH, Link DC, Graubert TA, DiPersio JF, Mardis ER, Wilson RK. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010 Dec 16;363(25):2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Kowarz E, Hofmann J, Renneville A, Zuna J, Trka J, et al. New insights to the MLL recombinome of acute leukemias. Leukemia. 2009;23:1490–1499. doi: 10.1038/leu.2009.33. [DOI] [PubMed] [Google Scholar]
- Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, Su X, Pui CH, Relling MV, Evans WE, Shurtleff SA, Downing JR. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Xin L, Sharov AA, Thomas M, Mowrer G, Meyers E, et al. Uncovering early response of gene regulatory networks in ESCs by systematic induction of transcription factors. Cell Stem Cell. 2009;5:420–433. doi: 10.1016/j.stem.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SJ, Gaymes T, Ladon D, Chronis C, Czepulkowski B, Wang R. Reducing MCM levels in human primary T cells during the G(0)-->G(1) transition causes genomic instability during the first cell cycle. Oncogene. 2010;29:3803–3814. doi: 10.1038/onc.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt SC, Bailey KJ, Freeland A. Reduced Mcm2 expression results in severe stem/progenitor cell deficiency and cancer. Stem Cells. 2007;25:3121–3132. doi: 10.1634/stemcells.2007-0483. [DOI] [PubMed] [Google Scholar]
- Ramírez J, Hagman J. The Mi-2/NuRD complex: a critical epigenetic regulator of hematopoietic development, differentiation and cancer. Epigenetics. 2009;4:532–536. doi: 10.4161/epi.4.8.10108. [DOI] [PubMed] [Google Scholar]
- Rebollo A, Schmitt C. Ikaros, Aiolos and Helios: transcription regulators and lymphoid malignancies. Immunol Cell Biol. 2003;81:171–175. doi: 10.1046/j.1440-1711.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- Remke M, Pfister S, Kox C, Toedt G, Becker N, Benner A, Werft W, Breit S, Liu S, Engel F, Wittmann A, Zimmermann M, Stanulla M, Schrappe M, Ludwig WD, Bartram CR, Radlwimmer B, Muckenthaler MU, Lichter P, Kulozik AE. High-resolution genomic profiling of childhood T-ALL reveals frequent copy-number alterations affecting the TGF-beta and PI3K-AKT pathways and deletions at 6q15-16.1 as a genomic marker for unfavorable early treatment response. Blood. 2009;114(5):1053–1062. doi: 10.1182/blood-2008-10-186536. [DOI] [PubMed] [Google Scholar]
- Santos J, Cerveira N, Correia C, Lisboa S, Pinheiro M, Torres L, et al. Coexistence of alternative MLL-SEPT9 fusion transcripts in an acute myeloid leukemia with t(11;17)(q23;q25) Cancer Genet Cytogenet. 2010;197:60–64. doi: 10.1016/j.cancergencyto.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Shima N, Alcaraz A, Liachko I, Buske TR, Andrews CA, Munroe RJ, et al. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat Genet. 2007;39:93–98. doi: 10.1038/ng1936. [DOI] [PubMed] [Google Scholar]
- Tefferi A. Novel mutations and their functional and clinical relevance in myeloproliferative neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia. 2010;24:1128–1138. doi: 10.1038/leu.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JP, 4th, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Williams RT, Sherr CJ. The INK4-ARF (CDKN2A/B) locus in hematopoiesis and BCR-ABL-induced leukemias. Cold Spring Harb Symp Quant Biol. 2008;73:461–467. doi: 10.1101/sqb.2008.73.039. [DOI] [PubMed] [Google Scholar]
- Yang Q, Kardava L, St Leger A, Martincic K, Varnum-Finney B, Bernstein ID, et al. E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. J Immunol. 2008;181:5885–5894. doi: 10.4049/jimmunol.181.9.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani VJ, Asou N, Jadayel D, Heward JM, Shipley J, Nacheva E, et al. Molecular cloning of complex chromosomal translocation t(8;14;12)(q24.1;q32.3;q24.1) in a Burkitt lymphoma cell line defines a new gene (BCL7A) with homology to caldesmon. Blood. 1996;87:3124–3134. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.