Abstract
Background
Structural abnormalities of the corpus callosum (CC), such as reduced size and increased shape variability, have been documented in individuals with fetal alcohol spectrum disorders (FASD). However, the regional specificity of altered CC structure, which may point to the timing of neurodevelopmental disturbances and/or relate to specific functional impairments, is unclear. Further, associations between facial dysmorphology and callosal structure remain undetermined.
Method
153 participants (age range 8–16) including 82 subjects with FASD and 71 non-exposed controls were included in this study. The structural magnetic resonance imaging (sMRI) data of these subjects was collected at 3 sites (Los Angeles and San Diego, California, and Cape Town, South Africa) and analyzed using classical parcellation schemes as well as more refined surface based geometrical modeling methods to identify callosal morphological alterations in FASD at high spatial resolution.
Results
Reductions in callosal thickness and area, specifically in the anterior third and the splenium, were observed in FASD compared to non-exposed controls. In addition, reduced CC thickness and area significantly correlated with reduced palpebral fissure length.
Conclusions
Consistent with previous reports, findings suggest an adverse effect of prenatal alcohol exposure on callosal growth and further indicate that fiber pathways connecting frontal and parieto-occipital regions in each hemisphere may be particularly affected. Significant associations between callosal and facial dysmorphology provide evidence for a concurrent insult to midline facial and brain structural development in FASD.
Keywords: fetal alcohol spectrum disorders, corpus callosum, facial dysmorphology, MRI
Introduction
The corpus callosum (CC) is the largest myelinated axonal pathway in the brain, consisting of over 190 million fibers (Tomasch, 1954), and the primary structure involved in the transmission of interhemispheric information. In animal models, the CC starts to form at 39 embryonic days (Sarnat, 1991) where the earliest callosal axons appear at 74 days and the genu and the splenium become recognizable by 84 days (Loeser and Alvord, 1968). Although the adult-like morphology is achieved by 115 days, the CC continues to change postnatally throughout childhood and early adulthood due to fiber myelination, reorganization, and pruning. Because alcohol is a teratogen that is known to affect brain white matter (Holzman et al., 1995) and that the majority of callosal fibers are formed prenatally, it has been speculated that the corpus callosum may be disproportionately affected by excessive prenatal alcohol exposure (Riley and McGee, 2005). Furthermore, accumulating neuropsychological findings support this assumption given the primary function of the CC in mediating interhemispheric communication (Pannek et al., 2010), which may impact deficiencies in attention, general intelligence, learning, verbal memory, executive function and social cognition observed in individuals exposed to alcohol prenatally (Fryer et al., 2009a, Vaurio, 2011).
Despite the converging evidence suggesting an adverse effect of prenatal alcohol exposure on CC development, the spatial profile of callosal dysmorphology and links with other known detrimental effects of exposure remain unclear. Consistent with animal research, studies have found children with fetal alcohol syndrome (FAS) or fetal alcohol spectrum disorders (FASD) to show defects in gross callosal morphology, including reduced overall CC size (Mattson, 1994, Riley et al., 1995) or complete absence of the CC (Jeret et al., 1987, Mattson, 1994, Riley et al., 1995). Specifically, the incidence of a complete absence of the CC (i.e. agenesis) among children prenatally exposed to alcohol approximates 6.8% (Riley et al., 1995), much higher than observed in normal as well as developmentally delayed populations (Jeret et al., 1986). Only a few studies have examined callosal structure in more detail in children with FASD, revealing reduced regional size of the CC as measured in the midsagittal section, most pronounced in the splenium (Sowell et al., 2001) and significant displacements and/ or shape alterations of the CC in children with FASD (Sowell et al., 2001, Bookstein et al., 2001, Bookstein et al., 2002). In addition, diffusion tensor imaging (DTI) studies have found microstructural anomalies in the CC (Ma et al., 2005, Wozniak et al., 2006), highlighting the apparent vulnerability of callosal structures to prenatal alcohol exposure. Despite these findings, the effect of prenatal alcohol exposure on more refined characteristics of callosal morphology, such as local thickness, has not yet been addressed.
Very little is known regarding the association between callosal morphological abnormalities and facial dysmorphology, a hallmark clinical indication of FASD. Specifically, well-controlled animal studies have demonstrated that alcohol exposure during early stages of pregnancy may contribute to concurrent disruptions in the development of midline facial features (e.g. long upper lip, abnormal philtrum, and short palpebral fissures) and forebrain structures (Sulik and Johnston, 1983, Godin et al., 2010). Notably, recent findings from the brain imaging literature suggest that facial dysmorphology may serve as an indicator of the underlying maldevelopment of the brain. For example, two studies have found significant correlations between facial anomalies and reduced volumes in frontal and in subcortical structures such as the thalamus (Astley et al., 2009, Roussotte et al., 2011). Thus, though findings from some DTI studies appear inconclusive regarding the relationships between facial characteristics and DTI measures of callosal integrity (Wozniak et al., 2006, Wozniak et al., 2009b), more refined aspects of CC macrostructure may serve as a more sensitive approach for clarifying the effects of prenatal alcohol exposure on the observed neurodevelopmental disturbances in FASD.
For the current study, we included a relatively large sample of alcohol-exposed children and adolescents recruited from three locations (i.e. Cape Town, South Africa, Los Angeles and San Diego, California). This sample is part of an ongoing project of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which aims to characterize the range of underlying neurodevelopmental abnormalities across the spectrum of FASD. The goal of the current study was to confirm previous findings of reduced midsagittal callosal areas, measured using a classically defined parcellation scheme, in FASD (Sowell et al., 2001) in a much larger and independent sample of prenatally exposed individuals. However, our primary goal was to extend these findings by using a more refined methodological approach (Luders et al., 2006) that allows the determination of highly localized changes in callosal thickness in FASD that may better characterize the nature and extent of CC dysmorphology in the syndrome. In addition, as a more exploratory aim, relationships between variation in regional callosal thickness and facial dysmorphology measures, which have not been addressed by previous studies, were evaluated. Based on findings discussed above and the functional role of the CC (Schulte and Muller-Oehring, 2010), we hypothesized that children and adolescents with FASD would exhibit reduced thickness and areas in multiple regions of the CC and that facial dysmorphology measures will be significantly correlated with reduced callosal thickness and area measurements.
Methods
Subjects
Structural magnetic resonance imaging (MRI) data were collected from 153 subjects at 3 sites: Los Angeles (LA, age range 8–16), Cape Town, South Africa (SA, age range 13–15), and San Diego (SD, age range 10–14). Subjects included 82 children and adolescents with FASD (23 from LA, 44 from SA, and 15 from SD) and 71 non-exposed controls (18 from LA, 35 from SA, and 18 from SD). As previously reported (Roussotte et al., 2011), the ethnic composition of participants varied by site. In LA and SD, while the majority of participants were classified as non-Hispanic White, many subjects classified as Asians, Native Hawaiians, and African Americans were also included. In SA, the majority of the participants were classified as “Cape Colored”, a racial category comprising people of mixed ancestry from intermarriages of Black African populations (primarily of Khoi and San tribal origins), European whites, and some Asians (May et al., 2007).
In LA, exposed subjects were recruited through the University of California Los Angeles Fetal Alcohol and Related Disorders Clinic, where exposure status was established through extensive interviews administered to the parents/ guardians as well as collateral information (e.g. social, medical and/or legal records). In SA, exposed subjects were recruited through the University of Cape Town. All SA subjects had been diagnosed previously as FAS or partial FAS from a population-based study of the epidemiology of FASD among first graders (May et al., 2007), and the diagnoses were reconfirmed for this study through maternal interview and questionnaires with the birth mothers. For 17 of the 44 FASD participants from SA, quantitative measures of the level of alcohol exposure were obtained from reports of the total number of drinks per week during each trimester and average number of drinks per occasion during each trimester (May et al., 2008; Roussotte et al., 2011). In SD, exposed participants were recruited through the San Diego State University Center for Behavioral Teratology and exposure status was determined through maternal reports and collateral records. Overall, the FASD sample was heavily exposed to alcohol prenatally (> 4 drinks/ occasion at least once/ week or > 13 drinks/ week) (Mattson et al., 2010). Across the sites, participants were excluded from the FASD group if evidence of other known causes of mental deficiency (e.g. chromosomal abnormalities, known exposure to other teratogenic agents) was present.
Non-exposed control subjects were recruited from ongoing studies at each site or specifically for this study through advertisements, word of mouth, and the use of national registers (Roussotte et al., 2011). Control participants were excluded if they were prenatally exposed to more than one drink per week on average, or more than two drinks on any one occasion during pregnancy. Additional exclusion criteria for both the control and FASD groups included significant head injury with loss of consciousness for more than 30 minutes, or significant physical or psychiatric disability. Furthermore, no exposed or control subjects with CC agenesis or partial agenesis were included in this study.
Facial and Cognitive Evaluations
Standardized methods were used to obtain facial dysmorphology scores on both FASD and non-exposed subjects by a trained member of the CIFASD blind to subject group membership (Jones et al., 2006, Hoyme et al., 2005, Roussotte et al., 2011). In brief, palpebral fissure length (PFL) was measured using a rigid ruler marked in millimeters held against the lower eyelid (Hoyme et al., 2005). Following a previously validated lip/philtrum guide (“lipometer”) (Hoyme et al., 2010, Astley and Clarren, 2000), the morphological characteristics of the philtrum were assessed and a score of 1 through 5 was assigned, with a higher score representing greater dysmorphology of the philtrum. Of the 153 subjects included, 93 subjects (58 FASD and 35 non-exposed subjects) received a PFL and 92 subjects (58 FASD and 34 non-exposed subjects) obtained a philtrum lipometer scores, and those within the FASD group were included in post-hoc analyses to examine associations between CC thickness and facial dysmorphology. As described in detail previously (Roussotte et al., 2011), all subjects eligible for neuropsychological testing were given a standardized test battery by trained examiners, including the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV; Wechsler, 2003), from which a Full Scale IQ (FSIQ) score was derived. The scores were obtained by the examiners and checked for accuracy by a second rater, and were available for all but one non-exposed control subject from SD.
MRI Data Acquisition
In LA, high-resolution T1-weighted sagittal volumes were collected from a 1.5 Tesla Siemens Sonata scanner, with repetition time (TR) = 1900 ms, echo time (TE) = 4.38 ms, flip angle = 15°, matrix size = 256 × 256 × 160 mm, field of view (FOV) = 256 mm, voxel size = 1 × 1 × 1 mm, and acquisition time = 8 min, 8 s. In SA, high-resolution T1-weighted sagittal volumes were collected from a 3 Tesla Siemens Allegra scanner, with TR = 2200 ms, TE = 5.16 ms, flip angle = 12°, matrix size = 256 × 256 × 160 mm, FOV = 256 mm, voxel size = 1 × 1 × 1 mm, and acquisition time = 7 min, 4 s. In SD, high-resolution T1-weighted sagittal volumes were collected from a 3 Tesla GE Signa Excite scanner, with TR = 7.8 ms, TE = 3.0 ms, flip angle = 12°, matrix size = 256 × 256 × 192 mm, FOV = 240 mm, voxel size = .938 × .938 × 1 mm, and acquisition time = 7 min, 26 s.
Image Processing
Several preprocessing steps were applied to the brain volumes including correction of signal intensity and magnetic field inhomogeneity artifacts, correction for head tilt and alignment by using a three-translation and three-rotation rigid-body transformation, and automated removal of extra-cortical tissue using FSL’s Brain Extraction Tool with manual correction of errors performed on a slice-by-slice basis on each brain slice through each image volume (Yang et al., 2010). Scalp editing was performed to exclude scalp and meninges and to include brain tissue, sulcal and subarachnoid CSF. Estimates for whole brain and whole brain gray/ white matter volume were obtained from these manually corrected scalp-edited image volumes.
After preprocessing, one rater (ORP), blind to group membership, delineated the CC using a three-step approach that has been validated in several previous publications (Di Paola et al., 2010, Freitag et al., 2009, Luders et al., 2006, Luders et al., 2010). Intraclass correlations determining the reliability of manual delineation were excellent (rI = .98). In brief, for analysis of CC thickness, the upper and lower callosal boundaries in the mid-sagittal section of each brain volume were manually outlined. Second, a new midline segment was automatically created by calculating the spatial average from 100 equidistance surface points representing the traces. Lastly, the distances between 100 surface points of the midline segment and the 100 corresponding surface points of the callosal top/bottom segments were automatically quantified. To obtain highly localized measures of callosal thickness for group comparisons, previously validated and detailed anatomical surface based mesh modeling methods were employed (Thompson et al., 1996). Regarding the CC area measurements, the callosal delineations were then further divided into five vertical partitions to represent the (1) anterior third (mainly genu and rostral body), (2) anterior midbody, (3) posterior midbody, (4) isthmus, and (5) splenium (Witelson, 1989, Luders et al., 2006). These callosal outlines were used to estimate callosal area measurements in mm2 for overall and each callosal segment.
Statistical Analysis
For demographic and cognitive variables, statistical analyses were conducted using SPSS 18.0. Age, FSIQ, total brain volume, and total white matter volume were compared between groups using independent two-sample t-tests and difference in gender was evaluated with Pearson Chi-square tests. For the comparison between FASD and non-exposed control group across three sites, general linear models were employed to test for group difference in callosal thickness and generate color-coded statistical maps illustrating where subjects with FASD differed significantly from typically developing control subjects. Age and gender were included as covariates for within-site analyses, and site as a covariate was additionally included for combined-site analyses. Permutation testing (with 10,000 permutations computed) was employed to control for multiple comparisons where the number of surface points showing significant differences in CC thickness between groups thresholded at p = .05 were compared with the number of surface points showing differences when individuals were randomly assigned to groups using the reduced model (Anderson and Ter Braak, 2003). For descriptive purposes and to confirm the location of the observed effects, additional group comparisons were conducted on CC total and segment area measurements. Specifically, the area measurements of total and five segments of CC were compared between the two groups using general linear models. Last, relationship between quantitative measures of prenatal alcohol exposure in the SA sample and CC area measures were also assessed using Pearson correlations.
Follow-up Analyses
To determine whether changes in callosal thickness and area measures observed in FASD group are also associated with measures of facial dysmorphology, these relationships were assessed using a multiple regression model within the FASD group only. Finally, for the 152 (82 FASD and 70 non-exposed controls) who were administered an IQ test, follow-up multiple regression analyses were performed to examine the relationship between intellectual functioning and CC thickness and area.
Results
Demographic, Cognitive, & Clinical Measures
Table 1 shows the demographic, cognitive, and clinical details of subjects. Across and within scan sites, groups did not differ from each other in age or gender (all p > .15). FASD subjects consistently showed lower FSIQ compared to control subjects across and within sites (all p < .001). In addition, FSIQ scores differed between scan sites. FASD subjects from SA site scored significantly lower than FASD subjects from the LA and SD subjects (both p < .001), whereas the mean FSIQ scores of FASD subjects in LA and SD site did not differ from each other (p = .86). For clinical measures, the frequency data of PFL and lipometer scores are reported in Figure S1. FASD subjects showed significantly smaller PFL and increased lipometer scores compared to non-exposed control subjects (F (1, 94) = 3.99, p = .049; F (1, 93) = 6.89, p = .01; respectively) across sites. However, no significant group difference in clinical measures was observed within site (all p > .06).
Table 1.
Demographic, clinical, and MRI measures of the FASD and Non-exposed controls (NC).
| Across Sites | Within Sites | |||||||
|---|---|---|---|---|---|---|---|---|
| Los Angeles | South Africa | San Diego | ||||||
| FASD (N = 82) |
NC (N = 71) |
FASD (N = 23) |
NC (N = 18) |
FASD (N = 44) |
NC (N = 35) |
FASD (N = 15) |
NC (N = 18) |
|
| Demographic Measures | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Age | 12.8(2.3) | 12.3(2.4) | 12.7(2.3) | 11.8(2.4) | 13.1(2.6) | 12.5(2.9) | 12.1(1.1) | 12.1(1.4) |
| Gender (Male/Female) | 43/26 | 28/30 | 14/9 | 7/11 | 26/18 | 18/17 | 11/4 | 10/8 |
| FSIQa | 64.8 (26.4) | 88.8 (26.1) | 83.1 (21.1) | 111.2 (20.7) | 46.4 (16.5) | 68.3 (12.2) | 78.3 (26.1) | 107.5 (17.6) |
| Clinical Measures | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| PFLb | 2.5 (.3) | 2.6(.1) | 2.6(.2) | 2.7(.2) | 2.4(.3) | 2.6(.1) | 2.6(.2) | 2.6(.1) |
| Lipometer c | 3.5(.6) | 3.2(.8) | 3.4(.6) | 3.2(.6) | 3.7(.7) | 3.3(1.1) | 3.4(.5) | 3.0(.5) |
| Full FAS Diagnosis (yes/ no) d | 32/75 | 0/71 | 3/16 | 0/18 | 26/44 | 0/35 | 3/15 | 0/18 |
| MRI Measures | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Total brain (cm3) | 986.5 (140.7) | 1081.6 (130.3) | 1028.7 (140.2) | 1111.0 (109.5) | 970.3 (149.0) | 1075.0 (139.8) | 969.4 (107.0) | 1065.0 (132.1) |
| Total white matter (cm3) | 479.3 (55.7) | 497.7 (48.3) | 494.0 (60.5) | 507.1 (53.8) | 472.0 (59.8) | 491.3 (52.9) | 478.3 (25.6) | 500.6 (30.4) |
Note:
FSIQ scores were available for 152 subjects (all but one NC subject from SD).
PFL and Lipometer measures were only available in 93 subjects (58 FASD and 35 NC subjects) including 29 subjects from LA (16 FASD and 14 NC control subjects), 40 subjects from SA (29 FASD and 11 NC subjects) and 24 subjects from SD (14 FASD and 10 NC control subjects).
Lipometer measure was available for all 93 subjects with FPL measures except one non-exposed control subject from LA.
Full FAS diagnosis was available for only 75 FASD subjects
Across sites, FASD subjects had smaller total brain and white matter volumes than non-exposed control subjects (F (1, 152) = 18.6, p < .001; F (1, 152) = 4.67, p = .032; respectively). Within sites, the total brain volumes of FASD subjects were found to be smaller than that of non-exposed control subjects within LA (F (1, 40) = 4.18, p = .048), SA (F (1, 78) = 10.17, p = .002), and SD (F (1, 32) = 5.07, p = .032). FASD subjects also had smaller total white matter volumes compared to non-exposed controls within SD site (F (1, 32) = 5.07, p = .032), but not LA (p = .48) or SA site (p = .14). Because this study examines CC morphology, which composed of mostly white matter, follow-up analyses were conducted for callosal areas and thickness controlling for total white matter volume, in addition to age, gender and site (when compared across sites).
FASD Effects on Surface-based Callosal Thickness Measurements
Analyses on surface-based callosal thickness measurements revealed significantly reduced thickness in the anterior third and the splenium in FASD subjects compared to non-exposed control subjects across the sites (Figure 1). The group difference remained significant after controlling for age, gender, and site, however adding total white matter volume as an additional covariate attenuated all group difference (Supplementary Materials, Figure S1). Within the LA site, significantly reduced thickness was observed in mid-section of the CC, including the anterior midbody, posterior midbody and the isthmus, in FASD compared to controls (Figure 1) and the difference remained significant after controlling for age, gender and total white matter volume (Figure S2). Within the SA site, significantly reduced thickness was found in the anterior third of the CC in FASD subjects compared to non-exposed control subjects (Figure 1), which remained significant after controlling for sex, age, and total white matter volume (Figure S2). However, localized thickness increases in the anterior and posterior midbody were also observed in FASD compared to control subjects (Figure S2). With the smallest FASD sample among the three sites, group comparisons conducted within the SD site did not yield any significant differences in callosal thickness (Figure 1 and S2).
Figure 1.
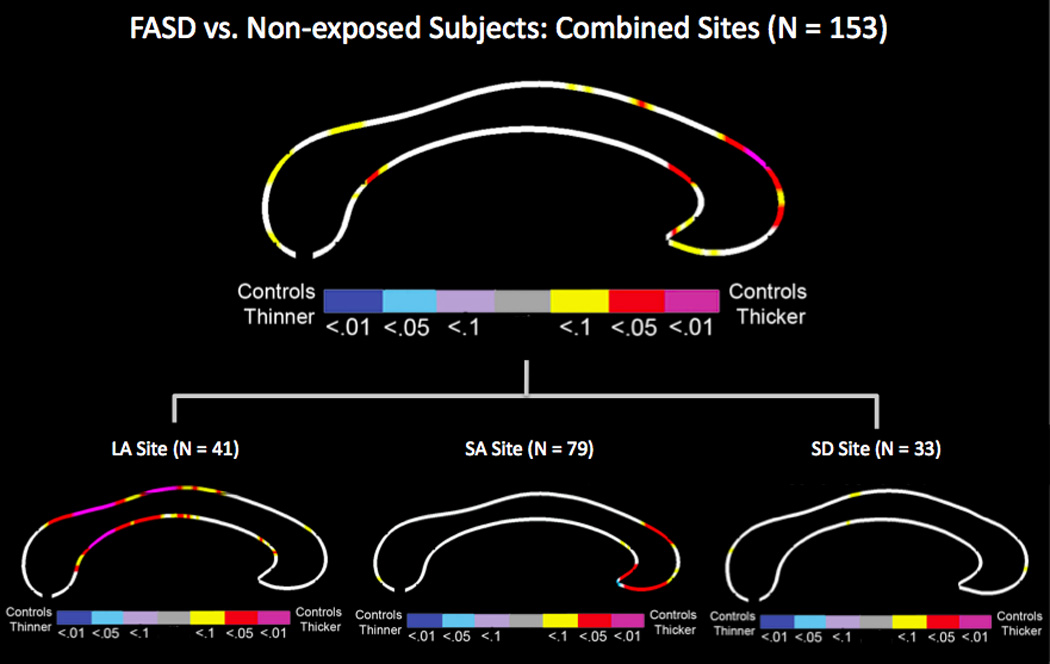
FASD effects on callosal thickness across three sites controlling for sex, age, and site as well as within Los Angeles (LA), South Africa (SA), and San Diego (SD) site controlling for sex and age. The color bar encodes the p value with hot-color indicating increased thickness and cold-color indicating decreased thickness in controls compared to FASD subjects.
FASD Effects on Area Measurements of CC
Across the sites, FASD subjects showed reduced areas in overall CC (F (1, 152) = 6.09, p = .015), and callosal segments of the anterior third (F (1, 152) = 6.75, p = .01) and the splenium (F (1, 152) = 5.14, p = .025) compared to non-exposed controls (Figure 2). Results remained significant after controlling for age, gender, and site, however became non-significant after controlling for total white matter volume (all p > .4). Within the LA site, FASD subjects showed reduced areas in overall CC (F (1, 40) = 6.0, p = .019), anterior midbody (F (1, 40) = 6.46, p = .015), posterior midbody (F (1, 40) = 6.17, p = .017), and the isthmus (F (1, 40) = 6.17, p = .017) compared to non-exposed controls, with trend-level significance for the anterior third (p = .078) and the splenium (p = .093). Within the SA site, reduced callosal areas in FASD subjects were observed specifically in the anterior third (F (1, 78) = 5.68, p = .02) and trend-level significance for the splenium (p = .06). Within the SD site, no significant difference was observed in any callosal areas in FASD, with only trend-level significance in the posterior midbody (p = .054). Follow-up analyses revealed that, after controlling for sex, age, and total white matter volume, FASD subjects showed reduced callosal areas compared to control subjects in overall (p = .037), anterior midbody (p = .03), posterior midbody (p = .033), and the isthmus (p = .041) within the LA site and in the posterior midbody (p = .046) within the SA site. Lastly, follow-up analyses on quantitative measures of prenatal alcohol exposure in the 17 SA subjects for whom such data were available revealed no significant correlations between CC thickness or area measures and total number of drinks per week or average drinks per occasion in all three trimesters (all p > .056).
Figure 2.
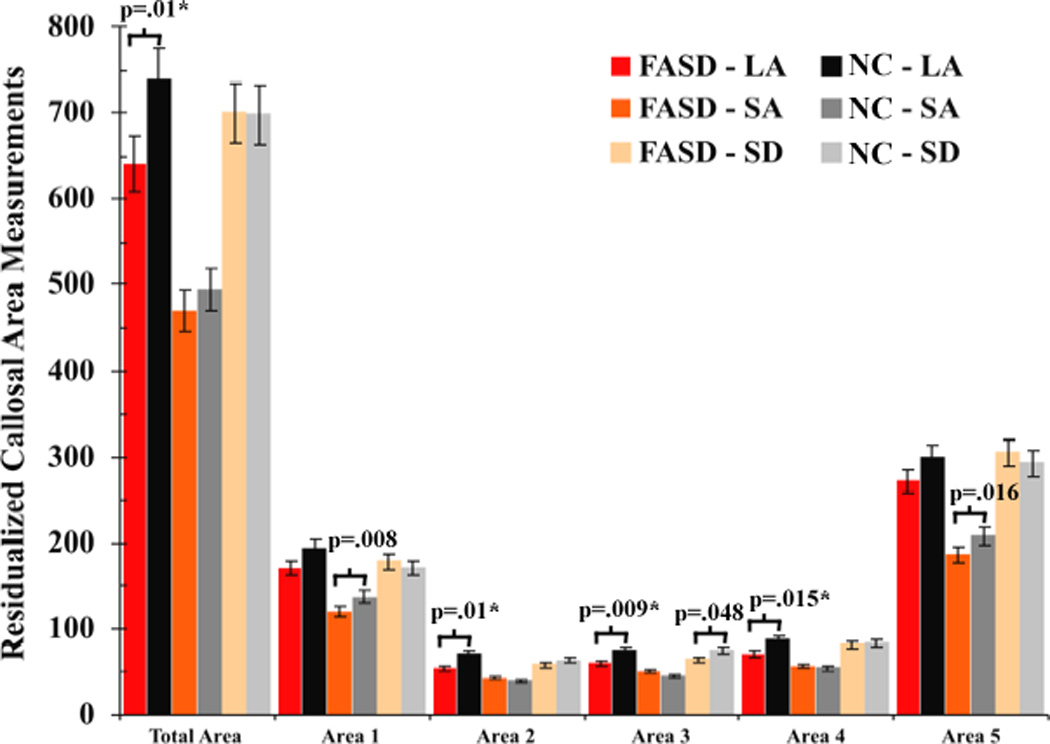
Average callosal area measurements of the total area, area 1 (anterior third including rostrum, genu and rostral body), area 2 (anterior midbody), area 3 (posterior midbody), area 4 (isthmus), and area 5 (splenium) of the FASD and non-exposed (NC) control subjects within each site, corrected for age, gender, and total white matter. The vertical lines represent the standard error bars. The p values indicate significant group difference in callosal areas while controlling for age and gender, while those remained significant after controlling for total white matter were marked with a “*”.
Facial Dysmorphology, Intellectual Functioning, and CC morphology
When data for the exposed participants were combined, callosal thickness and area measures were found significantly correlated with facial dysmorphology measures. Specifically, PFL scores were found to be significantly correlated with callosal thickness, specifically in the anterior third, isthmus and the splenium. In these regions, children within the FASD group with reduced CC thickness had smaller PFLs (Figure 3). However, no significant correlation was found between lipometer and callosal thickness. Post-hoc analysis confirmed that PFL scores significantly correlated with reduced area measurements of anterior third (r = .46, p < .001), isthmus (r = .39, p = .002), and the splenium (r = .37, p = .004). After controlling for age, gender, site, and total white matter volume, PFL scores remained significantly correlated with the area of the anterior third (r = .33, p = .018) and with a trend level of significance for the isthmus (p = .051) of the CC.
Figure 3.
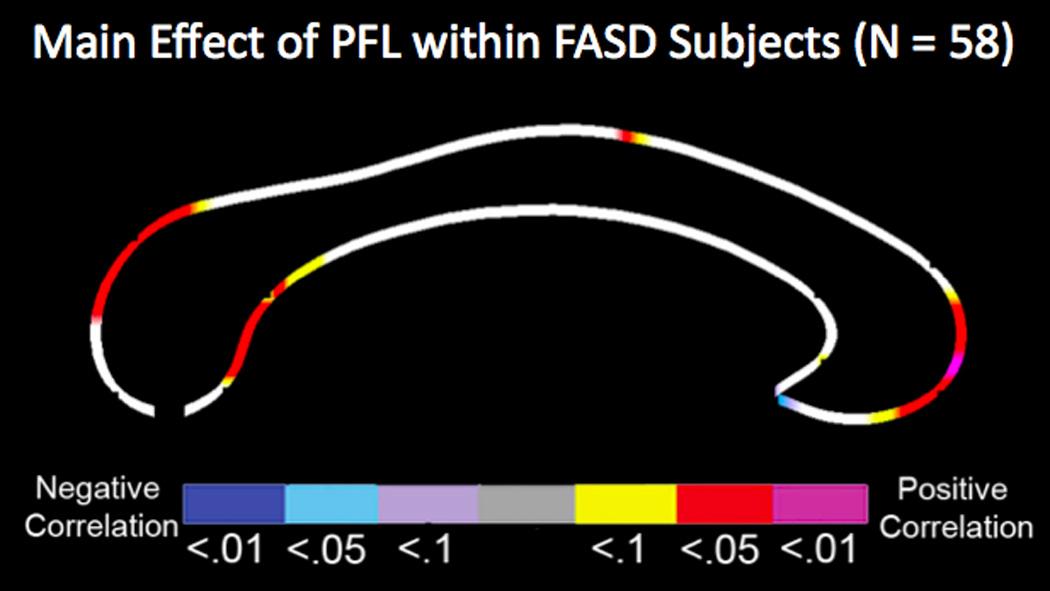
Relationships between regional callosal thickness and palpebral fissure length (PFL) within the FASD group, controlling for sex, age, total white matter, and site. The color bar encodes the p value with hot-color indicating positive correlation and cold-color indicating negative correlation.
Analyses of FSIQ showed significant positive associations with callosal thickness across sites, however the correlation was not significant after controlling for age, sex, total white matter volume, site and diagnosis (Figure S3). On the other hand, associations between CC areas measures and general intellectual functioning revealed significant positive correlations between FSIQ scores and total area (p = .03) and areas of the anterior third (p = .01) and isthmus of the CC (p = .017), controlling for age, sex, total white matter volume, site and diagnosis.
Discussion
In this study, we applied novel computational surface-based methods to calculate and compare callosal thickness at high spatial resolution in a relatively large cohort of 153 children and adolescents exposed to alcohol prenatally and non-exposed controls. We revealed significant FASD effects on callosal thickness and area measures in both anterior (genu/ rostral body) and posterior sections (isthmus/ splenium). These findings are consistent with previous reports that showed reduced CC area in FASD (Riley et al., 1995, Sowell et al., 2001, Swayze et al., 1997) and are in keeping with DTI studies of fetal alcohol effects on the white matter microstructure of the CC (Wozniak et al., 2009a, Wozniak et al., 2006, Lebel et al., 2008, Li et al., 2009, Ma et al., 2005, Sowell et al., 2008). Although controlling for total white matter volume served to attenuate the results of several analyses, many remained significant after controlling for age, gender, and total white matter suggesting that the developing corpus callosum may be especially sensitive to the damaging effects of alcohol relative to the brain in general.
Regardless of whether or not we covaried for total white matter, callosal abnormalities were most prominent in anterior callosal sections and the splenium of the CC when sites were combined. The anterior third includes the rostrum, genu, and the rostral body regions of the CC and contains fibers mainly project to the frontal lobes (Park et al., 2008). On the other hand, the posterior segments of the CC, particularly the splenium, allow the axons arising from the parietal and occipital cortex to pass between hemispheres (Park et al., 2008). Thus, reduced thickness and area in these CC segments may reflect the loss of some white matter pathways in the frontal, occipital and temporo-parietal association regions in individuals with prenatal alcohol exposure, and may be in support of previously reported reduced callosal size and shape abnormalities in FASD that seem to be especially prominent in the posterior callosal regions (Bookstein et al., 2001, Bookstein et al., 2002, Sowell et al., 2001). The localized findings in this study are consistent with observations of reduced white mater volumes localized within each hemisphere found in patients damaged by prenatal alcohol exposure compared to unaffected control subjects (Sowell et al., 2001), regions that project mainly through the posterior segments of the CC. The findings are also consistent with DTI studies showing increased mean diffusivity (MD) or low fractional anisotropy (FA) in posterior callosal segments as well as the genu of the CC (Wozniak et al., 2009a, Lebel et al., 2008, Li et al., 2009, Ma et al., 2005, Sowell et al., 2008). Taken together, findings suggest that thinner and reduced area in the CC, specifically the genu/ rostrum and splenial region, could indicate disrupted fiber pathways important for the development of frontal and parieto-occipital lobes which may contribute towards impaired neuropsychological performance observed in FASD.
Although findings suggest some variations in callosal morphological abnormalities in FASD across sites, such differences may be attributable to reduced power when examining sub-groups separately and differences in subject characteristics such as racial/ethnic composition, intellectual functioning, age distributions or severity of exposure. As discussed earlier, animal studies also showed smaller corpora callosal in prenatal alcohol-treated rats and mice than controls (Zimmerberg and Mickus, 1990, Livy and Elberger, 2001). Specifically, rats exposed to alcohol during a period of time that coincide with the first two trimesters of human gestation were found to show abnormalities in the number, size and distribution of the CC projection neurons (Miller and Vogt, 1984). However, the increase in the number and length of the callosal dendrites (reflected as increased cell body area in the CC) has been found to attenuate with age in the alcohol-exposed animals (Qiang et al., 2002). A similar but more complicated age-related interaction has been found in human callosal development (Luders et al., 2010). Specifically, there is greater callosal thickness growth in posterior regions in younger subjects, particularly between ages 9–12 years, followed by a more dominant growth of anterior segments (Luders et al., 2010). Thus, a disruption in this age-specific development in different callosal segments may explain why we observed more prominent deficits in the anterior regions in SA subjects, who were older on average, while LA subjects, who were younger on average, show deficits to be more posterior. In addition, it remains a possibility that FASD subjects from these demographically distinct sites may have unique behavioral or cognitive characteristics that contribute to the different pattern of reduced callosal thickness and area observed among sites. Thus, it would be important for future studies to include behavioral and cognitive measures to help further our understanding of the relationship between FASD and CC morphology.
Another key finding of the current study is the significant correlation between regional corpus callosum integrity and facial dysmorphology revealed by our exploratory analyses. Specifically, we found decreased PFL size to be associated with reduced thickness and area in the anterior third segment. This is consistent with previous reports which found children with FAS who had facial dysmorphology to have significantly lower FA in the CC than those without facial dysmorphology (Fryer et al., 2009b). Similarly, Li and colleagues showed that subjects without facial dysmorphology fell in between the non-exposed controls and subjects with dysmorphology in terms of the FA and MD measures in the CC (Li et al., 2009). However, some studies failed to find significant relationships between facial characteristics and DTI measures of callosal integrity (Wozniak et al., 2009b). Overall, findings support that midline facial anomalies and morphological alterations in midline brain structures such as the CC may occur as part of the concurrent processes during embryogenesis. However, future studies are needed to understand to what degree facial dysmorphology could be linked to underlying callosal pathology as a result of prenatal alcohol use (Sulik, 2005).
One limitation of this study is that the group effects of FASD on callosal morphology were influenced or not dissociable from total white matter volume. This presents an issue faced by several previous studies where findings appear similarly complicated by observations that not only is overall brain volume reduced in FASD (Archibald et al., 2001, Swayze et al., 1997), but total white matter is often found to be disproportionately reduced compared to gray matter also (Archibald et al., 2001, Lebel et al., 2008). Although it is possible that callosal structural changes are not independent of the more general microcephaly frequently observed in FASD, the reduced thickness and area of the CC reported here may still be reflective of callosal dysgenesis and even postnatal callosal developmental deficiencies. Another limitation of this study is that because of the nature of a large-scale cohort study conducted across demographically distinct locations, characteristics such as ethnicity, age, and clinical symptoms naturally varied between sites. However, by statistically controlling for gender, age and site in any analysis that combined sites, the impact of such difference was diminished to the extent possible. While we did not find significant relationship between CC thickness or area and the level of alcohol exposure during pregnancy, these finding should be interpreted with caution since information about quantity and frequency of alcohol exposure was not available for the majority of the FASD subjects. Future studies may thus better clarify whether quantitative measures of that indicate the level of prenatal alcohol exposure associate with the extent of brain structural abnormalities. Lastly, as in any human research, it remains a possibility that concurrent prenatal exposure to other teratogenic substances in addition to alcohol may contribute to the observed callosal morphological changes in FASD. However, well-controlled animal studies supported a link between alcohol and callosal abnormalities by demonstrating that prenatal alcohol exposure alone is sufficient to induce structural brain abnormalities (Sulik, 2005).
In summary, results from this relatively large cohort show that children and adolescents with histories of prenatal alcohol exposure exhibit significantly reduced thickness and area of the CC compared to non-exposed controls, and serve to confirm and extend earlier reports. Results further indicated the presence of an association between callosal morphological alterations and facial dysmorphology. Even when total white matter was accounted for, callosal thickness and area measures were still significantly correlated with facial dysmorphology among FASD subjects, especially in the anterior section. Findings may reflect a primary alcohol-induced deficiency or developmental delays in the growth of midbrain structures, namely the CC, in FASD, however, future studies are needed to clarify the exact impact of alcohol on developing brain, such as the duration of alcohol use or the timing of exposure, to fully understand the role of callosal anomalies in the etiology of FASD.
Supplementary Material
Acknowledgements
All or part of this work was performed in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. This work was also supported by NIDA R01DA017831, NIAAA U01 AA017122-01, NICHD R01 HD053893-01, NIAAA U24AA014811 and the March of Dimes 6-FY2008-50 (ERS), NIAAA U01 AA014834 (SNM), and U01 AA 11685 and R01 AA15134 (PAM). We would like to acknowledge the efforts in data collection of Suzanne Houston, Ariel Starr, and Genevieve Rodriguez in Los Angeles; Tania Badenhorst, Dominique Brand, Claire Corbett, Gosia Lapinska, and Karen Van Eden in Cape Town, South Africa; and Andria Norman, Jessica O’Brien, Kristina Hubbard, and Delilah Bolo in San Diego. We would also like to thank John Colby for technical assistance.
Footnotes
Disclosures: All authors report no conflicts of interests.
Reference
- Anderson MJ, Ter Braak CJF. Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation. 2003;73:85–113. [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine and Child Neurology. 2001;43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol-exposed individuals: Introducing the 4-Digit Diagnostic Code. Alcohol and Alcoholism. 2000;35:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Sampson PD, Streissguth AP, Connor PD. Geometric morphometrics of corpus callosum and subcortical structures in the fetal-alcohol-affected brain. Teratology. 2001;64:4–32. doi: 10.1002/tera.1044. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Luders E, Di Iulio F, Cherubini A, Passafiume D, Thompson PM, Caltagirone C, Toga AW, Spalletta G. Callosal atrophy in mild cognitive impairment and Alzheimer's disease: Different effects in different stages. Neuroimage. 2010;49:141–149. doi: 10.1016/j.neuroimage.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, Krick C, Konrad C. Total Brain Volume and Corpus Callosum Size in Medication-Naive Adolescents and Young Adults with Autism Spectrum Disorder. Biol Psychiatry. 2009;66:316–319. doi: 10.1016/j.biopsych.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Mattson SN, Tapert SF, Norman AL, Riley EP. Neuropsychological comparison of multiple groups of youth at risk for developing substance abuse. Alcoholism-Clinical and Experimental Research. 2009a;33:262A–262A. [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP. Characterization of White Matter Microstructure in Fetal Alcohol Spectrum Disorders. Alcoholism-Clinical and Experimental Research. 2009b;33:514–521. doi: 10.1111/j.1530-0277.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin EA, Dehart DB, Parnell SE, O'Leary-Moore SK, Sulik KK. Ventromedian forebrain dysgenesis follows early prenatal ethanol exposure in mice. Neurotoxicology and Teratology. 2010;33:231–239. doi: 10.1016/j.ntt.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman C, Paneth N, Little R, Pintomartin J. Perinatal brain injury in premature-infants born to mothers using alcohol in pregnancy. Pediatrics. 1995;95:66–73. [PubMed] [Google Scholar]
- Hoyme DB, Hoyme HE, Jones KL, Robinson L, Manning M, Bezuidenhout H, Marais AS, Kalberg W, Gossage J, May P. A south african mixed race lip/philtrum guide for diagnosis of fetal alcohol spectrum disorders. Journal of Investigative Medicine. 2010;58:274. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine Criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeret JS, Serur D, Wisniewski K, Fisch C. Frequency of agenesis of the corpus-callosum in the developmentally disabled population as determined by computerized-tomography. Pediatric Neuroscience. 1986;12:101–103. doi: 10.1159/000120229. [DOI] [PubMed] [Google Scholar]
- Jeret JS, Serur D, Wisniewski KE, Lubin RA. Clinicopathological findings associated with agenesis of the corpus-callosum. Brain & Development. 1987;9:255–264. doi: 10.1016/s0387-7604(87)80042-6. [DOI] [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, Chambers CD. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 2006;118:E1734–E1738. doi: 10.1542/peds.2006-1037. [DOI] [PubMed] [Google Scholar]
- Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J, Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcoholism-Clinical and Experimental Research. 2008;32:1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Li LC, Coles CD, Lynch ME, Hu XP. Voxelwise and Skeleton-Based Region of Interest Analysis of Fetal Alcohol Syndrome and Fetal Alcohol Spectrum Disorders in Young Adults. Hum Brain Mapp. 2009;30:3265–3274. doi: 10.1002/hbm.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Elberger AJ. Effect of prenatal alcohol exposure on midsagittal commissure size in rats. Teratology. 2001;63:15–22. doi: 10.1002/1096-9926(200101)63:1<15::AID-TERA1003>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Loeser JD, Alvord EC. Agenesis of corpus callosum. Brain. 1968;91:553. doi: 10.1093/brain/91.3.553. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The Development of the Corpus Callosum in the Healthy Human Brain. Journal of Neuroscience. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XY, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang DL, Hu XP. Evaluation of corpus callosum Anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcoholism-Clinical and Experimental Research. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Foround T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-RämÖ I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP CIFASD. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SNJ, Jernigan TL, Riley EP. MRI and prenatal exposure: Images provide insight into FAS. Alcohol Health and Research World. 1994;18:49–52. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Adnams CM, Hoyme HE, Jones KL, Robinson LK, Khaole NCO, Snell C, Kalberg WO, Hendricks L, Brooke L, Stellavato C, Viljoen DL. The epidemiology of fetal alcohol syndrome and partial FAS in a South African community. Drug and Alcohol Dependence. 2007;88:259–271. doi: 10.1016/j.drugalcdep.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais AS, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL. Maternal risk factors for fetal alcohol synfrome and partial fetal alcohol syndrome in South Africa: A third study. Alcohol Clin Exp Res. 2008;32:738–753. doi: 10.1111/j.1530-0277.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Vogt BA. The postnatal-growth of the callosal connections of primary and secondary visual-cortex in the rat. Developmental Brain Research. 1984;14:304–309. doi: 10.1016/0165-3806(84)90319-5. [DOI] [PubMed] [Google Scholar]
- Pannek K, Mathias JL, Bigler ED, Brown G, Taylor JD, Rose S. An automated strategy for the delineation and parcellation of commissural pathways suitable for clinical populations utilising high angular resolution diffusion imaging tractography. Neuroimage. 2010;50:1044–1053. doi: 10.1016/j.neuroimage.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang M, Wang MW, Elberge RAJ. Second trimester prenatal alcohol exposure alters development of rat corpus callosum. Neurotoxicology and Teratology. 2002;24:719–732. doi: 10.1016/s0892-0362(02)00267-2. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus-callosum in children prenatally exposed to alcohol. Alcoholism-Clinical and Experimental Research. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: An overview with emphasis on changes in brain and behavior. Experimental Biology and Medicine. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O'Connor MJ, Narr KL, Sowell ER. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat HB. In: Development disorders of the nervous system, in Neurology in Clinical Practice., Vol. II, Neurology in Clinical Practice. Bradley WG, Daroff RB, Fenichel GM, Marsden CD, editors. Boston: Butterworth-Heinemann; 1991. pp. 1258–1259. [Google Scholar]
- Schulte T, Muller-Oehring EM. Contribution of Callosal Connections to the Interhemispheric Integration of Visuomotor and Cognitive Processes. Neuropsychology Review. 2010;20:174–190. doi: 10.1007/s11065-010-9130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheime RSY. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. Journal of Neuroscience. 2008;28:1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates - Effects of heavy prenatal alcohol exposure. Neurology. 2001;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sulik KK. Genesis of alcohol-induced craniofacial dysmorphism. Experimental Biology and Medicine. 2005;230:366–375. doi: 10.1177/15353702-0323006-04. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC. Sequence of developmental alterations following acute ethanol exposure in mice - craniofacial features of the fetal alcohol syndrome. American Journal of Anatomy. 1983;166:257–269. doi: 10.1002/aja.1001660303. [DOI] [PubMed] [Google Scholar]
- Swayze VW, Johnson VP, Hanson JW, Piven J, Sato Y, Giedd JN, Mosnik D, Andreasen NC. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Tomasch J. Size, distribution, and number of fibres in the human corpus callosum. Anat. Rec. 1954;119:119–135. doi: 10.1002/ar.1091190109. [DOI] [PubMed] [Google Scholar]
- Vaurio LREP, Mattson SN. Neuropsychological Comparison of Children with Heavy Prenatal Alcohol Exposure and an IQ-Matched Comparison Group. Journal of the International Neuropsychological Society. 2011:1–11. doi: 10.1017/S1355617711000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The WSIC-IV technical and interpretive manual. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Witelson SF. Hand and sex-differences in the isthmus and genu of the human corpus-callosum - a postmortem morphological-study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, Lim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcoholism-Clinical and Experimental Research. 2006;30:1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, Nelson ML, Chang PN, Lim KO. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res. 2009a;33:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Muetzel RL, Mueller BA, McGee CL, Freerks MA, Ward EE, Nelson ML, Chang PN, Lim KO. Microstructural Corpus Callosum Anomalies in Children With Prenatal Alcohol Exposure: An Extension of Previous Diffusion Tensor Imaging Findings. Alcoholism-Clinical and Experimental Research. 2009b;33:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Nuechterlein KH, Phillips OR, Hamilton LS, Subotnik KL, Asarnow RF, Toga AW, Nar RKL. The contributions of disease and genetic factors towards regional cortical thinning in schizophrenia: the UCLA family study. Schizophrenia Research. 2010;123:116–125. doi: 10.1016/j.schres.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Mickus LA. Sex-differences in corpus-callosum - influence of prenatal alcohol exposure and maternal undernutrition. Brain Research. 1990;537:115–122. doi: 10.1016/0006-8993(90)90347-e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


