Abstract
Background
The acute locomotor effects of voluntary ethanol intake in mice (stimulation/sedation) might be important behavioral indicators of an animals’ propensity to engage in ethanol consumption and/or ethanol seeking behaviors. Using a binge-like ethanol intake model dubbed “Drinking-in-the-Dark”, we recently observed home cage locomotor stimulation in C57BL/6J mice during an acute ethanol intake session but acute home cage locomotor sedation following repeated ethanol exposures. To determine the role of novelty and/or ethanol history on these previously described locomotor effects, and to determine the relationship between these variables on locomotor activity immediately following DID intake, we conducted two separate experiments.
Methods
In experiment 1, mice were given access to either ethanol or water and locomotor activity was monitored immediately afterwards. In experiment 2, mice were given 13 days access to ethanol or water solution while home cage locomotor activity was monitored. On the 14th day, half of the water consuming animals received ethanol access for the first time. On the 15th day, all animals received ethanol access and locomotion was assessed afterwards in locomotor activity testing chambers.
Results
In experiment 1, locomotor activity following DID was positively associated with ethanol intake and BECs. In experiment 2, the group that received ethanol for the first time on the 14th day did not display locomotor stimulation. Locomotor activity following DID ethanol intake was positively associated with BECs in all groups regardless of ethanol history.
Conclusions
These results suggest that 1) DID-induced locomotor stimulation in the home cage may involve relative familiarity with the DID procedures and 2) locomotor stimulation immediately following DID is directly related to the relative concentration of ethanol in blood; an effect that is not altered by prior ethanol history. These data add new evidence of the pharmacological actions of binge-like ethanol intake, and provide a basis by which we may explore the motivation and consequences of such binge consumption.
A major goal of characterizing the nature of alcohol (ethanol)-induced behaviors is to determine how they might be involved in the propensity to engage in ethanol consumption and/or ethanol seeking behaviors. In mice, acute ethanol exposure induces many observable behavioral alterations that depend greatly on ethanol dose, genetic background, and the environmental and contextual parameters under which it is experienced. Furthermore, the relationship between these variables and the acute behavioral effects of ethanol often change dynamically as a function of previous ethanol history (i.e. tolerance/sensitization). To date, many studies have characterized acute behavioral responses to ethanol in mice (Crabbe et al., 1994). However, the vast majority of these studies have evaluated behavioral alterations following experimenter administered doses of ethanol. There have been relatively few studies that have evaluated the ability of voluntary ethanol consumption to directly produce (acute) alterations in behavior. And as might be expected, even fewer studies have evaluated how such alterations might change with repeated exposures.
We have recently published the results of work in which we used a binge-like ethanol intake model known as Drinking-in-the-Dark (DID) to determine the effects of such intake on concomitant locomotor activity (Linsenbardt et al., 2011). Interestingly, we observed an increase in locomotor behavior within the very first ethanol exposure session (stimulation) and a marked decrease in locomotor behavior (sedation) within the final session following approximately 2 additional weeks of daily DID drinking history. To better understand the nature of these previously characterized locomotor effects, we performed and now describe two separate experiments here. The first experiment was designed to address the relationship between the degree of ethanol exposure and subsequent locomotor behavior; locomotion immediately following binge ethanol intake. The second experiment was designed to address the possibility that novelty might have contributed to the previously observed locomotor stimulant response on the first day of DID access (during ethanol consumption), as well as to determine if a single or many ethanol exposures might differentially alter the DID-induced locomotor response observed in experiment 1 immediately following ethanol intake.
Methods
Animals
Male C57BL/6J mice (7-week old) were purchased from Jackson Laboratory (Bar Harbor, ME) and shipped to the animal facility at Indiana University – Purdue University Indianapolis (IUPUI). Upon arrival animals were acclimated to single housing in standard shoebox mouse cages and a 12 hour reverse light/dark cycle with lights off at 10:30 AM for at least a week prior to testing. All animals had ad lib access to food and water except during the ethanol access session when only ethanol was available, and (in some cases) during locomotor behavioral testing. All procedures were approved by the Purdue School of Science Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academic Press, 2003).
Ethanol Solutions
Ethanol drinking solution (20% v/v) was made with 190 proof ethanol purchased from Pharmco, Inc (Brookfield, CT) and regular tap water. Drinking solutions were made fresh daily.
Drinking in the Dark (DID)
DID procedures performed in our lab have been previously described (Moore et al., 2007). Three hours into the dark cycle each day, animals received access to an unsweetened 20% ethanol solution or tap water for 2 hours. Water bottles were replaced with ball-bearing sipper tubes filled with ethanol or tap water and fluid volumes were recorded to determine intake across DID access days for each individual animal.
Locomotor Activity Testing Chambers
Locomotor activity testing was conducted using the VersaMax Animal Activity Monitoring System (Accuscan Instruments Inc., Columbus, OH). Locomotor activity was detected by interruption of intersecting photocell beams evenly spaced along the walls of the 40×40 cm test chamber. This equipment was situated in sound-attenuating box chambers (inside dimensions, 53 cm across × 58 cm deep × 43 cm high) equipped with a house light and fan for ventilation and background noise. The locomotor activity testing equipment was interfaced with a Dell computer. Testing continued for 15 minutes during which time consecutive photocell beam interruptions were translated into distance traveled (cm) by the VersaMax computer program.
Home Cage Locomotor Apparatus
Home cage locomotion was monitored using a CI Multi-Device Interface (Columbus Instruments Inc., Columbus, OH) in conjunction with a Dell computer. Ambulatory activity was detected by the interruption of photocell beams (0.32 cm diameter; 875 nanometer wavelength; 160 hertz scan rate) positioned along the walls of standard shoebox mouse cages (18.4 cm wide X 29.2 cm long X 12.7 cm tall). For each home cage, two 33 cm long photocell sensor units (containing 12 recording photocell detector or emitters each spaced 2.54 cm apart) were positioned 27 cm apart along the long walls of the mouse cage, and two 24 cm long photocell sensor units (containing 8 recording photocell detector or emitters each spaced 2.54 cm apart) were positioned 32 cm apart along the short walls of the mouse cage. Data were collected in 1-min time intervals for a total of 2 hours during each DID session and translated into ambulatory counts using the provided software (version 1.4.0).
Blood Sampling
For the determination of blood ethanol concentrations (BECs), 50μl peri-orbital sinus bloods were drawn immediately following behavioral testing on the final test day. Samples were centrifuged and plasma was withdrawn and stored at −20°C. BECs were then determined using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
Procedures
Experiment 1: Locomotor Alterations Following DID
The goal of experiment 1 was to determine if a single acute DID ethanol exposure leads to alterations in locomotor activity. Furthermore, we wished to determine if the relative concentration of alcohol in blood was directly related to these locomotor alterations. The procedural timeline of this experiment is outlined in Table 1. Out of the 16 animals in this experiment, 8 were assigned to the acute ethanol group (WE) and the remaining 8 were assigned as water drinking controls (WW). Using standard DID procedures detailed above, both groups were given access to water solution for the first 2 days. The 1st day animals were habituated to the experimental procedures, and the 2nd day was used to generate baseline data. On the 3rd day, the WE group was given access to 20% ethanol solution in lieu of water and the WW group was again given water. On all three of these days, immediately following fluid access, all animals were removed from their cages and placed into the VersaMax locomotor monitoring apparatus (detailed above) for 15 minutes. Our decision to monitor animals for 15 minutes was to allow for a direct comparison to recently published work in which this time interval was used to monitor locomotion following experimenter administered ethanol after differential DID history (Linsenbardt et al., 2011). We were also limited by the number of VersaMax chambers in our lab (8); an issue that was particularly limiting given the strict time constraints of the limited access DID procedure. Thus, on each day animals were given access to 2 hours of fluid and then immediately tested for locomotion. Blood samples were taken immediately following locomotor testing on the final (3rd) day.
Table 1.
Experiment 1
| Treatment Group | Habituation/Baseline
|
Locomotion Following DID (not home cage)
|
|---|---|---|
| Days 1–2 | Day 3 | |
| Repeated Water (WW) | Water | Water |
| Acute Ethanol (WE) | Water | Ethanol |
Experiment 2: Role of Novelty and Ethanol History in DID-induced Locomotor Stimulation
The goal of experiment 2 was to determine if the relative novelty of the DID procedure and/or ethanol solution were responsible for the increase in locomotor activity/stimulation in the home cage (see Linsenbardt et al., 2011) and/or immediately following DID ethanol access (see results of experiment 1). The procedural timeline of this experiment is outlined in Table 2. The treatment groups for this experiment were similar to those in the previous experiment. However, in addition to the WW and WE groups, there was a group that received ethanol access throughout the entire experiment (EE). On the first 13 days of this experiment, the WW and WE groups were given access to water solution and the EE group was given access to ethanol solution using the DID procedures detailed above. On the 14th day, the WW and EE groups received access to water and ethanol solutions respectively. However, the WE group received ethanol solution on this day in lieu of water. Thus, the WE group had experience with the DID procedures and sipper tube for 13 days prior to its 1st ethanol exposure session. Home cage locomotor activity was recorded during each of these first 14 DID sessions.
Table 2.
Experiment 2
| Treatment Group | Daily DID/Home Cage Locomotion
|
DID/Home Cage Locomotion
|
Locomotion Following DID (not home cage)
|
|---|---|---|---|
| Days 1–13 | Day 14 | Day 15 | |
| Repeated Water (WW) | Water | Water | Ethanol |
| Acute Ethanol (WE) | Water | Ethanol | Ethanol |
| Repeated Ethanol (EE) | Ethanol | Ethanol | Ethanol |
At the onset of the ‘lights off’ phase of the light cycle on the 15th day, all mice were removed from their home cages and placed directly into the VersaMax locomotor activity monitoring chambers for 15 minutes to habituate them to the apparatus and procedure. After this 15 minute habituation session mice were immediately placed back into their home cages. Approximately 3 hours later, all animals were given access to ethanol solution following normal DID procedures. The WW group had access to ethanol for the first time on this day, the WE group had access to ethanol for the 2nd consecutive day, and the EE group had access to ethanol for the 15th consecutive day. Immediately after ethanol access on this day, all animals were placed into the VersaMax locomotor activity monitoring chambers for 15 minutes. This apparatus was used so that we could directly compare the locomotor effects following DID in this experiment to those results reported in experiment 1. Bloods were sampled immediately after removal from the chambers for determination of BECs. Home cage locomotor activity data was not collected on this final day.
Statistical Analysis
Mean water intake (the first 2 days) and locomotor activity (all 3 days) for experiment 1 were analyzed using a mixed 2-way analysis of variance (ANOVA) with day as the within subject’s factor and fluid assignment group as the between groups factor. Day 3 locomotor activity was also analyzed separately using a student’s T-test.
Mean water intake of the WW and WE groups on days 1–13 of experiment 2 were analyzed using a mixed two-way ANOVA with days as the within subject’s factor and fluid assignment as the between groups factor. To determine between groups differences in ethanol intake on day 14 (EE vs. WE) a student’s T-test was conducted. Home cage locomotor activity data on days 1 and 14 were analyzed by two-way ANOVA with day as the within subjects factor and fluid group assignment as the between groups factor. Linear regression analysis was also conducted on home cage locomotor activity across all 14 days for the WW and EE groups in order to describe the changes in locomotion in the EE group compared to water drinking (WW) controls. Locomotor data collected immediately following DID ethanol access on day 15 was analyzed by one-way ANOVA with fluid assignment as the between groups factor.
For both experiments, Pearson’s correlation was used to measure the linear relationship between intake, BEC, and locomotor activity. Tukey post-hoc tests were performed when appropriate. Data were considered significant at p<0.05.
Results
Experiment 1
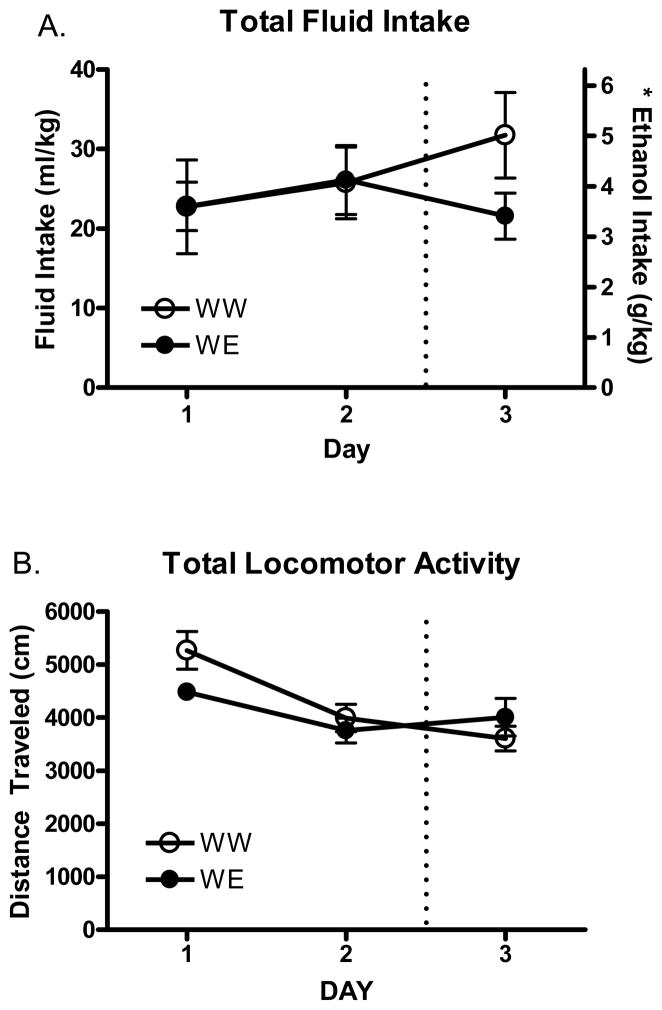
Ethanol and water consumption on days 1–3 can be seen in Figure 1. There were no differences in water consumption across the first 2 days (Figure 1a; p>.05). Analysis of locomotor activity following DID access across all 3 days (Figure 1b) revealed a significant main effect of day [F(2, 28)=17.7 p<.0001] and a significant day*fluid group interaction [F(2, 28)=4.4 p<.05]. However, post hoc tests did not indicate any differences between groups. Furthermore, separate analysis (t-test) of day 3 for both intake and locomotion indicated no differences (p>.05). Thus, there were no differences in baseline, or DID-induced locomotor effects when analyses of group means were conducted.
Figure 1.
Total fluid intake and locomotor activity across days. A. Total water and ethanol intake in mice across 3 daily DID sessions. Mice from the WW group (N=8) received water on all 3 days and mice in the WE group (N=8) received water on the first 2 days and ethanol on the final (3rd) day. B. Total locomotor activity in mice immediately following intake across 3 daily DID sessions. * Ethanol intake in the WE group only.
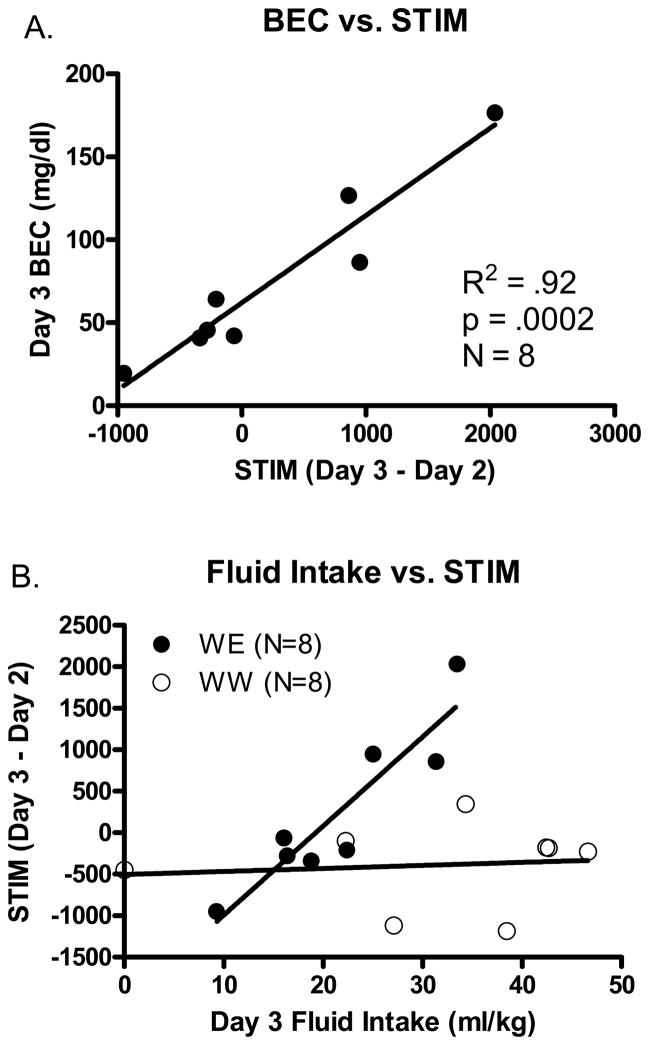
When the relationships between these data variables were analyzed as a function of individual differences, a different picture emerged (see Figure 2). Consistent with much data from our lab using the current DID procedures, ethanol intake was positively associated with BEC (data not shown; R2=.90; N=8; p=.0003). Interestingly, BEC was also positively associated with day 3 locomotor activity (R2=.65; N=8; p=.016) as well as the difference between day 3 locomotion and day 2 baseline locomotion (Figure 2a; R2=.92; N=8; p=.0002); the latter comparison the one we believe is the most meaningful for this experimental design. Furthermore, fluid intake was significantly positively associated with locomotor activity in the ethanol consuming WE group (R2=.84; N=8; p=.0014) but not in the water consuming WW control group (R2=.01; N=8; p>.05; Figure 2b). Thus, although the cause of these relationships is not immediately clear, it is possible that the concentration of ethanol in blood in some way interacts with (or drives) the observed locomotor alterations.
Figure 2.
Relationship between intake, locomotion, and BEC on day 3. A. The STIM response (day3-day2) is significantly positively associated with BEC. B. Fluid intake is significantly positively associated with The STIM response (day3-day2).
Experiment 2
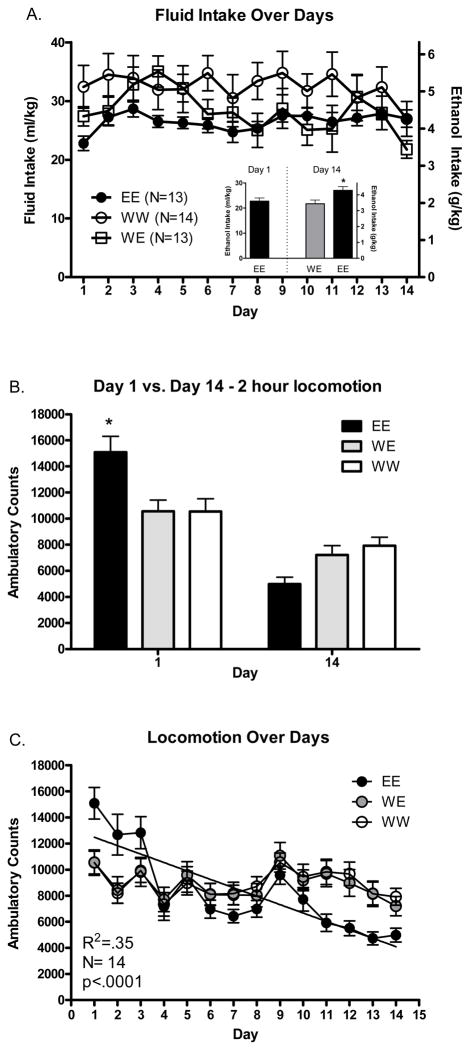
Ethanol and water consumption on days 1–14 can be seen in Figure 3a. There were no significant differences in water consumption over the first 13 days between the WW and WE groups. Analysis of EE and WE ethanol consumption on day 14 indicated that the EE group drank significantly more ethanol than the WE group on this day (t=2.51; df=24; p<.05). Importantly, there was no difference in mean ethanol intake between the EE group on day 1 and the WE group on day 14; the days that correspond to the first ethanol exposure for each group respectively.
Figure 3.
Results of fluid intake and home cage locomotor activity across days. A. Total fluid intake across the first 14 days. Inset reflects mean ethanol intake in the WE (N=13) group on day 14 and the EE (N=13) group on days 1 and 14. B. Total home cage locomotor activity on days 1 and 14. C. There is a significant negative relationship between total home cage locomotor activity and day in the EE group but not the WW (N=14) group. Only the regression line of the EE group is shown.
Home cage locomotor activity on days 1 and 14 can be seen in Figure 3b. Analysis revealed a significant main effect of day [F(1, 37)=97.7 p<.0001] and a significant day*group interaction [F(2, 37)=19.2 p<.0001]. Post hoc tests confirmed that the EE group had significantly higher locomotor activity than both the water consuming groups on day 1 (p<.05). However, there were no significant differences in locomotor activity between any of the groups on day 14 (p>.05). Thus, while the relative increase in locomotor activity in the EE group on day 1 replicates previous findings (Linsenbardt et al., 2011), the lack of stimulation in the WE group on day 14 suggests that the relative novelty of the DID procedure plays a role in this phenomenon.
Analysis of home cage locomotor activity on days 1–14 can be seen in Figure 3c. Analysis revealed a significant main effect of day [F(13, 481)=23.5 p<.0001] and a significant day*group interaction [F(26, 481)=8.2 p<.0001]. To evaluate differences in the change in locomotor activity between the EE and WW groups across days, we analyzed the daily home cage locomotor activity scores using linear regression analysis. Results of this analysis revealed a significant negative relationship between locomotor activity and day in the EE group (Figure 3c; R2=.35; N=14; p<.0001) indicating a progressive decrease in locomotor activity over days, and a non-significant relationship in the WW group (R2=.00; N=14; p=.73; regression line not shown).
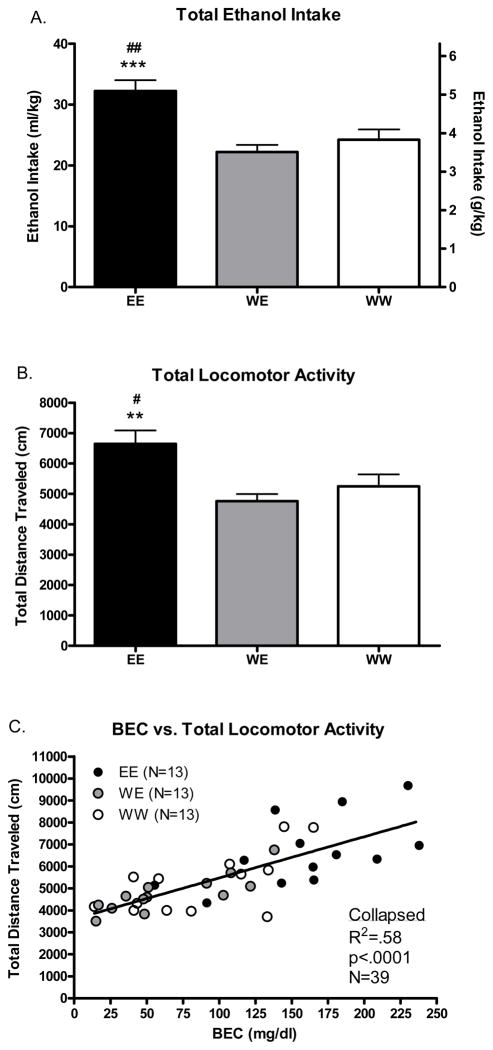
Day 15 data can be seen in Figure 4. One animal from the WW group was not tested on this day after it became apparent that the ethanol bottle had leaked due to a small crack. Results of total ethanol intake indicated a significant effect of group [Figure 4a; F(2, 38)=11.5 p=.0001] which post hoc test confirmed was due to relatively higher drinking in the EE group compared to the WE (p<.001) and WW (p<.01) groups. Results of total locomotor activity also indicated a significant effect of group [Figure 4b; F(2, 38)=7.2 p<.01] with post hoc test confirming that this difference was due to relatively higher locomotor activity in the EE group compared to the WE (p<.01) and WW (p<.05) groups. There were no differences between groups in locomotor activity collected during the habituation session (p=.37). Group means of the habituation data were: EE = 5741±290, WE = 5129±347, WW = 5330±284.
Figure 4.
Ethanol intake, locomotor activity and BECs on day 15. A. Total ethanol intake on day 15. B. Total locomotor activity immediately following ethanol intake session. C. Total locomotor activity and BEC are significantly positively associated.
Following up on the first experiment, we again evaluated the nature of relationships between the variables of interest. Ethanol intake was positively associated with BECs when collapsing on fluid group (R2=.75; N=39; p=.0003) and when analyzed separately in the EE (R2=.49; N=13; p<.01), WE (R2=.65; N=13; p<.01), and the WW (R2=.72; N=13; p<.001) group (data not shown). Consistent with the first experiment, BECs were also positively associated with locomotor activity on this day when collapsing on fluid group (Figure 4c; R2=.58; N=39; p<.0001) and when analyzed separately in the EE (R2=.34; N=13; p<.05), WE (R2=.42; N=13; p<.05), and the WW (R2=.69; N=13; p<.001) group. These results support data from experiment 1 where we observed a stimulant response immediately following a single DID ethanol session in ethanol naïve animals. These results also suggest that the relationship between BEC and locomotion does not change as a function of previous ethanol experience.
Discussion
Consistent with previous research using the DID model, mice reliably consumed large quantities of ethanol when given 2 hours of access during their peak arousal period. This intake elicited blood ethanol concentrations known to produce behavioral intoxication (Linsenbardt et al., 2011, Moore et al., 2007) and was associated with alterations in locomotor activity within drinking sessions (home cage), and immediately following drinking sessions (VersaMax). Both the novelty of the DID procedures as well as the relative degree of prior ethanol exposure were influential in some of the observed locomotor alterations.
Experiment 1 – Locomotion Following DID
There were no mean differences between the ethanol and water groups in locomotor activity immediately following intake on day 3. However, our within-subject analysis of the relationship between locomotor activity, intake, and BEC was suggestive of distinct group differences.
First, BECs (mg/dl) immediately following locomotor testing in the WE animals were positively associated with locomotion on day 3. BECs were also positively associated with day 3 locomotor activity when each individuals baseline locomotion (day 2) was taken into account (‘STIM’). This result suggests that the relative concentration of ethanol in blood resulting from an acute ethanol binge might directly mediate subsequent locomotor behavior in the VersaMax apparatus; those with the highest BECs exhibiting relative increases in locomotor behavior compared to the more sober individuals showing no change or slight decreases compared to the previous day’s baseline measure.
This result was further supported by the finding that amount of ethanol consumed (ml/kg) was positively associated with the STIM response. Because ethanol intake has consistently been found to be positively associated BECs using identical procedures (Linsenbardt and Boehm, 2009, Linsenbardt et al., 2011), this result was not surprising. Together these data suggest that the relative blood ethanol concentration induced by an acute ethanol binge might directly mediate subsequent locomotor behavior. However, as with all correlational findings, the relationship between these variables may be due to other factors that were not measured. Likewise, it is possible that an individual animal’s locomotor activity might drive and predict ethanol intake rather than ethanol intake and subsequent blood ethanol concentration predicting locomotion. To be certain, organisms with the propensity to display increased spontaneous locomotor behavior might also be expected to show increases in other motor-related behaviors, regardless of the index. However, that water intake was not associated with locomotor activity argues against such a possibility.
It was rather unexpected that animals placed in the VersaMax chambers following the very first ethanol exposure did not display between-groups stimulation given the significant positive relationship between ethanol consumption and locomotor activity. Although this may seem to provide additional support for the hypothesis that habituation to the DID procedures alters the locomotor response to ethanol, we believe there are several details of both experiments 1 and 2 that make this interpretation (for locomotor data collected following intake only) less likely. In those groups of animals that were placed in the chambers following the first ethanol exposure (WE group Exp1 and WW group Exp2), there were some mice that drank very little ethanol and some whose drinking was considerable. Given the significant positive relationship between intake, BEC, and locomotion, had there been more animals drinking sufficient volumes of ethanol on this first ethanol exposure day, we believe there would have been a significant between- groups stimulation. In support of this, the EE animals in experiment 2 that had many prior ethanol exposures drank much more, had higher BECs, and did display a significantly higher locomotor response than the other two groups with 1 or no previous ethanol experience (see below).
Experiment 2 – Home Cage Locomotion
Consistent with recently published results from our lab(Linsenbardt et al., 2011), the first DID ethanol session in the EE group on day 1 elicited a significant home cage locomotor stimulant response, whereas the final (14th) ethanol exposure in this group elicited locomotor sedation. A detailed discussion of these findings can be found in the above mentioned manuscript (Linsenbardt et al., 2011).
We incorporated the WE group in this study to determine if novelty of the DID procedures and/or ethanol solution might play a role in the acute stimulant response observed on the first ethanol access session in naïve EE mice. Interestingly, the stimulant response was absent in the WE group that received ethanol access for the first time on day 14, suggesting that novelty was indeed at least partially responsible for the acute stimulant effect observed in the EE mice during the 1st ethanol access session on day 1. It is difficult to know precisely how ethanol might have contributed to this effect. One possibility is that familiarity with DID procedures led to alterations in the amount of ethanol intake between these two groups. However, the data did not indicate differences in mean ethanol intake between the EE group on day 1 and the WE group on day 14 (Figure 3A inset). Another possibility is that familiarity with the DID procedures in the WE group led to alterations in the rate of ethanol consumption. Certainly, the rate of ethanol consumption dictates the rate and peak of ethanol concentration in brain; factors which presumably have direct impact on ethanol-induced behaviors (Eckardt et al., 1998). This possibility is generally supported by comparisons with the EE group. The EE group displayed a progressive decrease in locomotor behavior with each successive DID session compared to water drinking WW controls (Figure 3D). This progressive decrease in locomotor activity could not be completely explained by increased total ethanol intake because mean intake in this group plateaued following the first few days of access (Figure 3A). Thus, if the WE group consumed ethanol more rapidly on day 14 than the EE group on day 1, the differences in home cage locomotor activity could be attributed to differential ethanol pharmacokinetics driven by this rate difference. Experiments evaluating the micro structure of fluid consumption between ethanol and water consuming groups under similar conditions might provide support for or against this possibility.
Experiment 2 - Locomotion Following DID
Day 15 of experiment 2 replicated the findings from experiment 1 that a single DID ethanol exposure induces locomotor alterations that are positively associated with the degree of BEC. Furthermore, the relationship between BEC and locomotor activity was also evident in those groups that had experienced 1 (WE) or many (EE) previous ethanol exposures. These effects have several implications worth discussion. First, that one or many (14) prior ethanol exposures did not alter the positive relationship between locomotor activity and BEC suggests that this effect is not directly modulated solely by the novelty of “feeling” ethanol’s effects. Furthermore, there were no indications that tolerance altered the observed relationship between BEC and locomotion despite the fact that 14 days of DID ethanol exposure is capable of eliciting behavioral tolerance (Linsenbardt et al., 2011). Although purely speculative, these collective results might suggest that the increased locomotor activity following DID sessions is driven by some degree of ethanol-induced anxiolysis (Kalant, 1990). That is, those animals with the highest BECs might also be less anxious in the relatively novel environment and therefore displayed more exploratory behavior. Indeed, ethanol has been shown to elicit anxiolysis in mice using 2 different apparatus designed for this purpose; the elevated plus maze and light-dark box (Boehm et al., 2002). Importantly, the degree to which the Versamax locomotor apparatus induces anxiety under the experimental conditions we employed is not known. Nonetheless, the relationship between locomotor activity and BEC in the present study may generally reflect the anxiolytic effects of ethanol.
Experiments 1 and 2: Home Cage Locomotion vs. Locomotion Following DID
There are 2 critical distinctions between locomotor activity recorded during intake versus locomotor activity recorded immediately following intake that we believe make data collected under these two conditions dissociable and unique: 1) the different contexts in which the activity was recorded (home cage vs. VersaMax) and 2) the duration of the locomotor test (120 min vs. 15 min). Each daily home cage assessment captures the locomotor response to the sipper tube and the locomotor consequences of ethanol consumed during a single 120 minute access session. This is in contrast to the VersaMax assessment which captures the initial locomotor response to a larger relatively novel environmental context in animals that continue to ‘feel’ the physiological effects of ethanol; effects they were already experiencing while in the home cage. That animals given many ethanol exposures showed no difference in locomotion (EE; Day 14), but robust locomotor stimulation when placed into the VersaMax environment (EE; Day 15) might be explained by these procedural differences alone. However, work evaluating the role of environmental context on behavioral responses to other drugs of abuse might provide some insight into the differential locomotor effects we observed with ethanol. For example, a unique environmental context has been shown to enhance the acute psychomotor activating effects of both amphetamine (Badiani et al., 1995) and morphine (Paolone et al., 2003) in comparison to motor activation experienced in home cage. Follow up studies evaluating the neuroanatomical basis of these effects suggests that these types of differential drug responses are due to the engagement of different corticostriatal circuits depending on the context in which the drugs were experienced (Badiani and Robinson, 2004). This differential engagement of brain systems may also explain why the first exposure to ethanol in the home cage induced locomotor stimulation only when the DID procedures were completely novel (EE Day1) but not following 13 previous daily experiences with the procedures (WE Day 14). That is, the novel DID procedures may have recruited similar brain circuits as those activated when in unique environmental contexts. Similar context-dependent differences in corticostriatal activation were found when evaluating alterations in locomotor activation following repeated drug exposure (Badiani and Robinson, 2004) and might explain why home cage locomotor activity was generally lower in animals with many ethanol exposures (EE day 14) compared to ethanol naïve animals (WE day 14). Additionally, the stress associated with the novel DID procedures may have contributed to these differences as has been previously observed with amphetamines (Anisman et al., 1985). Indeed, the home cage locomotor activity in the WW group on day 1 was generally higher than on the 14 DID session; an effect which might support this theory.
Conclusion
The current studies suggest that 1) DID-induced locomotor stimulation in the home cage may involve relative familiarity with the DID procedures and 2) locomotor stimulation immediately following DID is directly related to BEC; an effect which is not altered by prior ethanol history. Although the mechanisms behind these findings cannot be fully explained by the current data, they add new evidence of the pharmacological actions of binge-like ethanol intake, and provide a basis by which we may explore the motivation and consequences of such binge consumption using the DID model.
Acknowledgments
This work was supported by NIAAA grant #’s AA015434 (SLB), AA016789 (SLB), and AA07462 (DNL).
References
- ANISMAN H, HAHN B, HOFFMAN D, ZACHARKO RM. Stressor invoked exacerbation of amphetamine-elicited perseveration. Pharmacol Biochem Behav. 1985;23:173–83. doi: 10.1016/0091-3057(85)90552-0. [DOI] [PubMed] [Google Scholar]
- BADIANI A, BROWMAN KE, ROBINSON TE. Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res. 1995;674:291–8. doi: 10.1016/0006-8993(95)00028-o. [DOI] [PubMed] [Google Scholar]
- BADIANI A, ROBINSON TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15:327–39. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- BOEHM SL, 2ND, REED CL, MCKINNON CS, PHILLIPS TJ. Shared genes influence sensitivity to the effects of ethanol onlocomotor and anxiety-like behaviors, and the stress axis. Psychopharmacology (Berl) 2002;161:54–63. doi: 10.1007/s00213-002-1000-y. [DOI] [PubMed] [Google Scholar]
- CRABBE JC, GALLAHER ES, PHILLIPS TJ, BELKNAP JK. Genetic determinants of sensitivity to ethanol in inbred mice. Behav Neurosci. 1994;108:186–95. doi: 10.1037//0735-7044.108.1.186. [DOI] [PubMed] [Google Scholar]
- ECKARDT MJ, FILE SE, GESSA GL, GRANT KA, GUERRI C, HOFFMAN PL, KALANT H, KOOB GF, LI TK, TABAKOFF B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- KALANT H. Stress-related effects of ethanol in mammals. Crit Rev Biotechnol. 1990;9:265–72. doi: 10.3109/07388558909036738. [DOI] [PubMed] [Google Scholar]
- LINSENBARDT DN, BOEHM SL., 2ND Agonism of the endocannabinoid system modulates binge-like alcohol intake in male C57BL/6J mice: involvement of the posterior ventral tegmental area. Neuroscience. 2009;164:424–34. doi: 10.1016/j.neuroscience.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINSENBARDT DN, MOORE EM, GRIFFIN KD, GIGANTE ED, BOEHM SL., 2ND Tolerance to Ethanol’s Ataxic Effects and Alterations in Ethanol-Induced Locomotion Following Repeated Binge-Like Ethanol Intake Using the DID Model. Alcohol Clin Exp Res. 2011;35:1246–55. doi: 10.1111/j.1530-0277.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE EM, SERIO KM, GOLDFARB KJ, STEPANOVSKA S, LINSENBARDT DN, BOEHM SL., 2ND GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–13. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAOLONE G, BURDINO R, BADIANI A. Dissociation in the modulatory effects of environmental novelty on the locomotor, analgesic, and eating response to acute and repeated morphine in the rat. Psychopharmacology (Berl) 2003;166:146–55. doi: 10.1007/s00213-002-1321-x. [DOI] [PubMed] [Google Scholar]