Abstract
Background
Cyclin-dependent kinases (CDKs) regulate cell proliferation and coordinate the cell cycle checkpoint response to DNA damage. Although inhibitors with varying selectivity to specific CDK family members have been developed, selective CDK4/6 inhibitors have emerged as the most attractive antineoplastic agents because of the importance of CDK4/6 activity in regulating cell proliferation and the toxic effects associated with inhibition of other CDKs (eg, CDK1 and CDK2).
Methods
FVB/N wild-type mice (n = 13) were used to evaluate carboplatin-induced myelosuppression in bone marrow by complete blood cell counts after treatment with the CDK4/6 inhibitor PD0332991. Genetically engineered murine models of retinoblastoma (Rb)-competent (MMTV-c-neu) and Rb-incompetent (C3-TAg) breast cancer (n = 16 MMTV-c-neu mice in the carboplatin plus vehicle control group, n = 17 MMTV-c-neu mice in the carboplatin plus PD0332991 group, n = 17 C3-TAg mice in the carboplatin plus vehicle control group, and n = 14 C3-TAg mice in the carboplatin plus PD0332991 group) were used to investigate the antitumor activity of PD0332991 alone or in combination with chemotherapy. All statistical tests were two-sided.
Results
Coadministration of PD0332991 with carboplatin compared with carboplatin alone in FVB/N wild-type mice increased hematocrit (51.2% vs 33.5%, difference = 17.7%, 95% confidence interval [CI] = −26.7% to −8.6%, P < .001), platelet counts (1321 vs 758.5 thousand cells per μL, difference = 562.5 thousand cells per μL, 95% CI = −902.8 to −222.6, P = .002), myeloid cells (granulocytes and monocytes; 3.1 vs 1.6 thousand cells per μL, difference = 1.5 thousand cells per μL, 95% CI = −2.23 to −0.67, P < .001), and lymphocytes (7.9 vs 5.4 thousand cells per μL, difference = 2.5 thousand cells per μL, 95% CI = −4.75 to −0.18, P = .02). Daily administration of PD0332991 exhibited antitumor activity in MMTV-c-neu mice as a single agent. However, the combination of carboplatin plus PD0332991 decreased antitumor activity compared with carboplatin alone in Rb-competent mice (mean percent change in tumor volume at day 21 = −52.6% vs 3.7% for carboplatin and carboplatin plus PD0332991, respectively, difference = 56.3%, 95% CI = −109.0% to −3.6%, P = .04). In contrast, Rb-deficient tumors in C3-Tag mice were resistant to PD0332991, and coadministration of PD0332991 plus carboplatin had no effect on in vivo tumor growth (mean percent change in tumor volume at day 21 = 118.8% and 109.1% for carboplatin and carboplatin plus PD0332991, respectively, difference = 9.7%, 95% CI = −183.5% to 202.9%, P = .92). Finally, in tumor-bearing mice, coadministration of PD0332991 with carboplatin provided statistically significant protection of platelets (P = .04).
Conclusion
We believe that the present data support a possible role for CDK4/6 inhibitors in a majority of patients with advanced cancer: to either inhibit tumor growth in CDK4/6-dependent tumors or ameliorate the dose-limiting toxicities of chemotherapy in CDK4/6-indepdendent tumors. Our data also suggest CDK4/6 inhibitors should not be combined with DNA-damaging therapies, such as carboplatin, to treat tumors that require CDK4/6 activity for proliferation.
CONTEXT AND CAVEATS
Prior knowledge
Cyclin-dependent kinases (CDKs) are key regulators of the cell cycle, and studies have indicated that selective CDK4/6 inhibitors may have antitumor activity without the toxic effects observed when pan-CDK inhibitors are used. The CDK4/6 inhibitor, PD0332991, has been previously shown to protect hematopoietic cells from ionizing radiation.
Study design
The ability of PD0332991 to protect hematopoietic cells from cytotoxic chemotherapy was investigated in wild-type mice and tumor-bearing genetically engineered mice. Two genetically engineered mouse models of breast cancer were used to investigate the antitumor effects of combining PD0332991 with chemotherapy.
Contribution
The CDK4/6 inhibitor protected hematopoietic cells from carboplatin-induced cytotoxicity and had antitumor activity as a single agent in a HER2-positive mouse model of breast cancer (MMTV-c-neu) but did not in a retinoblastoma (Rb)-deficient model of human basal-like breast cancer (C3-TAg). However, when combined with chemotherapy drugs, PD0332991 decreased antitumor activity in Rb-competent mice compared with chemotherapy alone.
Implications
Tumors can be either CDK4/6-dependent or independent, and some patients may benefit from treatment with a CDK4/6 inhibitor either as an anti-neoplastic agent or as a chemoprotectant. However, selective CDK4/6 inhibition may decrease the efficacy of chemotherapeutic drugs whose mechanism is dependent on cell cycle activity for proliferation in tumors that are CDK4/6-dependent.
Limitation
For this study, genetically engineered mouse models of breast cancer were used, which do not fully represent the genetic heterogeneity observed in the regulation of the cell cycle in different cancers.
From the Editors
Cyclin-dependent kinases (CDKs) mediate cell cycle progression, regulating transition from G1 to S phase and G2 to M phase. CDK activity is tightly controlled throughout the cell cycle by posttranscriptional modifications as well as the expression of cyclins and CDK inhibitors. There are four proliferative CDKs: CDK1, which predominantly regulates the transition from G2 to M phase, and CDK2/4/6, which regulate the transition from G1 to S phase (1,2). Recent work has shown that there is considerable redundancy among the CDKs that regulate the transition from the G1 to S phase, and many proliferating cells can use any of these kinases. However, certain cells in the developing and adult mammal (eg, hematopoietic stem and progenitor cells [HSPCs] and pancreatic beta cells) absolutely require the activity of CDK4/6 for proliferation (3–10).
Inactivation of the G1 to S phase checkpoint through several events such as deletion of Rb or cyclin-dependent kinase inhibitor 2A (CDKN2A), CDK4 point mutation, or cyclin D1 (CCND1) amplification is a characteristic feature of human malignancy (11). Several of these lesions specifically activate CDK4 and/or CDK6, suggesting a subset of these tumors may require CDK4/6 activity for proliferation. Alternatively, some cell cycle regulatory events such as loss of Rb or human papillomavirus E7 expression would be predicted to obviate a cancer cell’s need for CDK4/6 activity for G1 to S traversal (11–15). Therefore, in general terms, cancers can be defined as either CDK4/6-dependent or CDK4/6-independent on the basis of molecular events that compromise the G1 checkpoint in a given tumor. Because so many diverse human cancers harbor genetic events that activate CDK4/6, it has been hypothesized that selective CDK4/6 inhibitors may have broad antitumor activity in human malignancies. The finding that most normal proliferating cells can use CDK2 or CDK4/6 for proliferation (4,5,9,16) also suggests that selective CDK4/6 inhibitors will not exhibit toxic effects, such as myelosuppression and enteropathy, which are induced by agents that nonspecifically inhibit the cell cycle such as pan-CDK inhibitors (17).
There have been extensive efforts to specifically target CDK4/6 (18,19), which have led to the development of an array of small-molecule CDK inhibitors of varying selectivity. These studies have led to the recent development of a few potent and selective CDK4/6 inhibitors. One of these agents, PD0332991 (15,20), has entered human clinical testing in estrogen receptor–positive breast cancer, myeloma, and other cancers likely to be CDK4/6 dependent (21,22). Two trials of other CDK4/6 inhibitors (NVP-LEE011 and LY2835219) have begun enrolling cancer patients for phase I clinical testing. The selective CDK4/6 inhibitors have demonstrated statistically significant antitumor activity in preclinical models of melanoma, glioblastoma, and breast cancer (eg, Rb-competent cell lines and xenografted tumors), with only minimal toxic effects during long-term treatment in rodents. As predicted, these agents have not exhibited activity in cell lines harboring genetic deletion of the CDK-target Rb (8,23–26). Although these data have suggested a role for selective CDK4/6 inhibitors as antineoplastic agents, we recently showed that murine melanomas characterized by a loss of CDKN2A were not dependent on CDK4/6 activity (8). Therefore, neither intact Rb function nor the presence of CDK4-activating lesions such as CDKN2A loss necessarily predicts CDK4/6 dependence.
Although targeted agents have transformed clinical oncology, traditional cytotoxic drugs and ionizing radiation remain the mainstay of curative cancer therapy. Myelosuppression continues to represent the major dose-limiting toxic effect of these cytotoxic treatments, resulting in considerable morbidity and mortality, and frequent reductions in chemotherapy dose intensity, which may compromise disease control and patient survival (27). To address this need, the US Food and Drug Administration has approved drugs for chemotherapy-induced anemia (ie, epoetin-α and darbepoetin-α) and neutropenia (ie, filgrastim and pegylated filgrastim), but these injectable biological agents are associated with substantial costs and morbidity (28–34). Therefore, myelosuppression is a major problem in cancer care and will remain a challenge even in the era of targeted therapies.
We have recently shown that selective CDK4/6 inhibitors could afford marked hematopoietic protection from lethal doses of ionizing radiation through the induction of “pharmacological quiescence” in early HSPCs (8). Because of these observations, we hypothesized that pharmacological quiescence might also protect the bone marrow from myelosuppression induced by cytotoxic chemotherapy. However, this strategy may also result in tumor protection, which has limited the use of other agents like amifostine and dexrazoxane as antitumor agents (35). In our study, we sought to combine pharmacological quiescence induced by the CDK4/6 inhibitor PD0332991 with effective cytotoxic chemotherapy in two well-defined autochthonous murine models of breast cancer with a compromised G1 checkpoint caused by different genetic events.
Materials and Methods
Compounds
PD0332991 (15,20) was synthesized by the Center for Integrative Chemical Biology and Drug Discovery at the University of North Carolina (Chapel Hill, NC). Carboplatin (Hospira Inc, Lake Forest, IL), doxorubicin (Bedford Laboratories, Bedford, OH), etoposide (Teva Parenteral Medicines Inc, Irvine, CA), paclitaxel (Ivax Pharmaceuticals Inc, Doral, FL), camptothecin (Sigma-Aldrich, St Louis, MO), and staurosporine (Sigma-Aldrich) were obtained from their respective manufacturers and handled according to the manufacturer's recommendations.
Cell line
Telomerized human diploid fibroblasts (tHDFs), a human foreskin fibroblast line immortalized with expression of human telomerase (36,37), were grown in an incubator at 37°C in a humidified atmosphere of 5% CO2 and cultured in Dulbecco's modified Eagle media containing 10% fetal bovine serum, 100 U/mL penicillin/streptomycin (Invitrogen, Carlsbad, CA), and 1× Glutamax (Invitrogen). The tHDF cells were previously engineered with a retrovirus encoding E2F-1 fused to a modified hormone-binding domain from the estrogen receptor, which upon 4-hydroxy tamoxifen treatment induces ARF (36). On receipt of the tHDF cells on November 14, 2007, they were authenticated by treatment with 4-hydroxy tamoxifen as previously described (36), and ARF induction was verified by real-time polymerase chain reaction (data not shown).
Assessment of DNA Damage and Apoptosis by γ-H2AX Flow Cytometry and Caspase Activation
For the γ-H2AX assay, 30 000 cells were plated per well in 12-well plates. For the caspase 3/7 assay, 1000 cells were plated per well in 96-well white wall clear bottom plates. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 for 24 hours. Cells were then incubated at 37°C in a humidified atmosphere of 5% CO2 with 10, 30, 100, or 300 nM PD0332991 or dimethyl sulfoxide (Sigma-Aldrich) vehicle control for 16 hours. Cells were then treated with chemotherapy (100 μM carboplatin, 1 μM doxorubicin, 5 μM etoposide, 156 nM camptothecin, or 250 nM paclitaxel) or 1.5 nM staurosporine. For γ-H2AX, cells were harvested for analysis 8 hours after exposure to chemotherapy or staurosporine.
For the γ-H2AX assay, tHDF cells were fixed and stained using the H2A.X Phosphorylation Assay Kit (Flow Cytometry; Millipore, Temecula, CA) by the manufacturer's instructions. γ-H2AX-positive tHDF cells were then quantified using a CyAn ADP Analyzer (Beckman Coulter, Indianapolis, IN) and FlowJo analysis software (Version 7.2.2; Tree Star, Ashland, OR). For the in vitro caspase 3/7 assay, tHDF cells were analyzed directly in the 96-well plates 24 hours after chemotherapy or staurosporine treatment. Caspase 3/7 activation was measured using the Caspase-Glo 3/7 Assay System (Promega, Madison, WI) by following the manufacturer's instructions.
Mice
All mouse experiments, treatments, and housing were done in the University of North Carolina Mouse Phase I Unit (Chapel Hill, NC) with the approval of the University of North Carolina Institutional Animal Care and Use Committee. No more than five mice were housed per cage with ad libitum access to standard chow and water. All mice were drug and procedure naive before the start of the studies described here. All mice were killed by CO2 inhalation followed by cervical dislocation.
For studies of wild-type mice, 8- to 12-week-old FVB/N female mice (The Jackson Laboratory, Bar Harbor, ME) were used. To evaluate whether pharmacological quiescence would protect mice from chemotherapy-induced myelosuppression, we first used wild-type non–tumor-bearing FVB/N mice to minimize potential complexities resulting from the impact of a tumor on the health of the mice. In addition, for these initial analyses in non–tumor-bearing FVB/N mice (n = 13 mice per group), we used a single dose of 90 mg/kg carboplatin to illicit pancytopenia to minimize non-hematological complications from the cumulative exposure to carboplatin seen in the lower 75 mg/kg carboplatin regimen given weekly for 3 weeks that was used in our efficacy studies.
The C3-TAg transgenic mouse model (The Jackson Laboratory) of basal-like breast cancer (BBC) (38) and the MMTV-c-neu mouse model (The Jackson Laboratory) of luminal breast cancer (39) were both on the FVB/N background. These mice were used because they represent models of Rb-competent (MMTV-c-neu model) and Rb-incompetent (C3-TAg model) breast cancer. Also, they have been extensively characterized in previous studies, including expression profiling, which have shown that they represent their human breast cancer subtype (40). The MMTV-c-neu model of breast cancer expresses c-neu (the mouse ortholog of human HER2) driven by the mouse mammary tumor virus (MMTV) promoter. Previous reports in murine (41–44) and human HER2-positive breast cancer (45–47) suggest that these tumors require CDK4 and CCND1 for progression and maintenance. The C3-TAg model expresses the simian virus 40 large T antigen under the control of the rat prostatic steroid binding protein C3 (1) promoter. Previous expression profiling studies suggest the C3-TAg model is the best known murine model of human BBC (40,48). The molecular genetics between this model and its human counterpart are similar, as the T-antigen binds and inactivates Rb and p53 (12–14), and human BBC is frequently deficient for both Rb and p53 (14,48–52).
To evaluate myelosuppression, peripheral blood was collected by tail vein nick for complete blood cell analysis, which was performed using a HEMAVET HV950 multispecies hematology system (Drew Scientific, Waterbury, CT) by following the manufacturer's instructions. C3-TAg (11 mice in the control group, seven mice in the PD0332991 group, 17 mice in the carboplatin plus vehicle control group, and 14 mice in the carboplatin plus PD0332991 group) and MMTV-c-neu (19 mice in the control group, seven mice in the PD0332991 group, 16 mice in the carboplatin plus vehicle control group, and 17 mice in the carboplatin plus PD0332991 group) mice were examined weekly to assess tumor development by palpation. Mice were enrolled in the study when tumor volumes were approximately 50–60 mm3 by caliper measurement. Following enrollment, tumor response was assessed by weekly caliper measurement. Data were normalized to tumor size at the time of therapy initiation, and volumes were calculated by the formula volume = [(width)2 × length]/2. Tumor-bearing mice were killed, as described above, at the indicated times because of morbidity, tumor ulceration, or a tumor size of more than 1.5 cm in diameter.
Irradiation Study
To investigate PD0332991 and/or erythropoietin (Epo; Amgen, Thousand Oaks, CA)-mediated hematological protection around the time of myelosuppressive radiation, mice were pretreated with 150 mg/kg PD0332991 or vehicle control by oral gavage 30 minutes before exposure to 6.5 Gy of total body irradiation (TBI) (seven mice for the control group, eight mice for the Epo group, eight mice for the PD0332991group, and six mice for the PD0332991 plus Epo group). Seventy-two hours after irradiation, mice received daily subcutaneous injections of Epo or normal saline control for three consecutive days. For these experiments, TBI (6.5 Gy) was used to cause bone marrow damage rather than chemotherapy because of its ability to induce pronounced anemia, which is the therapeutic indication for Epo.
Bone Marrow and Tumor Cell Proliferation by Flow Cytometry and Bone Marrow Caspase Activation
For HSPC proliferation experiments, young adult female FVB/N mice were treated with a single dose of 150 mg/kg PD0332991 by oral gavage. Mice were then killed at 0, 24, 36, and 48 hours following PD0332991 administration, and bone marrow was harvested (n = 3 mice per time point), as previously described (8). Four hours before the bone marrow was harvested, mice were treated with 100 µg of EdU by intraperitoneal injection (Invitrogen). Bone marrow mononuclear cells were harvested and immunophenotyped using previously described methods (8). In brief, HSPCs and myeloid progenitors were identified by expression of lineage markers (L), Sca1 (S), and c-Kit (K). HSPCs express the L−K+S+immunophenotype, whereas myeloid progenitors express the L−K+S−immunophenotype.
For tumor proliferation experiments, tumor-bearing MMTV-c-neu and C3-TAg mice (n = 3 mice per group) were treated with a single dose of 150 mg/kg PD0332991 by oral gavage, followed by three doses of 100 µg of EdU by intraperitoneal injection at 16, 19, and 22 hours after PD0332991 treatment. Mice were then killed 24 hours after PD0332991 administration, and tumors were excised for further analysis. Single cell suspensions were prepared from the excised tumors using the GentleMacs Tissue Dissociator (Miltenyi Biotec, Auburn, CA) per the manufacturer's protocol. The HSPCs and tumor cells were processed using the Click-iT EdU Alexa Fluor-647 Flow Cytometry Kit (Invitrogen). Flow cytometry was performed using a CyAn ADP Analyzer (Beckman Coulter) and FlowJo analysis software (Version 7.2.2; Tree Star).
To measure caspase activation following chemotherapy exposure, young adult female FVB/N mice were studied. Four groups of mice (n = 3 mice per group) were treated with vehicle control or 150 mg/kg PD0332991 by oral gavage, 90 mg/kg carboplatin by intraperitoneal injection, or 150 mg/kg PD0332991 by oral gavage plus 90 mg/kg carboplatin by intraperitoneal injection. Twenty-four hours after treatment, mice were killed, and the bone marrow was harvested. Total bone marrow cells were counted and then 20 000 cells per well were plated in quadruplicate in 96-well plates. Apoptosis was measured by using the Caspase-Glo 3/7 Assay System (Promega) as per the manufacturer's recommendations.
Statistical Analysis
Comparisons between two treatment groups were made using student t test, and comparisons between more than two treatment groups were made using one-way analysis of variance with Bonferroni correction for multiple comparisons, when appropriate. To analyze the γ-H2AX flow cytometry data, χ2 test was used. Log-rank tests were used in Kaplan–Meier analyses, and hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) were calculated. Nonlinear regression analysis was done to determine whether there was a statistically significant difference between tumor growth curves for mice that were administered different treatments. P values of less than .05 were considered statistically significant. All statistical analyses and calculations were performed using GraphPad Prism 5 (GraphPad Software Inc, La Jolla, CA). All statistical tests were two-sided. We decided that a 10% effect would be clinically relevant and determined that groups of mice of the size described above would be large enough to detect this effect. Ultimately, the effect was larger than expected, and the P values were statically significant.
Results
Effect of Pharmacological Quiescence Induced by CDK4/6 Inhibition at the Time of Exposure to DNA-Damaging Agents
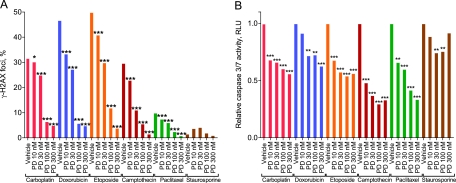
To determine if pharmacological quiescence afforded resistance to chemotherapeutic agents with differing mechanisms of action, we developed an in vitro model using tHDFs (a human foreskin fibroblast line immortalized with expression of human telomerase). These cells are highly CDK4/6 dependent for proliferation as demonstrated by their complete G1 arrest following treatment with PD0332991 (8). Treatment with PD0332991 to affect a cell cycle arrest before treatment with several DNA-damaging agents (eg, carboplatin, doxorubicin, etoposide, and camptothecin) or an antimitotic agent (paclitaxel) attenuated DNA damage as measured by the level of phosphorylated forms of the H2AX histone (γ-H2AX), a marker of DNA double-strand breaks (Figure 1, A). Treatment with staurosporine, a pan-kinase inhibitor, did not result in appreciable γ-H2AX formation (Figure 1, A). Additionally, treatment of tHDF cells with PD0332991 before carboplatin, doxorubicin, etoposide, camptothecin, and paclitaxel exposure elicited a robust decrease in caspase 3/7 activation in a dose-dependent manner (Figure 1, B). These data indicate that a transient cell cycle arrest, induced by CDK4/6 inhibition, protects CDK4/6-senstive cells from the toxicity of a variety of commonly used cytotoxic chemotherapy agents that are associated with myelosuppression.
Figure 1.
Ability of pharmacological quiescence to protect against chemotherapy-induced γ-H2AX formation and apoptosis. The tHDF cells were treated with varying concentrations of PD0332991 (PD) before treatment with 100 μM carboplatin, 1 μM doxorubicin, 5 μM etoposide, 156 nM camptothecin, 250 nM paclitaxel, or 1.5 nM staurosporine. The tHDF cells were then assayed for A) γ-H2AX formation and B) caspase 3/7 activation. γ-H2AX formation data measured by flow cytometry represent at least 20 000 gated events. Caspase 3/7 data are presented as the average ratio from a single experiment of 1000 cells per well in quadruplicate. P values were calculated by a two-sided χ2 test (γ-H2AX formation data) or one-way analysis of variance (caspase 3/7 data) with Bonferroni correction for multiple comparisons. All data are representative of three independent experiments. *P < .05, **P < .01, ***P < .001. RLU = relative light units; tHDF = telomerized human diploid fibroblast.
Effect of Pharmacological Quiescence on Chemotherapy-Induced Myelosuppression In Vivo
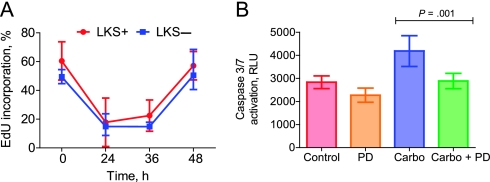
On the basis of the finding that pharmacological quiescence of HSPCs in vivo provides protection from a lethal dose of TBI (8), we sought to determine if pharmacological quiescence would also protect mice from chemotherapy-induced myelosuppression. For these experiments, a detailed understanding of the temporal effects of pharmacological quiescence on HSPCs was required to ensure that the cell cycle remained arrested throughout the period of chemotherapy exposure. We measured the kinetics of pharmacological quiescence induction and reversal in HSPCs from 8- to 12-week-old female FVB/n mice after a single dose of 150 mg/kg PD0332991 administered by oral gavage (Figure 2, A). Hematopoietic progenitors were determined by the LKS immunophenotype. EdU incorporation was reduced after CDK4/6 inhibitor treatment (EdU incorporation at baseline, 24 hours, and 36 hours = 60.4%, 17.8%, and 22.5%, respectively; difference at 24 hours = 42.6%, 95% CI = 28.8% to 56.4%; difference at 36 hours = 37.9%, 95% CI = 26.8% to 49.0%) and resulted in a robust suppression of proliferation in early HSPCs (L−K+S+) (Figure 2, A). Differentiated myeloid progenitors (L−K+S−) were similarly sensitive to CDK4/6 inhibition (EdU incorporation at baseline, 24 hours, and 36 hours = 49.4%, 14.9%, and 14.9%, respectively; difference at 24 hours = 34.5%, 95% CI = 19.7% to 49.3%; difference at 36 hours = 34.5%, 95% CI = 25.6% to 43.4%) (Figure 2, A). EdU incorporation in both fractions returned to baseline levels by 48 hours after oral gavage with PD0332991 (Figure 2, A). These data show that a single oral dose of a potent and selective CDK4/6 inhibitor can produce reversible pharmacological quiescence in HSPCs and myeloid progenitors that lasts longer than 36 hours.
Figure 2.
Pharmacodynamic assessment of CDK4/6 inhibition in the bone marrow. A) FVB/N mice (n = 3 mice per group) were treated with a single dose of 150 mg/kg PD0332991 by oral gavage to assess the temporal effect of transient CDK4/6 inhibition on bone marrow arrest and was quantitated by EdU incorporation at the indicated times. Four hours before bone marrow harvest, mice were treated with 100 µg of EdU. After the mice were killed by CO2 inhalation followed by cervical dislocation, bone marrow was harvested and sorted by expression of lineage markers (L), c-KIT (K), and Sca1 (S) to evaluate the temporal impact of CDK4/6 inhibition on specific cell populations of the bone marrow: hematopoietic stem and progenitor cells (L−K+S+) and myeloid progenitors (L−K+S−). Error bars represent 95% confidence intervals. B) To assess the effect of transient CDK4/6 inhibition on carboplatin-induced apoptosis in the bone marrow, FVB/N mice (three mice per group) were treated with vehicle control, 150 mg/kg PD0332991 by oral gavage, 90 mg/kg carboplatin by intraperitoneal injection, or 150 mg/kg PD0332991 oral gavage plus 90 mg/kg carboplatin by intraperitoneal injection. Twenty-four hours after treatment, mice were killed, bone marrow was harvested, and apoptosis was measured by caspase 3/7 activation. Results shown represent a single experiment, in which each mouse represents a unique biological replicate and four technical replicates from each mouse were analyzed. Error bars represent 95% confidence intervals. Two-sided P values were calculated by student t test. Carbo = carboplatin; CDK = Cyclin-dependent kinases; PD = PD0332991; RLU = relative light units.
To directly measure the effect of transient pharmacological quiescence on chemotherapy-induced bone marrow toxicity, we determined the ability of CDK4/6 inhibitors to prevent carboplatin-induced myelosuppression. We chose carboplatin for in-depth in vivo analysis because of its widespread clinical use; propensity to cause dose-limiting myelosuppression, including thrombocytopenia; and evidence of antitumor activity in several breast cancer genetically engineered murine models (GEMMs). Consistent with the literature (53), we established that a single dose of 90 mg/kg carboplatin in 8- to 12-week-old female FVB/n mice resulted in measurable pancytopenia 14 days after administration after testing several dosing regimens (data not shown). Pharmacokinetic parameters for carboplatin were unchanged when coadministered with PD0332991 (Supplementary Figure 1 and Supplementary Table 1, available online). Mice treated with carboplatin alone had a statistically significant increase in apoptosis, whereas coadministration of PD0332991 completely inhibited carboplatin-induced apoptosis in HSPCs (L−K+S+) (carboplatin alone = 4185 relative light units [RLU], carboplatin plus PD0332991 = 2884 RLU, difference = 1301 RLU, 95% CI = 615.4 to 1985, P = .001) (Figure 2, B). These data indicate that transient pharmacological quiescence provided direct protection of HSPCs from the cytotoxic effects of DNA-damaging chemotherapy in vivo.
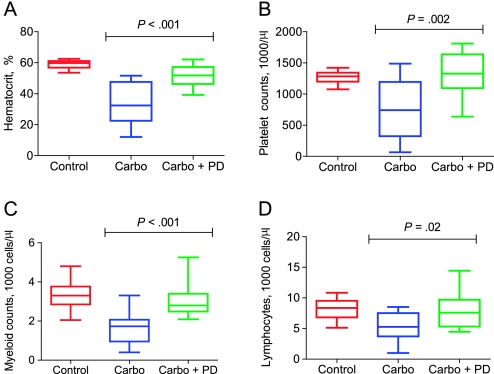
We next evaluated whether this acute protection of HSPCs measured 24 hours after carboplatin exposure would translate into reduced myelosuppression post-chemotherapy in FVB/n mice. Using the single-dose 90 mg/kg carboplatin regimen, we found coadministration of PD0332991 with carboplatin provided marked quadrilineage hematopoietic protection at day 14 post-chemotherapy (Figure 3, A–D). Specifically, coadministration of PD0332991 with carboplatin compared with single-agent carboplatin treatment resulted in increased hematocrit (51.2% vs 33.5%, difference = 17.7%, 95% CI = 26.7% to 8.58 %, P < .001), platelet counts (1321 vs 758.5 thousand cells per μL, difference = 562.5 thousand cells per μL, 95% CI = 902.8 to 222.6, P < .002), myeloid cells (granulocytes and monocytes; 3.1 vs 1.6 thousand cells per μL, difference = 1.5 thousand cells per μL, 95% CI = 2.23 to 0.67, P < .001), and lymphocytes (7.9 vs 5.4 thousand cells per μL, difference = 2.5 thousand cells per μL, 95% CI = 4.75 to 0.18, P = .02). As the kinetics of cell count recovery differ for each lineage after a DNA-damaging exposure, it is likely that even more pronounced protection would have been observed for the individual lineages at other time points after chemotherapy. These data show that pharmacological quiescence protects HSPCs from carboplatin-induced DNA damage and leads to accelerated count recovery post-chemotherapy.
Figure 3.
Ability of transient pharmacological quiescence to provide quadrilineage protection from chemotherapy-induced myelosuppression. FVB/N wild-type mice (n = 13 mice per group) were treated with vehicle control, 90 mg/kg carboplatin by intraperitoneal injection, or 90 mg/kg carboplatin by intraperitoneal injection plus 150 mg/kg PD0332991 by oral gavage. Complete blood cell counts were analyzed on day 14. A) hematocrit levels and the number of B) platelets, C) myeloid cells, and D) lymphocytes were determined. Boxes represent the 5%–95% distribution, whiskers represent minimum and maximum values, and the middle bar represents the median. Student's t test was done to calculate two-sided P values. Carbo = carboplatin; PD = PD0332991.
An important consideration is how pharmacological quiescence compares with existing approaches to reduce myelosuppression. Therefore, we tested the hematological protection afforded by PD0332991 and/or Epo. Consistent with a previous report (54), three daily doses of Epo administered by subcutaneous injection starting 3 days post-TBI did not produce a statistically significant effect on red blood cell (RBC) recovery at 21 days post-TBI (mean RBC count after control = 3.36 million cells per μL vs mean RBC count after Epo treatment = 2.82 million cells per μL; difference = 0.54 million cells per μL, 95% CI = −1.49 to 2.55) (Supplementary Figure 2, available online). As reported previously (8), however, single-agent PD0332991 led to higher RBCs after ionizing radiation, as was observed in our study. In addition to affording quadrilineage rather than RBC-specific protection, the combined treatment of PD0332991 at the time of TBI and Epo post-TBI, provided the greatest effect on RBC recovery (mean RBC count after PD0332991 and Epo treatment = 5.99 million cells per μL vs mean RBC count after treatment with vehicle = 3.36 million cells per μL; difference = 2.63 million cells per μL, 95% CI = 0.46 to 4.80, P = .02) (Supplementary Figure 2, available online). This observation suggests that CDK4/6 inhibition around the time of TBI increases RBC progenitor number and/or function and that these surviving erythroid progenitors are then the substrate for the erythropoiesis-stimulating effects of Epo. These data indicate that pharmacological quiescence synergizes with established cytokine-based approaches to reduce myelosuppression after a DNA-damaging event.
Efficacy of CDK4/6 Inhibitors in HER2-Driven Breast Tumors
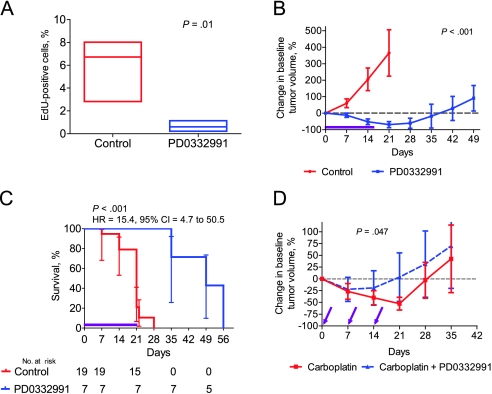
We next studied the effects of pharmacological quiescence when used in GEMMs of breast cancer. We first examined a HER2-driven model of breast cancer (39), which expresses c-neu (the mouse ortholog of human HER2) driven by the MMTV promoter. We chose this model because previous studies in murine (41–44) and human HER2-positive breast cancer (45–47) suggest that these tumors require CDK4 and CCND1 for progression and maintenance. MMTV-neu mice were generated and observed post-lactation, with tumors observed with a median latency of approximately 25 weeks. Mice were enrolled in therapy studies when tumors reached a standard size (50–60 mm3) that permitted easy serial assessment. Treatment of mice with established tumors with PD0332991 for 24 hours caused a near-complete cessation of tumor proliferation in vivo as assessed by EdU incorporation (Figure 4, A). Tumor-bearing mice were continuously treated with PD0332991 added to their chow (100 mg/kg/d). Continuous treatment with PD0332991 (100 mg/kg/d) led to a marked reduction in tumor volume during a 21-day course of therapy (mean percent change in tumor volume at day 21 = 365.5% vs −69.4% for control and PD0332991, respectively, difference = 434.9%, 95% CI = 243.8% to 626%, P < .001) (Figure 4, B) with improved median survival (21 days for mice treated with the vehicle control vs 49 days for mice treated with PD0332991; HR of survival = 15.4, 95% CI = 4.7 to 50.5, P <.001) (Figure 4, C). Nonlinear regression analysis demonstrated that the tumor growth curve for PD0332991-treated mice was statistically significantly different from that of control mice (P < .001). Several tumors displayed complete tumor regression (Supplementary Figure 3, available online), and no resistance to PD0332991 was noted during a 21-day course of therapy. These data show that this HER2-driven GEMM is “addicted” to CDK4/6 activity for proliferation.
Figure 4.
The use of CDK4/6 inhibitors in a CDK4/6-dependent genetically engineered murine model of breast cancer. A) A single dose of 150 mg/kg PD0332991 (n = 4) or vehicle control (n = 3) was administered by oral gavage to tumor-bearing MMTV-c-neu mice. Twenty-four hours later, tumors were excised, and the mean tumor EdU labeling, a measure of proliferation, was assessed. Boxes represent the 5%–95% distribution, whiskers represent the minimum and maximum values, and the middle bar represents the median number of proliferating cells labeled with EdU. Nonlinear regression analysis was used to compare the change in baseline tumor volume for the two groups during the course of the study. B–C) Tumor-bearing MMTV-c-neu mice (PD0332991, n = 7; control, n = 19) were treated with either PD0332991 (100 mg/kg/d) delivered in chow or standard chow (control) for 21 days (purple line). B) Tumor volumes were recorded weekly and graphed as the percent change in baseline tumor volume. Error bars represent the 95% confidence intervals (CI). Nonlinear regression analysis was used to compare the change in baseline tumor volume for the two groups during the course of the study. C) Kaplan–Meier curves for the control and PD0332991-treated groups are shown. The error bars represent the 95% CIs. The hazard ratio (HR) and 95% CI were calculated, and the P value was calculated using a two-sided log-rank test. D) Tumor-bearing MMTV-c-neu mice were randomly assigned to each of the study groups (carboplatin plus vehicle control, n = 16, or carboplatin plus PD0332991, n = 17). Mice received carboplatin at 75 mg/kg by intraperitoneal injection with or without PD0332991 (150 mg/kg by oral gavage) once a week for 3 weeks (purple arrows). Error bars represent 95% confidence intervals. Student t test was done to calculate two-sided P values for EdU incorporation in (B) and (D). All statistical tests were two-sided.
Efficacy of Coadministration of PD0332991 and Chemotherapy in HER2-Driven Tumors
We next sought to determine the effects of concomitant CDK4/6 inhibitors on chemotherapy response in HER2-driven MMTV-neu mice. Although carboplatin has not traditionally been used in the first-line treatment of human HER2-positive breast cancer, it is active in this disease and is increasingly used in the metastatic setting (55). Tumor-bearing mice were treated with weekly intraperitoneal injection of 75 mg/kg carboplatin with or without 150 mg/kg PD0332991 administered by oral gavage at the time of carboplatin administration. Both agents demonstrated activity as a single agent in this model by decreasing the mean tumor volume at day 21 (mean percent change in tumor volume = −69.4%, 95% CI = −87.5% to −51.3% for P0332991 and −52.6%, 95% CI = −66.1% to −39.2% for carboplatin) (Figure 4, B and D), leading to the hypothesis that a synergistic reduction in tumor volume may result when the agents are coadministered. Instead, however, CDK4/6 inhibitor coadministered with carboplatin led to in vivo tumor protection (mean percent change in tumor volume at day 21 = −52.6% vs 3.7% for carboplatin and carboplatin plus PD0332991, respectively; difference = −56.3%, 95% CI = −109.0% to −3.6%, P = .04) (Figure 4, D). Nonlinear regression analysis demonstrated that the tumor growth curve for carboplatin plus PD0332991 treated mice was statistically significantly different from carboplatin only treated mice (P = .047). A similar reduction of the antitumor efficacy of doxorubicin, another highly active agent in human HER2-positive breast cancer, was also noted in the MMTV-neu mice (Supplementary Figure 4, available online). These results suggest that just as pharmacological quiescence can protect HSPCs, a pharmacological reduction in tumor proliferation can also markedly reduce the efficacy of cytotoxic agents such as carboplatin and doxorubicin that induce tumor cell death in a cell cycle–specific manner.
Efficacy of CDK4/6 Inhibitors in Rb-Deficient Breast Tumors
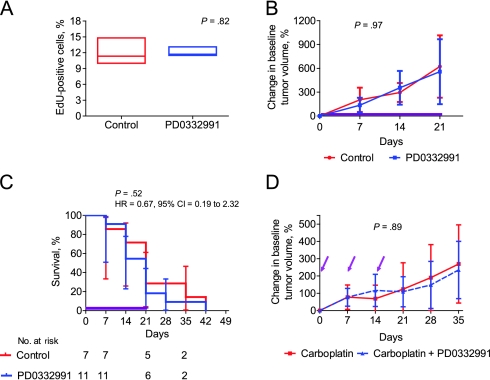
We next tested CDK4/6 inhibitors in the well-defined C3-TAg breast tumor model (38), which previous expression profiling studies have shown is the best known murine model of human BBC (40,48). As in the MMTV-c-neu mice, we tested the effects of CDK4/6 inhibitors alone or in combination in tumor-bearing mice. In accord with the lack of functional Rb, a 24-hour course of PD0332991 did not affect tumor proliferation as measured by EdU incorporation (untreated mean number of EdU-positive cells = 11.8%; PD0332991 vs mean number of EdU-positive cells = 12.0%; difference = 0.2%, 95% CI = −2.3% to 1.8%) (Figure 5, A). Likewise, a continuous 21-day course of PD0332991 treatment in chow (100 mg/kg/d) did not reduce tumor growth (Figure 5, B) or extend median survival (21 days for mice treated with vehicle control vs 21 days for mice treated with PD0332991; HR = 0.67, 95% CI = 0.19 to 2.32, P = .52) (Figure 5, C). These data show that this Rb-deficient GEMM model of BBC is not dependent on CDK4/6 activity.
Figure 5.
The use of CDK4/6 inhibitors in a CDK4/6-independent genetically engineered murine model of breast cancer. A) A single dose of 150 mg/kg PD0332991 (n = 4) or vehicle control (n = 6) was administered by oral gavage to tumor-bearing C3-TAg mice. Twenty-four hours later, tumors were excised, and the mean tumor EdU labeling, a measure of proliferation, was assessed. Boxes represent 5%–95% distribution, whiskers represent the minimum and maximum values, and the middle bar represents the median number of proliferating cells labeled with EdU. Student t test was done to calculate two-sided P value for EdU incorporation. B–C) Tumor-bearing C3-TAg mice (PD0332991, n = 7; control, n = 11) were treated with either PD0332991 (100 mg/kg/d) delivered in chow or standard chow (control) for 21 days (purple line). B) Tumor volumes were recorded weekly and graphed as the percent change in baseline tumor volume. Error bars represent the 95% confidence intervals. Nonlinear regression analysis was used to compare the change in baseline tumor volume for the two groups during the course of the study. C) Kaplan–Meier curves for the control and PD0332991-treated groups are shown. The dashed lines indicate the 95% confidence intervals. D) Tumor-bearing MMTV-c-neu mice were randomly assigned to each of the study groups (carboplatin plus vehicle control, n = 17, or carboplatin plus PD0332991, n = 14). Mice were administered 75 mg/kg carboplatin by intraperitoneal injection with or without PD0332991 (150 mg/kg by oral gavage) once a week for 3 weeks (purple arrows). Error bars represent 95% confidence intervals. Nonlinear regression analysis was used to compare the change in baseline tumor volume for the two groups during the course of the study in (B) and (D). All statistical tests were two-sided.
Efficacy of Coadministration of PD0332991 and Chemotherapy in Rb-Deficient Tumors
To determine the effects of CDK4/6 inhibitors on the chemotherapy response in the C3-TAg breast tumor model of BBC, we examined the efficacy of concomitant carboplatin and CDK4/6 inhibitors in this model. Carboplatin is a highly active and commonly used agent in human BBC (56), and previous studies in the University of North Carolina Mouse Phase I Unit had established that this agent, given weekly by intraperitoneal injection, exhibited statistically significant single-agent activity in the C3-TAg model (data not shown). Consistent with the finding that CDK4/6 inhibition did not modulate tumor proliferation in this model (Figure 5, A), a single dose of CDK4/6 inhibitor coadministered with weekly 75 mg/kg carboplatin had no effect on in vivo tumor growth (mean percent change in tumor volume at day 21 = 118.8% and 109.1% for carboplatin and carboplatin plus PD0332991, respectively, difference = 9.7%, 95% CI = −183.5% to 202.9%, P = .89) (Figure 5, D). Additionally, coadministration of carboplatin and PD0332991 reduced thrombocytopenia (Supplementary Figure 5, available online; P = .04) in C3-TAg mice, as was observed in wild-type mice (Figure 1, D). These results suggest that pharmacological quiescence does not protect tumors whose proliferation is resistant to CDK4/6 inhibition, indicating that CDK4/6 inhibitors could be safely used to reduce myelosuppression in tumors whose proliferation is not modulated by CDK4/6 inhibition (eg, Rb-deficient tumors).
Discussion
In this study, we demonstrate that autochthonous breast tumors can be either CDK4/6 dependent or independent. Consistent with previous studies (41–47), a potent and selective CDK4/6 inhibitor demonstrated considerable activity in a HER2-driven GEMM. In contrast, inhibition of CDK4/6 did not produce a therapeutic benefit in the Rb-deficient C3-TAg breast cancer model nor, as we previously reported, in a p16INK4a-deficient melanoma GEMM (8). Correspondingly, Puyol et al. (57) recently showed that a CDK4/6 inhibitor had variable activity in a RAS-driven GEMM of lung cancer, with some tumors responding but most exhibiting resistance. These murine findings are consistent with the limited reported experience of CDK4/6 inhibitors in humans (eg, modest but important response rates in certain tumor types like myeloma, mantle cell lymphoma, and teratoma) (58–60).
We believe the evidence suggests that a subset of human tumors will show substantial dependence on CDK4/6 activity, and a clinical test to readily identify CDK4/6-dependent tumors would facilitate the clinical use of these agents as antineoplastics. Our data and other work suggest that Rb loss portends resistance to these agents (8,23,24,26). Increased expression of nonmutant p16INK4a, commonly observed in many tumor types (61), also appears to signify resistant disease given that p16INK4a is a potent inhibitor of CDK4/6 (24,62). Importantly, it is unlikely that mere CCND1 overexpression or p16INK4a loss will broadly indicate CDK4/6 addiction; consistent with our findings that p16INK4a-deficient melanoma is resistant in vivo (8). In accord with these views, a phase II clinical trial has been initiated in non–small cell lung cancer that selects patients for treatment with PD0332991 using Rb expression and p16INK4a deletion as predictors of response (www.clinicaltrials.gov, NCT01291017). The present data, however, demonstrate how CDK4/6 inhibitors can be used for therapeutic benefit in tumors, whether CDK4/6 dependent or independent, if a reliable means to discern resistant and sensitive tumors can be developed.
In particular, we show that CDK4/6 inhibitors provided meaningful therapeutic benefit even in tumors that were refractory to CDK4/6 inhibition. Consistent with previous work with ionizing radiation, we showed a marked protective effect of pharmacological quiescence on chemotherapy-induced myelosuppression. In vitro, this protection extended to a number of cell cycle–specific DNA-damaging agents as well as taxanes. Furthermore, by identifying CDK4/6-resistant tumors (eg, Rb-deficient), we have shown successful bone marrow protection without compromising dose intensity or reducing tumor kill. Several retrospective and prospective randomized trials have shown that reductions in the chemotherapy dose intensity compromises long-term disease control and survival (27). Despite compelling data, surveys in the United States and elsewhere have reported that dose reductions and delays frequently occur in clinical practice because of myelosuppression, even in the potentially curative setting.
Current therapeutic approaches to minimize myelosuppressive effects rely on the use of growth factors (ie, granulocyte colony-stimulating factor or Epo) and have substantial limitations. These expensive injectable biologics each target a single hematopoietic cell lineage and have been associated with long-term toxic effects. Granulocyte colony-stimulating factor and its derivatives have been associated with a modest increase in the risk of myelodysplasia and secondary leukemia (63,64). Similarly, the association of Epo and derivatives with increased mortality, thrombosis, and tumor progression has recently garnered a Black Box warning from the US Food and Drug Administration, which indicates that medical studies have shown that the drug carries a substantial risk of serious or even life-threatening adverse effects. Additionally, there are no available approaches to limit chemo-induced thrombocytopenia or lymphopenia. Limiting chemo-induced thrombocytopenia, in particular, is a clinically significant unmet need (65) of particular importance in breast cancer treated with dose-dense regimens (66) and (67). The pharmacological quiescence approach therefore appears to offer the advantage of quadrilineage protection as opposed to treating a single lineage after myelosuppression has occurred. Importantly, the fear of tumor protection has been a major limitation to the use of other adjunctive measures, but the well-understood mechanism of action of pharmacological quiescence limits this concern. By treating patients whose cancers are resistant to CDK4/6 inhibition (eg, Rb-deficient), chemotherapy-induced myelosuppression can be ameliorated without compromising cancer cell death.
The finding that cell cycle modulation affects the toxicity of DNA-damaging agents such as carboplatin and doxorubicin has implications for their use in human tumors. For example, we show that doxorubicin, carboplatin, and PD0332991 are effective in the MMTV-c-neu model, but that concurrent treatment of the DNA-damaging agents with a selective CDK4/6 inhibitor results in reduced antitumor efficacy. Therapeutic antagonism between cytostatic biologic agents and cytotoxic chemotherapy has been suggested previously in other settings. For example, concurrent administration of the epidermal growth factor receptor tyrosine kinase inhibitors gefitinib or erlotinib with chemotherapy in several phase III clinical trials has proven no more effective than chemotherapy alone (68–71). Upon subset analysis, it has been suggested that patients with wild-type epidermal growth factor receptor (the majority of patients) treated with erlotinib plus chemotherapy had the worst outcomes of any treatment group (72,73). Our data similarly suggest that CDK4/6 inhibitors should not be paired with cytotoxic agents in tumors dependent on CDK4/6 activity for proliferation.
A major hurdle for the routine incorporation of CDK4/6 inhibitors into current treatment paradigms will be the ability to prospectively discern CDK4/6-dependent vs CDK4/6-independent cancers. Although there are common genetic alterations that predict resistance to CDK4/6 inhibition (ie, Rb loss), an incomplete understanding of cancer cell cycle regulation prevents full coverage across the spectrum of human cancers. Therefore, this approach would only be of clinical use if paired with a reliable “companion diagnostic” that provides results in clinical real time. Such a test of CDK4/6 dependence would likely combine mutational analysis (eg, Rb and p16INK4a) with tests of mRNA and protein expression. For example, using a panel of over 30 breast cancer cells, Finn et al. (24) identified a deferential gene expression pattern between CDK4/6-sensitive and CDK4/6-resistant cells. Given the fear of unintentionally protecting a patient's cancer, however, pharmacological quiescence will only be of clinical value if this concern can be comprehensively addressed.
To the point above, our study is not without limitations. Although we used two established preclinical models of breast cancer with a well-defined understanding of their dependence on CDK4/6 and Rb, the genetic lesions in these tumors do not fully represent the diverse genetic heterogeneity in cell cycle regulation seen across all cancers. Therefore, the present work provides proof of the potential therapeutic uses of CDK4/6 inhibitors in patients with CDK4/6-depedent and CDK4/6-independent tumors; however, considerable work is still needed to define genetic or other biomarkers that can be used to prospectively identify a specific tumor's dependence on CDK4/6. Additionally, in this study, we only evaluated tumors that were either sensitive or intrinsically resistant to CDK4/6 inhibition and did not evaluate tumor models “transiently” sensitive or with acquired resistance to CDK4/6 inhibition. We believe there are therapeutic strategies for employing CDK4/6 inhibitors in these tumors; however, this study did not evaluate these potential clinical scenarios.
In summary, we believe that a compelling argument can be made for the use of CDK4/6 inhibitors in most human cancers, which generally appear to come in four types based on their dependence on CDK4/6 activity and sensitivity to cytotoxic agents (Table 1). Group I tumors are “durably” CDK4/6 dependent (eg, Cyclin D–amplified mantle cell lymphoma), and in this group, CDK4/6 inhibitors could be used as antineoplastic agents, as demonstrated in our study and that of Leonard et al. (58). Group II tumors are fully CDK4/6 independent (eg, Rb-null small cell lung cancer), and we showed that in these patients, CDK4/6 inhibitors may be used to prevent myelosuppression. Group III tumors are transiently CDK4/6 dependent but rapidly develop resistance as is common in other therapeutics (59). We believe that CDK4/6 inhibitors could be of use in this setting to synchronize tumor cells in the cell cycle, allowing for increased tumor cell death with other cytotoxic agents. Proof of concept for this approach has been suggested by Di Liberto et al. (74) in multiple myeloma, where cell cycle synchronization appears to boost the efficacy of bortezomib. Lastly, Group IV tumors are fully CDK4/6 resistant but insensitive to cytotoxic agents. Although we believe this class of tumor is rare, it is unlikely that CDK4/6 inhibitors would be of use in these tumors. We believe that the present data support a possible role for CDK4/6 inhibitors in a majority of patients with advanced cancer: to inhibit tumor growth, ameliorate the dose-limiting toxicities of chemotherapy or ionizing radiation, or to synchronize tumors for increased cell death mediated by other therapeutic agents.
Table 1.
Predicted clinical scenarios for the use of cyclin-dependent kinase (CDK)-4/6 inhibitors*
| Tumor group | Response to CDK4/6 inhibitors | Possible molecular features | Example cancer | Clinical use of CDK4/6 inhibitors |
| Group I | Durably sensitive | D-type cyclin amplification | Mantle cell lymphoma | Antineoplastic [Leonard et al. (58) and this study] |
| Group II | Resistant | Rb loss, E7 expression, MYC amplification | BBC, SCLC, HPV-positive head and neck carcinoma | Pharmacological quiescence (this study) |
| Group III | Transiently sensitive | D-type cyclin overexpression | Multiple myeloma, GBM | Pharmacological synchronization (adjunct to agents that kill in cell cycle–dependent manner) (74) |
| Group IV | Resistant (but insensitive to cytotoxic agents) | Unknown | Unknown | None |
BBC = basal-like breast cancer; SCLC = small cell lung cancer; HPV = human papillomavirus; GBM = glioblastoma multiforme.
Funding
This work was supported by the University of North Carolina Lineberger Comprehensive Cancer Center Mouse Phase I Unit, and grants from the Golfers Against Cancer Foundation (to N.E.S and C.M.P), the Ellison Medical Foundation (to N.E.S), the Burroughs Wellcome Fund (1006673 to N.E.S), the National Institutes of Health (RO1 P01-ES014635 and UO1-CA141576 to N.E.S; P50-CA58223-09A1 and RO1-CA148761 to C.M.P), the University Cancer Research Fund from the University of North Carolina Lineberger Comprehensive Cancer Center (to N.E.S and W.C.Z), and the American Cancer Society (PF-10-239-01-TBG to P.J.R).
Supplementary Material
Footnotes
The study sponsors did not have any role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the article; or the decision to submit the article for publication. The University of North Carolina has filed a patent based on this work. P. J. Roberts, J. E. Bisi, J. C. Strum, and N. E. Sharpless are co-inventors on this patent, which has been licensed to G-Zero Therapeutics, a company co-founded by N. E. Sharpless and K.-K. Wong. P. J. Roberts, J. E. Bisi, and J. C. Strum are consultants for G-Zero Therapeutics. We wish to thank Dr Jian Jin and the Center for Integrative Chemical Biology and Drug Discovery at the University of North Carolina Eshelman School of Pharmacy (Chapel Hill, NC), for providing PD0332991 and compound-related expertise.
References
- 1.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 2.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28(33):2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 3.Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13(20):1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 4.Kozar K, Ciemerych MA, Rebel VI, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118(4):477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Malumbres M, Sotillo R, Santamaria D, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118(4):493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Ortega S, Prieto I, Odajima J, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35(1):25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 7.Rane SG, Dubus P, Mettus RV, et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet. 1999;22(1):44–52. doi: 10.1038/8751. [DOI] [PubMed] [Google Scholar]
- 8.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010;120(7):2528–2536. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey MR, Krishnamurthy J, Pei XH, et al. Expression of p16Ink4a compensates for p18Ink4c loss in cyclin-dependent kinase 4/6-dependent tumors and tissues. Cancer Res. 2007;67(10):4732–4741. doi: 10.1158/0008-5472.CAN-06-3437. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui T, Hesabi B, Moons DS, et al. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol. 1999;19(10):7011–7019. doi: 10.1128/mcb.19.10.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Canc Rev. 2001;1(1):222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- 12.Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24(52):7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 13.Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11(1):15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- 14.Deeb KK, Michalowska AM, Yoon CY, et al. Identification of an integrated SV40 T/t-antigen cancer signature in aggressive human breast, prostate, and lung carcinomas with poor prognosis. Cancer Res. 2007;67(17):8065–8080. doi: 10.1158/0008-5472.CAN-07-1515. [DOI] [PubMed] [Google Scholar]
- 15.Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–1438. [PubMed] [Google Scholar]
- 16.Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18(22):2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- 17.Blagden S, de Bono J. Drugging cell cycle kinases in cancer therapy. Curr Drug Targets. 2005;6(3):325–335. doi: 10.2174/1389450053765824. [DOI] [PubMed] [Google Scholar]
- 18.Collins I, Garrett MD. Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr Opin Pharmacol. 2005;5(4):366–373. doi: 10.1016/j.coph.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 19.de Carcer G, Perez de Castro I, Malumbres M. Targeting cell cycle kinases for cancer therapy. Curr Med Chem. 2007;14(9):969–985. doi: 10.2174/092986707780362925. [DOI] [PubMed] [Google Scholar]
- 20.Toogood PL, Harvey PJ, Repine JT, et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48(7):2388–2406. doi: 10.1021/jm049354h. [DOI] [PubMed] [Google Scholar]
- 21.Chen-Kiang S, Di Liberto M, Louie T, et al. Targeting Cdk4/6 in combination therapy of chemoresistant multiple myeloma. ASCO Meeting Abstracts. 2008;26(15 suppl):8503. [Google Scholar]
- 22.Slamon DJ, Hurvitz SA, Applebaum S, et al. Phase I study of PD 0332991, cyclin-D kinase (CDK) 4/6 inhibitor in combination with letrozole for first-line treatment of patients with ER-positive, HER2-negative breast cancer. ASCO Meeting Abstracts. 2010;28(15 suppl):3060. [Google Scholar]
- 23.Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29(28):4018–4032. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- 24.Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaud K, Solomon DA, Oermann E, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70(8):3228–3238. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiedemeyer WR, Dunn IF, Quayle SN, et al. Pattern of retinoblastoma pathway inactivation dictates response to CDK4/6 inhibition in GBM. Proc Natl Acad Sci U S A. 2010;107(25):11501–11506. doi: 10.1073/pnas.1001613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyman GH. Chemotherapy dose intensity and quality cancer care. Oncology (Williston Park). 2006;20(14) suppl 9:16–25. [PubMed] [Google Scholar]
- 28.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28(33):4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- 29.Lyman GH. Balancing the benefits and costs of colony-stimulating factors: a current perspective. Semin Oncol. 2003;30(4) suppl 13:10–17. doi: 10.1016/s0093-7754(03)00312-9. [DOI] [PubMed] [Google Scholar]
- 30.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362(9392):1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 31.Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 2003;4(8):459–460. doi: 10.1016/s1470-2045(03)01163-x. [DOI] [PubMed] [Google Scholar]
- 32.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23(25):5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 33.Smith RE, Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26(7):1040–1050. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- 34.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25(9):1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 35.Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27(1):127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 36.Brookes S, Rowe J, Ruas M, et al. INK4a-deficient human diploid fibroblasts are resistant to RAS-induced senescence. EMBO J. 2002;21(12):2936–2945. doi: 10.1093/emboj/cdf289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruas M, Gregory F, Jones R, et al. CDK4 and CDK6 delay senescence by kinase-dependent and p16INK4a-independent mechanisms. Mol Cell Biol. 2007;27(12):4273–4282. doi: 10.1128/MCB.02286-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maroulakou IG, Anver M, Garrett L, Green JE. Prostate and mammary adenocarcinoma in transgenic mice carrying a rat C3(1) simian virus 40 large tumor antigen fusion gene. Proc Natl Acad Sci U S A. 1994;91(23):11236–11240. doi: 10.1073/pnas.91.23.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54(1):105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- 40.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8(5):R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411(6841):1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 42.Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell. 2006;9(1):13–22. doi: 10.1016/j.ccr.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Reddy HK, Mettus RV, Rane SG, Grana X, Litvin J, Reddy EP. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 2005;65(22):10174–10178. doi: 10.1158/0008-5472.CAN-05-2639. [DOI] [PubMed] [Google Scholar]
- 44.Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9(1):23–32. doi: 10.1016/j.ccr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 45.An HX, Beckmann MW, Reifenberger G, Bender HG, Niederacher D. Gene amplification and overexpression of CDK4 in sporadic breast carcinomas is associated with high tumor cell proliferation. Am J Pathol. 1999;154(1):113–118. doi: 10.1016/S0002-9440(10)65257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samady L, Dennis J, Budhram-Mahadeo V, Latchman DS. Activation of CDK4 gene expression in human breast cancer cells by the Brn-3b POU family transcription factor. Cancer Biol Ther. 2004;3(3):317–323. [PubMed] [Google Scholar]
- 47.Takano Y, Takenaka H, Kato Y, et al. Cyclin D1 overexpression in invasive breast cancers: correlation with cyclin-dependent kinase 4 and oestrogen receptor overexpression, and lack of correlation with mitotic activity. J Cancer Res Clin Oncol. 1999;125(8–9):505–512. doi: 10.1007/s004320050309. [DOI] [PubMed] [Google Scholar]
- 48.Herschkowitz JI, He X, Fan C, Perou CM. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 2008;10(5):R75. doi: 10.1186/bcr2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8(3):235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 50.Foulkes WD, Brunet JS, Stefansson IM, et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 2004;64(3):830–835. doi: 10.1158/0008-5472.can-03-2970. [DOI] [PubMed] [Google Scholar]
- 51.Gauthier ML, Berman HK, Miller C, et al. Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell. 2007;12(5):479–491. doi: 10.1016/j.ccr.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Treskes M, Boven E, van de Loosdrecht AA, et al. Effects of the modulating agent WR2721 on myelotoxicity and antitumour activity in carboplatin-treated mice. Eur J Cancer. 1994;30A(2):183–187. doi: 10.1016/0959-8049(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 54.Vittorio PV, Whitfield JF, Rixon RH. The radioprotective and therapeutic effects of imidazole and erythropoietin on the erythropoiesis and survival of irradiated mice. Radiat Res. 1971;47(1):191–198. [PubMed] [Google Scholar]
- 55.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24(18):2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 56.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(Suppl 2):S73–81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puyol M, Martin A, Dubus P, et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell. 18(1):63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 58.Leonard JP, LaCasce A, Smith MR, et al. Cdk4/6 Inhibitor PD 0332991 Demonstrates Cell Cycle Inhibition Via FLT-PET Imaging and Tissue Analysis in Patients with Recurrent Mantle Cell Lymphoma. ASH Annual Meeting Abstracts. 2008;112(11):264. [Google Scholar]
- 59.Niesvizky R, Lentzsch S, Badros AZ, et al. A Phase I Study of PD 0332991: Complete CDK4/6 Inhibition and Tumor Response In Sequential Combination with Bortezomib and Dexamethasone for Relapsed and Refractory Multiple Myeloma. Blood (ASH Annual Meeting Abstracts) 2010;116(21):860. [Google Scholar]
- 60.Vaughn DJ, Flaherty K, Lal P, et al. Treatment of growing teratoma syndrome. N Engl J Med. 2009;360(4):423–424. doi: 10.1056/NEJMc0808558. [DOI] [PubMed] [Google Scholar]
- 61.Romagosa C, Simonetti S, Lopez-Vicente L, et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30(18):2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 62.Konecny GE, Winterhoff B, Kolarova T, et al. Expression of p16 and Retinoblastoma Determines Response to CDK 4/6 Inhibition in Ovarian Cancer. Clin Cancer Res. 2011;17(6):1591–1602. doi: 10.1158/1078-0432.CCR-10-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99(3):196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 64.Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25(3):292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 65.Vadhan-Raj S. Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents. Semin Hematol. 2009;46(1 Suppl 2):S26–32. doi: 10.1053/j.seminhematol.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Untch M, Mobus V, Kuhn W, et al. Intensive dose-dense compared with conventionally scheduled preoperative chemotherapy for high-risk primary breast cancer. J Clin Oncol. 2009;27(18):2938–2945. doi: 10.1200/JCO.2008.20.3133. [DOI] [PubMed] [Google Scholar]
- 67.Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: results from a randomized trial. J Natl Cancer Inst. 2005;97(23):1724–1733. doi: 10.1093/jnci/dji398. [DOI] [PubMed] [Google Scholar]
- 68.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25(12):1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]
- 69.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 1. J Clin Oncol. 2004;22(5):777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial–INTACT 2. J Clin Oncol. 2004;22(5):785–794. doi: 10.1200/JCO.2004.07.215. [DOI] [PubMed] [Google Scholar]
- 71.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23(25):5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 72.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23(25):5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 73.Gandara D, Narayan S, Lara PN, Jr., et al. Integration of novel therapeutics into combined modality therapy of locally advanced non-small cell lung cancer. Clin Cancer Res. 2005;11(13 Pt 2):5057s–5062s. doi: 10.1158/1078-0432.CCR-05-9012. [DOI] [PubMed] [Google Scholar]
- 74.Di Liberto M, Huang X, Bretz J, et al. Induction of Metabolic Impairment In Prolonged Early G1 Arrest Induced by CDK4/CDK6 Inhibition Sensitizes Myeloma Cells for Proteasome Inhibitor Killing During Subsequent S Phase Synchronization. Blood. 2010;116(21):2989. (ASH Annual Meeting Abstracts) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.