Abstract
Background
Adjuvant tamoxifen therapy is effective for postmenopausal women with endocrine-responsive breast cancer. Cytochrome P450 2D6 (CYP2D6) enzyme metabolizes tamoxifen to clinically active metabolites, and CYP2D6 polymorphisms may adversely affect tamoxifen efficacy. In this study, we investigated the clinical relevance of CYP2D6 polymorphisms.
Methods
We obtained tumor tissues and isolated DNA from 4861 of 8010 postmenopausal women with hormone receptor–positive breast cancer who enrolled in the randomized, phase III double-blind Breast International Group (BIG) 1-98 trial between March 1998 and May 2003 and received tamoxifen and/or letrozole treatment. Extracted DNA was used for genotyping nine CYP2D6 single-nucleotide polymorphisms using polymerase chain reaction–based methods. Genotype combinations were used to categorize CYP2D6 metabolism phenotypes as poor, intermediate, and extensive metabolizers (PM, IM, and EM, respectively; n = 4393 patients). Associations of CYP2D6 metabolism phenotypes with breast cancer-free interval (referred to as recurrence) and treatment-induced hot flushes according to randomized endocrine treatment and previous chemotherapy were assessed. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical tests were two-sided.
Results
No association between CYP2D6 metabolism phenotypes and breast cancer-free interval was observed among patients who received tamoxifen monotherapy without previous chemotherapy (P = .35). PM or IM phenotype had a non-statistically significantly reduced risk of breast cancer recurrence compared with EM phenotype (PM or IM vs EM, HR of recurrence = 0.86, 95% CI = 0.60 to 1.24). CYP2D6 metabolism phenotype was associated with tamoxifen-induced hot flushes (P = .020). Both PM and IM phenotypes had an increased risk of tamoxifen-induced hot flushes compared with EM phenotype (PM vs EM, HR of hot flushes = 1.24, 95% CI = 0.96 to 1.59; IM vs EM, HR of hot flushes = 1.23, 95% CI = 1.05 to 1.43).
Conclusions
CYP2D6 phenotypes of reduced enzyme activity were not associated with worse disease control but were associated with increased hot flushes, contrary to the hypothesis. The results of this study do not support using the presence or absence of hot flushes or the pharmacogenetic testing of CYP2D6 to determine whether to treat postmenopausal breast cancer patients with tamoxifen.
CONTEXT AND CAVEATS
Prior knowledge
Women with hormone receptor–positive breast cancer are treated with tamoxifen. Because cytochrome P450 2D6 (CYP2D6) metabolizes tamoxifen to clinically active metabolites, it is suggested that pharmacogenetic testing of CYP2D6 polymorphisms to identify patients with reduced tamoxifen metabolism phenotypes may predict poorer responsiveness to tamoxifen.
Study design
DNA from tumor tissues of postmenopausal breast cancer patients who participated in the Breast International Group (BIG) 1-98 trial and received adjuvant tamoxifen and/or letrozole treatment was used for CYP2D6 genotyping. CYP2D6 metabolism phenotypes were classified as poor, intermediate, and extensive metabolizers (PM, IM, and EM, respectively), and associations with breast cancer-free interval and treatment-induced hot flushes were assessed according to randomly assigned endocrine treatment and previous chemotherapy.
Contribution
CYP2D6 metabolism phenotypes showed no association with breast cancer-free interval among patients who received tamoxifen without previous chemotherapy. The PM and IM phenotypes showed an increased rate of tamoxifen-induced hot flushes compared with EM phenotype, which was contrary to the hypothesis.
Implications
Phenotypes of reduced CYP2D6 enzyme activity were not associated with worse disease control or reduced tamoxifen-induced hot flushes. Results do not support the pharmacogenetic testing of CYP2D6 to predict the efficacy of tamoxifen in postmenopausal hormone receptor–positive breast cancer patients.
Limitations
BIG 1-98 trial did not collect data on concomitant medications. Therefore, this study could not address whether patients should avoid concomitant medication of tamoxifen and CYP2D6 inhibitors. Some misclassification of metabolism phenotypes may have occurred.
From the Editor
For decades, the standard adjuvant endocrine therapy for postmenopausal women with estrogen receptor (ER)– and/or progesterone receptor (PgR)–positive breast cancer was 5 years of the selective ER modulator tamoxifen, which improved disease-free survival and reduced the annual breast cancer death rate by 31% (1). Recent reports from the Breast International Group (BIG) 1-98 trial have shown that adjuvant therapy with the aromatase inhibitor letrozole given as a single agent for 5 years improves disease-free and overall survival compared with 5 years of tamoxifen in this population (2–5). However, there may be groups of patients, for example, those at lower risk for recurrence, for whom tamoxifen or a sequence of the two agents represents a reasonable choice (4,6), and others for whom the availability and/or side effects of aromatase inhibitor therapy make tamoxifen the preferable treatment. Thus, there is considerable interest in defining the population of patients who have the greatest chance of benefiting from tamoxifen.
Early clinical investigation of the pharamacogenetics of tamoxifen metabolism showed promise that a pharmacogenetic testing of Cytochrome P450 2D6 (CYP2D6) phenotype to identify patients with reduced tamoxifen metabolism could predict poorer responsiveness to tamoxifen in terms of disease recurrence (7). A modeling study further suggested that patients with a phenotype of extensive tamoxifen metabolism may receive equal benefit from tamoxifen as from an aromatase inhibitor (8). However, in more than one dozen subsequent clinical investigations, the results are conflicting (9,10), and the evidence base is inconclusive. With CYP2D6 pharmacogenetic testing now clinically available, there is uncertainty among patients, health-care providers, health authorities, and insurers about its utility for patient care.
The underlying hypothesis is that CYP2D6 polymorphisms leading to reduced CYP2D6 enzyme activity result in lower plasma concentrations of endoxifen, which adversely affects tamoxifen efficacy. Tamoxifen has relatively weak affinity for ER and undergoes extensive primary and secondary metabolism, principally by the highly polymorphic enzyme of the CYP2D6 gene (11,12), to form clinically active metabolites 4-hydroxytamoxifen and endoxifen (4-hydroxy-N-desmethyltamoxifen), which have 30- to 100-fold greater affinity for ER compared with tamoxifen (13,14). Endoxifen is believed to be the most clinically active metabolite (15–17). Patients’ plasma concentrations of tamoxifen, endoxifen, and 4-hydroxytamoxifen vary widely, but studies have shown that CYP2D6 reduced metabolism phenotypes to be associated with lower endoxifen levels (16,18,19). Lower endoxifen levels are also hypothesized to result in fewer or less severe tamoxifen-induced hot flushes (7,20,21).
We investigated the clinical relevance of CYP2D6 metabolism phenotype in the BIG 1-98 trial. We hypothesized that CYP2D6 phenotypes of reduced CYP2D6 enzyme activity would be associated with worse disease control among tamoxifen-treated, postmenopausal hormone receptor–positive breast cancer patients and would be associated with reduced onset of tamoxifen-induced hot flushes. Unlike previous studies that mostly focused on patients who received tamoxifen without previous chemotherapy, our investigations separated patients according to previous chemotherapy use.
Methods
Patients
The BIG 1-98 study is an international, randomized, phase III double-blind trial comparing 5 years of monotherapy with tamoxifen (20 mg daily) or with letrozole (2.5 mg daily) or a sequential therapy of 2 years of one of these agents (same daily dose) followed by 3 years of the other (2–5) among postmenopausal women with ER- and/or PgR-positive, operable invasive breast cancer (Supplementary Figure 1, available online). A total of 8010 women were enrolled between March 1998 and May 2003. Centers participated in one of two randomization options (two-arm and four-arm): from March 1998 through March 2000, women were randomized to receive only letrozole or only tamoxifen for 5 years; and from April 1999 through May 2003, women were randomly assigned to one of four study treatments.
All participants provided written informed consent. Ethics committees and relevant health authorities approved the protocol. Trial participants were followed clinically at baseline, every 6 months for the first 5 years during blinded study drug dispensing, and yearly thereafter. Specific adverse events were listed on the case report forms and graded according to the Common Toxicity Criteria (CTC) v2.0 at study visits, including hot flushes (CTC grade 1 = mild or no more than one per day; grade 2 = moderate and greater than one per day) and night sweating (CTC grade 1 = mild and occasional; grade 2 = frequent or drenching).
Tissue Collection, DNA Extraction, and CYP2D6 Genotyping
Retrospective tissue collection was carried out by the International Breast Cancer Study Group (IBCSG) and the Danish Breast Cancer Collaborative Group between 1998 and 2010 in accordance with institutional guidelines and national laws. BIG 1-98 Collaborative Group members who submitted tumor blocks are listed in Supplementary Appendix (available online). Funding was provided to participating institutions by the BIG 1-98 trial's pharmaceutical partner, Novartis, to partially cover associated costs. The IBCSG Biological Protocols Working Group approved this project. All material processing and genotyping were done without the knowledge of patients’ treatment assignments or outcomes.
Formalin-fixed paraffin-embedded primary breast cancer tissue blocks for 5786 patients were assessed for adequacy of invasive tumor material to extract nucleic acids. In 5166 tumor blocks from patients, an area that was representative of the invasive tumor component was identified, and one or two 1-mm cores were punched in this area. DNA was isolated for 4861 of 8010 trial patients (Figure 1). Genomic DNA was extracted using the QIAamp DNA formalin-fixed paraffin-embedded tissue kit (Qiagen, Valencia, CA) according to manufacturer's instructions. DNA was eluted with 60 μL of sterile distilled water, quantified, and quality controlled according to the 260/280 nm ratio using the Infinite M 200 NanoQuant (Tecan, Mannedorf, Switzerland) and aliquoted at a concentration of 10 ng/μL.
Figure 1.
Flow diagram of the Breast International Group (BIG) 1-98 trial participants included in the study. Collection of tumor tissue blocks, DNA extraction, CYP2D6 genotyping, and patient cohorts for analysis are shown. BCFI = breast cancer-free interval; CYP2D6 = Cytochrome P450 2D6; ER = estrogen receptor; FFPE = formalin-fixed paraffin-embedded.
The DNA samples from patients were genotyped for nine CYP2D6 single-nucleotide polymorphisms (SNPs) that were selected to maximize CYP2D6 phenotype prediction by capturing approximately 99% of CYP2D6 genotypes: the most common null allele CYP2D6*4 (1846G>A [rs3892097]); three SNPs that together determine CYP2D6*2, CYP2D6*10, and CYP2D6*41 alleles and rule out the possibility of CYP2D6*5 allele (ie, 4180G>C [rs1135840], 100C>T [rs1065852], and 2988G>A [rs28371725]); and five SNPs for determining four less common alleles CYP2D6*3, CYP2D6*6, CYP2D6*7, and CYP2D6*17 (ie, 2549delA [rs35742686], 1707delT [rs5030655], 2935A>C [rs5030867], 1023C>T [rs28371706], and 2850C>T [rs16947]) (22) (Supplementary Table 1, available online). Samples were genotyped in a Clinical Laboratory Improvement Amendments (CLIA)–accredited laboratory using polymerase chain reaction (PCR)–based methods: the GenomeLab SNPstream Genotyping System (Beckman Coulter, Brea, CA); and the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). For SNPstream genotyping, we used three primers for each SNP; two primers for the initial PCR reaction and one for the single-base extension reaction were designed and grouped into 48-plexed panels. Using 2–10 ng of genomic DNA, a 48-plex PCR was carried out in 384-well plate to amplify an approximately 100 bp region flanking each SNP. The reaction was treated with ExoSAP reagent to remove any leftover PCR primers and deoxynucleotide triphosphates (dNTPs). The 48-plex extension primer pool was then added to the same PCR plate. A single-base extension reaction was performed to incorporate a differentially labeled fluorescent dNTP to the SNP position. The extension reaction was transferred to a Tag Array and spatially resolved to distinguish the 48 SNPs. All reagents were preformulated and included in the GenomeLab SNPware Reagent kit (Beckman Coulter Inc, Fullerton, CA). The GenomeLab SNPstream Genotyping System Software Suite v2.3 (Beckman Coulter Inc) was used for array imaging and genotype calling. For the CYP2D6*4 (1846G>A [rs3892097]) and CYP2D6*41 (2988G>A [rs28371725]) SNPs, commercially available pre-designed TaqMan assays (Applied Biosystems) were used and run on the 7900HT Fast Real-Time PCR System. The optimization was done using Applied Biosystems’ TaqMan probes, in conjunction with the KlearKall Mastermix (KBioscience, Beverly, MA). The PCR conditions were as follows: 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute and then hold at 4°C. For CYP2D6*41, the cycle number was increased to 45 cycles and for CYP2D6*4, the cycle number was increased to 60 cycles.
As described previously (23), we categorized patients according to predicted CYP2D6 metabolism phenotypes based on genotype combinations: poor metabolizer (PM) phenotypes were homozygous or compound heterozygous for CYP2D6*3, CYP2D6*4, CYP2D6*6, or CYP2D6*7 alleles (PM alleles); intermediate metabolizer (IM) phenotypes carried either homozygous CYP2D6*41 alleles (IM alleles) or a CYP2D6*41 allele in combination with a PM allele (ie, IM/IM or IM/PM alleles, respectively), or were heterozygous carriers of one PM or IM allele with an extensive metabolizer (EM) allele (ie, heterozygous for extensive metabolizer allele [hetEM]); EM phenotypes were characterized by the absence of PM and IM alleles.
Statistical Analysis
Breast cancer-free interval (BCFI) was analyzed among patients who were randomly assigned to 5 years of tamoxifen or letrozole monotherapy and was defined from random assignment to the first breast cancer event (local, regional, or distant recurrence, or a new invasive contralateral breast cancer; henceforth referred to as recurrence) ignoring second (non-breast) cancers (24). In the absence of an event, BCFI was censored at the last follow-up visit, death without a previous cancer event (3.5% of patients), or at selective crossover from tamoxifen to letrozole after dissemination of the primary trial results in 2005 (2). Although the updated trial analysis at a median follow-up of 76 months implemented an inverse probability of censoring weighted analysis (25), which estimated the relative benefit of letrozole vs tamoxifen monotherapy that would have been observed in the absence of selective crossover of 25% of patients assigned tamoxifen, it was reasonably approximated by an unweighted analysis with censoring at the time of selective crossover to letrozole (4,5). For this investigation of tamoxifen metabolism, the primary analysis used the unweighted censored analysis approach. BCFI was estimated using the Kaplan–Meier method. Cox proportional hazards modeling, stratified by two- or four-arm randomization option, was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs). The model included the three-way interaction of treatment, previous neoadjuvant or adjuvant chemotherapy use, and CYP2D6 phenotype. The model also adjusted for characteristics at random assignment (race [white vs all other], local therapy [mastectomy vs breast-conserving surgery], tumor size [≤2 vs >2 cm], tumor grade [1, 2, 3], nodal status [positive vs negative], peritumoral vascular invasion [absent, present, not assessed], HER2 status [positive vs negative], Ki-67 labeling index [<14% vs ≥14% immunostained cells]). The primary hypothesis tested the association of CYP2D6 metabolism phenotype (2 df Wald test) with BCFI in tamoxifen-treated patients without previous chemotherapy, with secondary tests in the other three subgroups defined by randomized endocrine treatment and previous chemotherapy use. There was no adjustment for multiple hypothesis testing. The proportional hazards assumption was assessed by testing for genotype-by-time interaction overall and within each of the four subgroups.
Time to onset of hot flushes or night sweats (henceforth referred to as hot flushes) within the first 2 years of treatment was defined from random assignment to the first report of new or worsening events of any grade within 23 months of random assignment because most treatment-induced hot flushes began during that period (5). Patients were analyzed according to the assigned treatment for the first 2 years of the 5-year treatment, which may have been monotherapy or sequential therapy (Figure 1). Cumulative incidence was estimated as one minus the Kaplan–Meier estimate. A Cox model for hot flush onset was adjusted for characteristics at random assignment (race [white vs all other], age [<60, 60–64, 65–69, ≥70 years], body mass index [<18.5, 18.5–29.9, >30 kg/m2], history of hormone replacement therapy use [never, within last 3 months, ≥3 months ago], and hot flushes [presence vs absence]).
Among the 1250 patients assigned to tamoxifen monotherapy, with 14% events and 37.5% prevalence of reduced CYP2D6 metabolism phenotype (PM or IM), there was 80% power (two-sided α = 0.05) to detect a hazard ratio of 1.55 comparing PM or IM vs EM phenotypes.
Results are presented in accordance with REMARK criteria (26). All statistical tests were two-sided, and P values less than .05 were considered statistically significant.
Results
A total of 4861 postmenopausal women with hormone receptor–positive breast cancer were genotyped for CYP2D6. Most (98%) patients were white, 43% had lymph node–positive disease, and 77% had no previous chemotherapy. Characteristics of the patients and their disease according to availability of DNA for genotyping were comparable (Table 1). Tumor DNA samples from 4861 patients were used for genotyping, and CYP2D6 metabolism phenotype could be estimated for 4393 patients, which were included in the analysis (Figure 1). The classification of CYP2D6 metabolism based on previously reported genotype combinations (23) showed 8.3% PM, 29.5% IM, and 62.2% EM phenotypes (Table 2). Fewer than 20% of trial patients discontinued their assigned treatment before 5 years for discretionary reasons (ie, other than breast cancer recurrence, second non-breast cancer, or selective crossover). Discontinuation was not associated with CYP2D6 phenotype (P = .97; data not shown).
Table 1.
Characteristics of patients in the BIG 1-98 trial according to availability of DNA for genotyping*
| Characteristic | DNA for CYP2D6 genotyping† |
|
| No (n = 3149 patients) | Yes (n = 4861 patients) | |
| Two-arm or four-arm randomization, % | 66 | 84 |
| Median follow-up, mo | 73 | 72 |
| Postmenopausal, % | 100 | 100 |
| White race, % | 97 | 98 |
| Age, median (IQR), y | 61 (55–67) | 61 (56–67) |
| BMI, median (IQR), kg/m2 | 26 (22–29) | 26 (22–29) |
| Mastectomy, % | 46 | 42 |
| Previous (neo)adjuvant chemotherapy, % | 29 | 23 |
| Lymph node positive, % | 38 | 43 |
| Tumor size > 2 cm, % | 34 | 39 |
| Tumor grade 2 or 3, % | 60 | 67 |
| Peritumoral vascular invasion present | 18 | 17 |
| Centrally assessed tumor features‡ | ||
| ER absent, % | 2 | 1 |
| HER2 positive, % | 7 | 6 |
| Ki-67 LI of immunostained cells, median (IQR), % | 10 (5–16) | 12 (7–19) |
Eligible for enrollment in the randomized, phase III double-blind Breast International Group (BIG) 1-98 trial were postmenopausal women with hormone receptor–positive operable invasive breast cancer. BMI = body mass index; CYP2D6 = Cytochrome P450 2D6; ER = estrogen receptor; IQR = interquartile range; LI = labeling index.
Genomic DNA was isolated from formalin-fixed paraffin-embedded primary breast cancer tissue blocks from 4861 BIG 1-98 trial patients. Genotyping for nine CYP2D6 single-nucleotide polymorphisms was done using polymerase chain reaction–based methods.
Centrally assessed tumor features were available for a subset of the trial patients (1515 of 3149 patients with no DNA for genotyping; 4776 of 4861 patients with DNA available for genotyping).
Table 2.
CYP2D6 genotyping and prevalence of CYP2D6 metabolism phenotype in BIG 1-98 trial participants*
| CYP2D6 allele† | Assessable, No. | Polymorphic alleles, No. (%) | Genotype, % |
|||
| SNP | Homozygous | Heterozygous | Wild-type | |||
| CYP2D6*4 | 1846G>A (rs2892097) | 3828 | 1444 (18.9) | 8.6 | 20.5 | 70.9 |
| CYP2D6*2, *4, *10, *41 | 4180G>C (rs1135840) | 0 | — | — | — | — |
| CYP2D6*10,*4 | 100C>T (rs1065852) | 0 | — | — | — | — |
| CYP2D6*41 | 2988G>A (rs28371725) | 3842 | 643 (8.4) | 4.2 | 8.4 | 87.4 |
| CYP2D6*3 | 2549delA (rs35742686) | 3012 | 80 (1.3) | 0.4 | 1.9 | 97.7 |
| CYP2D6*6 | 1707delT (rs5030655) | 2707 | 101 (1.9) | 0.2 | 3.3 | 96.5 |
| CYP2D6*7 | 2935A>C (rs5030867) | 2767 | 0 | 0.0 | 0.0 | 100.0 |
| CYP2D6*17 | 1023C>T (rs28371706) | 0 | — | — | — | — |
| 2850C>T (rs16947) | 2285 | 1550 (33.9) | 16.2 | 35.4 | 48.4 | |
| CYP2D6 metabolism phenotype‡, No. (%) | ||||||
| Patients classified | — | 4393 (100.0) | — | — | — | — |
| Poor metabolizer | — | 365 (8.3) | — | — | — | — |
| Intermediate metabolizer | — | 1294 (29.5) | — | — | — | — |
| Extensive metabolizer | — | 2734 (62.2) | — | — | — | — |
BIG = Breast International Group; CYP2D6 = Cytochrome P450 2D6; SNP = single-nucleotide polymorphism; — = not applicable.
CYP2D6*4 (1846G>A; rs2892097) and CYP2D6*41 (2988G>A; rs28371725) were genotyped in all 4861 patient DNA samples; other alleles were genotyped in 3691 patient DNA samples. One hundred seventy-nine patient DNA samples failed CYP2D6 genotyping. Genotyping was done using polymerase chain reaction–based methods.
Patients were categorized into predicted metabolism phenotypes as follows: poor metabolizer (PM) phenotypes were homozygous or compound heterozygous for CYP2D6*3, CYP2D6*4, CYP2D6*6 or CYP2D6*7 alleles (PM alleles); intermediate metabolizer (IM) phenotypes carried either homozygous CYP2D6*41 alleles (IM alleles) or a CYP2D6*41 allele in combination with a PM allele (ie, IM/IM or IM/PM alleles, respectively; n = 215 patients; 5%), or were heterozygous carriers of one PM or IM allele with an extensive metabolizer (EM) allele (heterozygous for EM allele or hetEM; n = 1079 patients; 24.5%); EM phenotypes were characterized by the absence of PM and IM alleles.
Association Between CYP2D6 Phenotypes and Breast Cancer–Free Interval
A total of 2537 of 4393 patients were assigned to tamoxifen (n = 1243 patients) or letrozole (n = 1294 patients) monotherapy, in whom we investigated the association of CYP2D6 metabolism phenotype with BCFI (Figure 1). Because previous studies (7,9,10) mostly focused on patients who had received tamoxifen without previous chemotherapy, our investigations separated patients according to previous chemotherapy use.
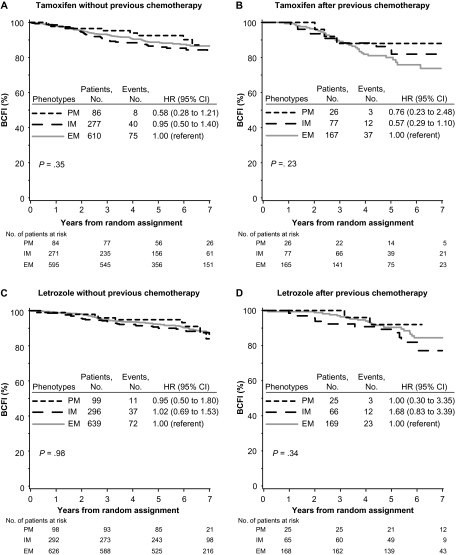
Among tamoxifen-treated patients without previous chemotherapy, no association between CYP2D6 metabolism phenotype and BCFI was noted (P = .35) (Figure 2, A). The PM and IM phenotypes, individually as well as combined, had a non-statistically significantly reduced risk of breast cancer event compared with the EM phenotype (PM vs EM, HR of recurrence = 0.58, 95% CI = 0.28 to 1.21; IM vs EM, HR of recurrence = 0.95, 95% CI = 0.50 to 1.40; PM and IM combined vs EM, HR of recurrence = 0.86, 95% CI = 0.60 to 1.24) (Figure 2, A and Supplementary Table 2, available online). No association was also noted among tamoxifen-treated patients with previous chemotherapy (P = .23) (Figure 2, B).
Figure 2.
Kaplan–Meier estimates of breast cancer-free interval (BCFI) according to CYP2D6 metabolism phenotype, endocrine treatment, and previous chemotherapy use in the Breast International Group (BIG) 1-98 trial. Outcome of 1243 patients randomly assigned to 5 years of tamoxifen monotherapy or 1294 patients randomly assigned to 5 years of letrozole monotherapy in the BIG 1-98 trial is shown. Based on CYP2D6 genotyping, metabolism phenotypes were classified as poor metabolizer (PM), intermediate metabolizer (IM), and extensive metabolizer (EM). A) Tamoxifen without previous chemotherapy. B) Tamoxifen after chemotherapy. C) Letrozole without previous chemotherapy. D) Letrozole after chemotherapy. P values were calculated using two-sided Wald tests with 2 df for association of CYP2D6 metabolism phenotype with BCFI within an adjusted Cox proportional hazards model. CI = confidence interval; CYP2D6 = Cytochrome P450 2D6; HR = hazard ratio.
Among letrozole-treated patients without previous chemotherapy, no association between CYP2D6 metabolism phenotype and BCFI was noted (P = .98) (Figure 2, C). No association was also noted among letrozole-treated patients with previous chemotherapy (P = .34) (Figure 2, D). Univariate (unadjusted) and multivariable (adjusted) analyses of associations between CYP2D6 metabolism phenotype with BCFI for both tamoxifen- and letrozole-treated groups are summarized in Supplementary Table 2 (available online).
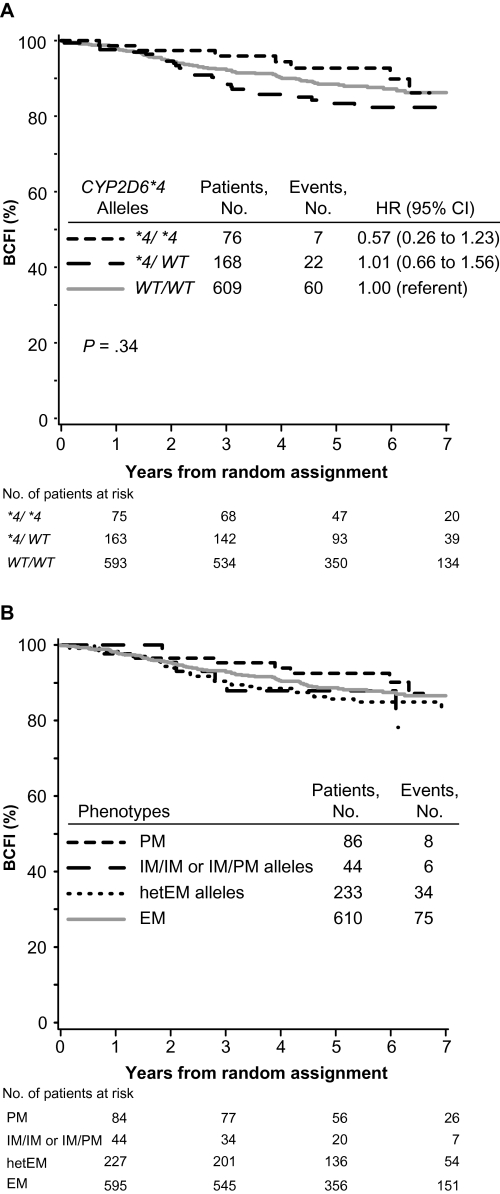
Some previous clinical investigations (7,9,10) classified patients’ CYP2D6 metabolism phenotype based solely on the most prevalent PM allele in white populations, CYP2D6*4. The associations with BCFI in tamoxifen-treated patients without previous chemotherapy based upon CYP2D6*4 were consistent with those based on the CYP2D6 metabolism phenotype (P = .34) (Figure 3, A). Patients who were homozygous (CYP2D6*4/*4, analogous to the PM phenotype) or heterozygous (CYP2D6*4/WT, analogous to the IM phenotype) for CYP2D6*4 variant allele had risks of breast cancer events that were not statistically significantly different from patients who were homozygous for wild-type alleles (WT/WT, analogous to the EM phenotype) (CYP2D6*4/*4 vs WT/WT, HR of recurrence = 0.57, 95% CI = 0.26 to 1.23; CYP2D6*4/WT vs WT/WT, HR of recurrence = 1.01, 95% CI = 0.66 to 1.56). Another point of inconsistency in the literature is whether patients who carry only one reduced or null function allele, that is, heterozygous carriers of one IM or PM allele (hetEM alleles), were classified as having IM or EM phenotype (9,10). In tamoxifen-treated patients without previous chemotherapy who had IM phenotype, the subgroup of patients with hetEM alleles had similar outcome as the subgroup of patients with IM/IM or IM/PM alleles (Figure 3, B).
Figure 3.
Kaplan–Meier estimates of breast cancer-free interval (BCFI) among tamoxifen-treated patients in the Breast International Group (BIG) 1-98 trial according to CYP2D6 metabolism phenotype. Outcome of 973 patients without previous chemotherapy who were randomly assigned to 5 years of tamoxifen monotherapy. P values were calculated using two-sided Wald tests with 2 df for association of CYP2D6 metabolism phenotype with BCFI within adjusted Cox proportional hazards models. A) Phenotype classification based only on CYP2D6*4 allele. Homozygous CYP2D6*4/*4 (shown as *4/*4) is analogous to the poor metabolizer (PM) phenotype; heterozygous CYP2D6*4/WT (shown as *4/WT) is analogous to the intermediate metabolizer (IM) phenotype; homozygous for wild-type alleles (WT/WT) is analogous to the extensive metabolizer (EM) phenotype. B) Phenotype classification with separation of two IM phenotype subgroups, defined as carriers of either homozygous CYP2D6*41 alleles or a CYP2D6*41 allele in combination with a PM allele (ie, IM/IM or IM/PM alleles, respectively), or defined as heterozygous carriers of one PM or IM allele and one EM allele (ie, heterozygous for EM alleles [hetEM]). CI = confidence interval; CYP2D6 = Cytochrome P450 2D6; HR = hazard ratio.
Association Between CYP2D6 Phenotypes and Treatment-Induced Hot Flushes
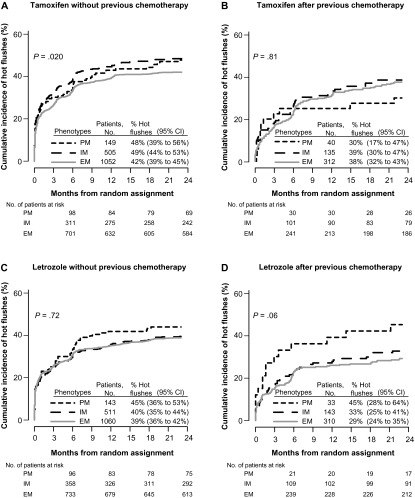
Among all 4393 patients assigned tamoxifen or letrozole monotherapy (n = 2537 patients) or sequential therapy (n = 1856 patients), we investigated new onset or worsening hot flushes during the first 2 years of therapy (Figure 1). Among the 1706 patients without previous chemotherapy who initiated treatment with tamoxifen, there was an association of CYP2D6 metabolism phenotype with hot flushes (P = .020) (Figure 4, A). The PM and IM phenotypes had a statistically significantly increased risk of tamoxifen-induced hot flushes compared with the EM phenotype (PM vs EM, HR of hot flushes = 1.24, 95% CI = 0.96 to 1.59; and IM vs EM, HR of hot flushes = 1.23, 95% CI = 1.05 to 1.43) (Supplementary Table 3, available online). The cumulative incidence of hot flushes within 2 years of starting tamoxifen was 48% (95% CI = 39% to 56%) in patients with PM phenotype and 49% (95% CI = 44% to 53%) in patients with IM phenotype as compared with 42% (95% CI = 39% to 45%) in patients with EM phenotype (Figure 4, A). Among tamoxifen-treated patients with previous chemotherapy, no association between CYP2D6 phenotype and hot flushes was noted (P = .81) (Figure 4, B).
Figure 4.
Cumulative incidence of new or worsening hot flushes during the first 2 years of treatment in the Breast International Group (BIG) 1-98 trial according to CYP2D6 metabolism phenotype. Results of all 4393 patients whose phenotypes were poor metabolizer (PM), intermediate metabolizer (IM), and extensive metabolizer (EM). All P values were calculated using two-sided Wald tests with 2 df for association of CYP2D6 metabolism phenotype with onset of hot flushes within an adjusted Cox proportional hazards model. A) Tamoxifen without previous chemotherapy. B) Tamoxifen after previous chemotherapy. C) Letrozole without previous chemotherapy. D) Letrozole after previous chemotherapy. CI = confidence interval; CYP2D6 = Cytochrome P450 2D6.
Patients in the letrozole-treated group without previous chemotherapy showed no association between CYP2D6 phenotype and hot flushes (P = .72) (Figure 4, C), and no association was also noted among patients with previous chemotherapy (P = .06) (Figure 4, D). Univariate (unadjusted) and multivariable (adjusted) analyses of associations between CYP2D6 metabolism phenotype and hot flushes for both tamoxifen- and letrozole-treated groups are summarized in Supplementary Table 3 (available online).
Discussion
In contrast to our hypothesis, we found no association of CYP2D6 metabolism phenotype with BCFI among postmenopausal patients treated with tamoxifen, with or without previous chemotherapy, in the BIG 1-98 trial. The patients who received 5 years of tamoxifen and were classified as having a PM or IM phenotype did not have poorer disease control than those classified as having EM phenotype. Based on our results, CYP2D6 pharmacogenetic testing is not justified to determine whether adjuvant tamoxifen should be given to postmenopausal women with endocrine-responsive breast cancer.
We did not observe a treatment-by-phenotype interaction in the study, and thus the magnitude of benefit of letrozole over tamoxifen was the same among EMs as in the study as a whole. This result refutes the modeling supposition of Punglia et al. (8) that EMs might receive similar or perhaps greater benefit from adjuvant tamoxifen than an aromatase inhibitor.
We found that the PM and IM phenotypes of CYP2D6 were not associated with reduced hot flushes, rather, we observed a greater incidence of tamoxifen-induced hot flushes in these patients. This finding is in contrast to the hypothesis that lower endoxifen levels are manifest by fewer or less severe tamoxifen-induced hot flushes, which was based on an observation that none of 13 patients with CYP2D6*4/*4 genotype (PM phenotype) had moderate or severe hot flushes (7). Two subsequent studies reported mixed results (20,21), with one study reporting the highest incidence of hot flushes in the IM group (21), consistent with our observations. If, as others have reported, there is an association of hot flushes to breast cancer outcome (27,28), then it is because of some other mechanism. Based on our results and others (21), the presence or absence of hot flushes should not be used to estimate tamoxifen metabolism when making treatment decisions.
Our results indicate that CYP2D6 metabolism phenotype is not the correct surrogate for predicting symptoms and outcome of tamoxifen-treated postmenopausal women, and conclude that the relationship of tamoxifen metabolism with symptoms and disease control is not adequately understood. The hypothesis underlying our investigation—that CYP2D6 polymorphisms leading to reduced enzyme activity result in lower endoxifen levels, which adversely affects tamoxifen efficacy—was not tested in our clinical study, as we did not measure endoxifen levels. We can only conclude that CYP2D6 metabolism phenotype does not predict tamoxifen efficacy.
The role of endoxifen in tamoxifen efficacy is controversial. Endoxifen has greater affinity for ER than tamoxifen (13,14), and endoxifen may be the most important tamoxifen metabolite, with higher plasma concentrations than 4-hydroxytamoxifen (15,16,18,19) and similar ER affinity (13,14). Furthermore, endoxifen appears to have a different mechanism of action from 4-hydroxytamoxifen by targeting ERα for degradation, as opposed to stabilizing ERα, and inhibiting estradiol-mediated upregulation of amphiregulin, a ligand of epidermal growth factor receptor (17). The interest in endoxifen is such that endoxifen citrate (29) and Z-Endoxifen hydrochloride (18) are being developed as therapeutics (clintrials.gov NCT01273168). However, plasma concentrations of tamoxifen and a primary metabolite N-desmethyltamoxifen are higher than both endoxifen and 4-hydroxytamoxifen (15,16,18,19), and tamoxifen and metabolites N-desmethyltamoxifen, didesmethyltamoxifen, and 4-hydroxytamoxifen have been estimated to nearly saturate ER with 99.94% occupancy (30). In addition, ERα degradation has not been evidenced in neoadjuvant tamoxifen trials (31). Thus, further elucidation of tamoxifen metabolism and efficacy are needed.
The Women's Healthy Eating and Living (WHEL) study of breast cancer survivors recently reported on the relationship between endoxifen levels and outcome in a cohort of 1370 women with ER-positive early breast cancer, who were enrolled in the WHEL study if recurrence free and within 4 years of diagnosis, and who had been taking tamoxifen for at least 4 months before blood collection (32). There was no evidence of an increasing hazard of disease recurrence with decreasing endoxifen levels (considered in quintiles), but an increased hazard in the lowest quintile (ie, among the 20% of patients with the lowest endoxifen levels) was observed, and the authors postulated a minimum critical concentration threshold for endoxifen, which was associated with an IM or PM phenotype, high body mass index, and lower tamoxifen concentrations. They concluded that metabolism phenotype alone may not be sufficient to predict an individual's tamoxifen efficacy, as about one-quarter of patients with PM phenotype had endoxifen levels above the threshold.
Prospective clinical trials are needed to better understand the relationship between CYP2D6 metabolism phenotype, active metabolite concentrations, and outcomes. An ongoing prospective trial in the United States is Eastern Cooperative Oncology Group (ECOG) Trial 3108 (clinicaltrials.gov NCT01124695). This phase II trial of 240 patients with metastatic breast cancer treated with single-agent tamoxifen will assess the relationship of CYP2D6 activity with progression-free survival and response, and of endoxifen concentration with response.
Differences in published clinical studies include alleles genotyped, lack of a standardized definition of CYP2D6 metabolism phenotype, and regional and time-linked variation in the concomitant use of CYP2D6 inhibitors with tamoxifen (9). These factors have been further complicated by varied study designs, patient populations and endpoint definitions, and use of mostly retrospective investigations in small clinical trials or convenience samples. The results have been correspondingly conflicting. Our study, together with a recent study in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial (33), overcome most of the shortcomings and draw similar conclusions.
This study has a few limitations. Despite being the largest investigation to date, with almost 5000 patients genotyped who were consistently treated and followed in a clinical trial, it does not address all clinical issues in relation to CYP2D6 genotype. One such issue is whether patients should avoid the concomitant administration of tamoxifen and CYP2D6 inhibitors, such as selective serotonin reuptake inhibitors. Information on concomitant medications was not collected in BIG 1-98 trial. It is commonly suggested that patients receiving tamoxifen should avoid potent inhibitors when possible (9–11), based not only on studies showing variation in plasma endoxifen levels according to a CYP2D6 metabolism phenotype definition that includes CYP2D6 inhibitor drugs but also on the purported association of phenotype with outcome. This suggestion is controversial (33,34), and our study cannot contribute to the clinical decision about whether to avoid CYP2D6 inhibitors with tamoxifen treatment. By genotyping only a small set of CYP2D6 SNPs, there may be some misclassification of metabolism phenotype. Others have reported increased hazard ratios of nearly 2.0 for PM and IM vs EM phenotypes (7,9,10). In order for our study to have obscured a true hazard ratio of 1.5 and observed a hazard ratio near 1.0, 75% of patients classified as EMs would have to have been misclassified. Another consideration in all clinical investigations of CYP2D6 is whether patients completed 5 years of tamoxifen. One study specifically studied the issue and observed higher discontinuation of tamoxifen at 4 months among patients with greater CYP2D6 activity scores (35). In BIG 1-98 trial, discontinuation of assigned treatment was not associated with CYP2D6 metabolism phenotype.
In conclusion, in the BIG 1-98 trial of postmenopausal women who received 5 years of adjuvant tamoxifen with or without previous chemotherapy, CYP2D6 phenotypes of reduced enzyme activity were not associated with worse disease control. CYP2D6 PM and IM phenotypes were not associated with reduced tamoxifen-induced hot flushes. Results suggest that CYP2D6 pharmacogenetic testing is not justified to determine whether tamoxifen should be given to postmenopausal women nor to withhold treatment with an aromatase inhibitor. The presence or absence of hot flushes should not be used in the clinical setting to estimate tamoxifen efficacy. Our conclusions are only in postmenopausal women. The role of CYP2D6 in premenopausal women is unknown; for premenopausal patients, investigation of pharmacogenetic testing is ongoing in the Tamoxifen and Exemestane Trial (TEXT; identifier NCT00066703 at clinicaltrials.gov) and Suppression of Ovarian Function Trial (SOFT; identifier NCT00066690 at clinicaltrials.gov).
Funding
The translational research, including DNA extraction and genotyping, was funded by Susan G. Komen for the Cure Promise Grant (KG080081 to GV, OP, MMR). The Breast International Group (BIG) 1-98 trial was funded by Novartis and coordinated by the International Breast Cancer Study Group (IBCSG). Other support for the IBCSG: Swedish Cancer Society; The Cancer Council Australia; Australian New Zealand Breast Cancer Trials Group; Frontier Science and Technology Research Foundation; Swiss Group for Clinical Cancer Research (SAKK); United States National Institutes of Health National Cancer Institute (CA-75362 to RDG); Cancer Research Switzerland/Oncosuisse; and the Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK).
Supplementary Material
Footnotes
The BIG 1-98 trial is registered at ClinicalTrials.gov (Identifier NCT00004205). We are indebted to the women, physicians, nurses, and data managers who participated in this clinical trial; to the many pathologists who submitted tumor blocks; to the BIG 1-98 Steering Committee; to Novartis for funding of the clinical trial and of the collection of tumor blocks; to the IBCSG for the design of the trial, coordination, data management, medical review, statistical support, and to the IBCSG Central Pathology Office for collection and processing of tumor blocks; to the BIG-198 Collaborative Group (members who submitted tumor blocks are listed in Supplementary Appendix, available online); to Susan G. Komen for the Cure Promise Grant.
Conflicts of interest: The BIG 1-98 trial was funded by Novartis, who contracted with the IBCSG for provision of services related to the conduct and management of the trial and provided partial support for collection and review of tumor blocks. Dr A. Goldhirsch, Dr A. S. Coates, and Dr R. D. Gelber are responsible for the scientific management of the IBCSG. Dr G. Viale is responsible for management of the IBCSG Central Pathology Office. Dr J. M. Rae receives research funding from Pfizer, Inc, and has served on an Advisory Board for Olema Pharmaceuticals. Dr B. Thürlimann owns stock in Novartis. The remaining authors have no conflicts to declare.
Role of trial sponsor: The BIG 1-98 trial sponsor, Novartis, contracted with the IBCSG to design the BIG 1-98 trial; collect, analyze, and interpret the data; and report the results. This report presents a study based on collected tumor material (collection partially funded by Novartis) and genotyping (funded by a Promise Grant from the Susan G. Komen for the Cure). The writing of this article did not include representatives from Novartis or Susan G. Komen for the Cure. The BIG 1-98 Steering Committee reviewed the article, suggested changes, and is responsible for the decision to publish. The Steering Committee includes one Novartis employee and one former Novartis employee among its 31 members.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.The Breast International Group (BIG) 1-98 Collaborative Group. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 3.Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007;25(5):486–492. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 4.The Breast International Group (BIG) 1-98 Collaborative Group. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361(8):766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colleoni M, Giobbie-Hurder A, Regan MM, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29(9):1117–1124. doi: 10.1200/JCO.2010.31.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viale G, Regan MM, Dell’Orto P, et al. Which patients benefit most from adjuvant aromatase inhibitors? Results using a composite measure of prognostic risk in the BIG 1-98 randomized trial. Ann Oncol. 2011;22(10):2201–2207. doi: 10.1093/annonc/mdq738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23(36):9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 8.Punglia RS, Burstein HJ, Winer EP, Weeks JC. Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Natl Cancer Inst. 2008;100(9):642–648. doi: 10.1093/jnci/djn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraldeschi R, Newman WG. The impact of CYP2D6 genotyping on tamoxifen treatment. Pharmaceuticals. 2010;3(4):1122–1138. doi: 10.3390/ph3041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins MJ, Stearns V. Pharmacogenetics of endocrine therapy for breast cancer. Annu Rev Med. 2011;62:281–293. doi: 10.1146/annurev-med-070909-182545. [DOI] [PubMed] [Google Scholar]
- 11.Brauch H, Mürdter TE, Eichelbaum M, Schwab M. Pharmacogenomics of tamoxifen therapy. Clin Chem. 2009;55(10):1770–1782. doi: 10.1373/clinchem.2008.121756. [DOI] [PubMed] [Google Scholar]
- 12.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310(3):1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 13.Jordan VC. Metabolites of tamoxifen in animals and man: identification, pharmacology, and significance. Breast Cancer Res Treat. 1982;2(2):123–128. doi: 10.1007/BF01806449. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MD, Zuo H, Lee KH, et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85(2):151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 15.Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55(5):471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97(1):30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabollite, endoxifen, is a potent antiestrogen that targets estrogen receptor α for degradation in breast cancer cells. Cancer Res. 2009;69(5):1722–1727. doi: 10.1158/0008-5472.CAN-08-3933. [DOI] [PubMed] [Google Scholar]
- 18.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 19.Borges S, Desta Z, Li L, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharm Ther. 2006;80(1):61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Bonanni B, Macis D, Maisonneuve P, et al. Polymorphism in the CYP2D6 tamoxifen-metabolizing gene influences clinical effect but not hot flashes: data from the Italian Tamoxifen Trial. J Clin Oncol. 2006;24(22):3708. doi: 10.1200/JCO.2006.06.8072. [DOI] [PubMed] [Google Scholar]
- 21.Henry NL, Rae JM, Li L, et al. for Consortium on Breast Cancer Pharmacogenomics Investigators Association between CYP2D6 genotype and tamoxifen-induced hot flashes in a prospective cohort. Breast Cancer Res Treat. 2009;117(3):571–575. doi: 10.1007/s10549-009-0309-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sikora MJ, Thibert JN, Salter J, Dowsett M, Johnson MD, Rae JM. High-efficiency genotype analysis from formalin-fixed, paraffin-embedded tumor tissues. Pharmacogenomics J. 2011;11(5):348–358. doi: 10.1038/tpj.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroth W, Goetz MP, Hamann U, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302(13):1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–2132. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 25.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in and AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 26.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23(36):9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 27.Cuzick J, Sestak I, Cella D, Fallowfield L, on behalf of the ATAC Trialists’ Group Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: a retrospective analysis of the ATAC trial. Lancet Oncol. 2008;9(12):1143–1148. doi: 10.1016/S1470-2045(08)70259-6. [DOI] [PubMed] [Google Scholar]
- 28.Mortimer JE, Flatt SW, Parker BA, et al. Tamoxifen, hot flashes and recurrence in breast cancer. Breast Cancer Res Treat. 2008;108(3):421–426. doi: 10.1007/s10549-007-9612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad A, Shahabuddin S, Sheikh S, et al. Endoxifen, a new cornerstone of breast cancer therapy: demonstration of safety, tolerability, and systemic bioavailability in healthy human subjects. Clin Pharmacol Ther. 2010;88(6):814–817. doi: 10.1038/clpt.2010.196. [DOI] [PubMed] [Google Scholar]
- 30.Dowsett M, Haynes BP. Hormonal effects of aromatase inhibitors: focus on premenopausal effects and interaction with tamoxifen. J Steroid Biochem Mol Biol. 2003;86(3–5):255–263. doi: 10.1016/s0960-0760(03)00365-0. [DOI] [PubMed] [Google Scholar]
- 31.Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–5116. doi: 10.1200/JCO.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Madlensky L, Natarajan L, Tchu S. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rae JR, Druzy S, Hayes DF, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(6):452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lash TL, Cronin-Fenton D, Ahern TP, et al. CYP2D6 inhibition and breast cancer recurrence in a population-based study in Denmark. J Natl Cancer Inst. 2011;103(6):1–12. doi: 10.1093/jnci/djr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rae JM, Sikora MJ, Henry NL, et al. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J. 2009;9(4):258–264. doi: 10.1038/tpj.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.