Abstract
Endothelial dysfunction precedes cardiovascular disease and is accompanied by mitochondrial dysfunction. Here we tested the hypothesis that diesel exhaust particulate extracts (DEPEs), prepared from a truck run at different speeds and engine loads, would inhibit genomic estrogen receptor activation of nuclear respiratory factor-1 (NRF-1) transcription in human umbilical vein endothelial cells (HUVECs). Additionally, we examined how DEPEs affect NRF-1 regulated TFAM expression and, in turn, Tfam-regulated mtDNA-encoded cytochrome c oxidase subunit I (COI, MTCO1) and NADH dehydrogenase subunit I (NDI) expression as well as cell proliferation and viability. We report that 17β-estradiol (E2), 4-hydroxytamoxifen (4-OHT), and raloxifene increased NRF-1 transcription in HUVECs in an ER-dependent manner. DEPEs inhibited NRF-1 transcription and this suppression was not ablated by concomitant treatment with E2, 4-OHT, or raloxifene, indicating that the effect was not due to inhibition of ER activity. While E2 increased HUVEC proliferation and viability, DEPEs inhibited viability but not proliferation. Resveratrol increased NRF-1 transcription in an ER-dependent manner in HUVECs, and ablated DEPE inhibition of basal NRF-1 expression. Given that NRF-1 is a key nuclear transcription factor regulating genes involved in mitochondrial activity and biogenesis, these data suggest that DEPEs may adversely affect mitochondrial function leading to endothelial dysfunction and resveratrol may block these effects.
Keywords: endothelial cells, diesel exhaust, diesel exhaust particles, NRF-1, mitochondrial activity, resveratrol
Introduction
Endothelial dysfunction precedes cardiovascular disease and is accompanied by mitochondrial dysfunction (Rocha et al. 2010). Air pollution has impacts on endothelial function and vascular tone, blood pressure, thrombosis, and blood pressure (Simkhovich et al. 2008). The induction of reactive oxygen species (ROS, e.g., H2O2) in the vasculature of animals exposed to particulate matter (PM (Brook et al. 2004)) in ambient air is thought to be due to the liberation of polyaromatic hydrocarbons (PAHs) and other organic components of the diesel exhaust particles (DEPs) (Simkhovich et al. 2008). Evidence linking inhaled DEPs to adverse health effects including cardiovascular disease has been reviewed (Monforton 2006). Although the mechanism by which DEPs gain access to the circulation is unclear, a small percentage of inhaled PMs have been found in circulation (Chao et al. 2011).
The cardiovascular system has been recognized as an important target of estrogen action through both genomic and non-genomic (membrane-initiated) pathways involving both estrogen receptors α and β: ERα and ERβ (reviewed in (Arnal et al. 2010; Chambliss et al. 2002)). We previously characterized rapid non-genomic responses of primary bovine aortic endothelial cells (BAEC) and human umbilical vein endothelial cells (HUVECs) to estradiol (E2), resveratrol, and organic diesel exhaust particulate extracts (DEPEs) collected from a diesel truck run at various speeds and engine loads (Klinge et al. 2005; Klinge et al. 2008; Sumanasekera et al. 2007a; Sumanasekera et al. 2007b). Our studies demonstrated that DEPEs from a truck run under increasing loads (L) stimulated phosphorylation of MAPK, AKT, and eNOS whereas DEPEs from the truck run at increasing speeds (S) did not affect MAPK alone, but inhibited E2-induced MAPK and endothelial nitric oxide synthase (eNOS) phosphorylation (Sumanasekera et al. 2007a). Higher polyaromatic hydrocarbon (PAH) concentrations in the DEPE L compared to the DEPE S samples correlated with the differences in cellular activities. DEPEs rapidly increased NO with the DEPE L sample acting additively with E2 and then inhibiting E2-induced NO with longer time of treatment. Our results and those from a study comparing the genomic activity of organic extracts from the particulate and gas phase of ambient air collected in downtown Toronto, Canada (Klein et al. 2006) indicate that the impact of air pollution, chemical composition, and biological activity is complex.
In experimental animals and humans, estrogens increase vasodilator tone (Miller and Duckles 2008). Studies of the effect of E2 in rat cerebral blood vessels revealed that E2 increased eNOS activity, by both genomic and nongenomic mechanisms, and mitochondrial energy production in vivo and in vitro (Duckles and Krause 2010; Miller and Duckles 2008; Razmara et al. 2008; Stirone et al. 2005). Chronic treatment of ovariectomized rats with E2 resulted in increased protein expression of nuclear respiratory factor-1 (NRF-1) in the cerebral blood vessels (Stirone et al. 2005). NRF-1 is a key nuclear transcription factor that regulates the expression of nuclear-encoded mitochondrial genes including other transcription factors, e.g., Tfam (Transcription factor A, mitochondrial) responsible for regulating transcription of mitochondrial encoded genes, e.g., cytochrome c oxidase subunit I (COI, MTCO1) and NADH dehydrogenase subunit I (NDI) (Kelly and Scarpulla 2004; Scarpulla 2006; Scarpulla 2008). For this reason, NRF-1 has been proposed to be an important integrator of nucleo-mitochondrial interactions (Scarpulla 2002). We identified NRF-1 as a primary estrogen response gene in MCF-7 breast adenocarcinoma and H1793 lung adenocarcinoma cells (Mattingly et al. 2008). The mechanism by which E2 increased NRF-1 transcription was by activation of E2-ERα interaction with an estrogen response element (ERE) in the 5' promoter of the NRF1 gene. The ability of NRF-1 to regulate mitochondrial activity is expected to be important in maintaining endothelial function in the vasculature, although no one has specifically addressed this question. Homozygous disruption of NRF-1 in mice results in embryonic lethality between days 3.5–6.5 and the blastocysts show reduced mitochondrial DNA (Huo and Scarpulla 2001).
Mitochondria play a critical role in vascular pathology with endothelial cells considered the “frontline against vascular disease” (Davidson and Duchen 2007). The goal of this study was to determine the impact of DEPEs, E2, the TAM metabolite 4-hydroxytamoxifen (4-OHT), RAL, and resveratrol on the expression of NRF-1 in HUVECs.
Materials and Methods
Chemicals
E2, 4-hydroxytamoxifen (4-OHT), raloxifene (RAL), pertussis toxin (PTX), PD98059, and Wortmannin were purchased from Sigma-Aldrich (St. Louis, MO, USA). The ERα and ERβ selective agonists, propyl pyrazole triol (PPT) (Stauffer et al. 2000) and diarylpropionitrile (DPN) (Meyers et al. 2001), respectively, and the pan ER antagonist ICI 182,780 were purchased from Tocris (Ellisville, MO, USA). All were dissolved in 100% ethanol (EtOH) that was solely in glass storage containers.
Diesel exhaust particulate extracts (DEPEs)
The detailed chemical composition of the DEPEs used in this study was previously reported (Sumanasekera et al. 2007a). In brief, 2-ton diesel-engine truck (made in Japan, 4,610 cc, direct injection type, 1999 model) was run on a chassis dynamometer under the loads of 0, 50, or 75 % of maximum (L0, L50, and L75; torque 0, 3, or 4.5 kN, respectively) and at vehicle speeds of 20, 50, or 80 km/h (S20, S50, and S80 with no added load) (Kizu et al. 2003b; Okamura et al. 2002; Okamura et al. 2004b). Diesel exhaust particles (DEP) were collected on polytetrafluoroethylene-coated borosilicate Emfab filters (product no. 7224 = TX40HI20WW), Pallflex Products (Putnam, CT, USA) as described previously (Sumanasekera et al. 2007a). The filters retain 99.9% of particles sizes ≥ 0.3 μm. The extraction of the organic constitutents and the chemical composition of the DEPEs was reported in (Sumanasekera et al. 2007a). A filter blank (FB) sample was prepared similarly from unused filters. DEPEs were provided by Dr. Ryoichi Kizu, Faculty of Pharmaceutical Sciences, Doshisha Women's College of Liberal Arts, Japan. All DEPEs were dissolved in EtOH.
Cell treatments
Human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex BioScience (Walkersville, MD, USA). HUVEC were used between P3–8 and were maintained in EGM-2 supplemented with hydrocortisone, human fibroblast growth factor, vascular epidermal growth factor, insulin growth factor-1, ascorbic acid, human epidermal growth factor, gentamicin sulfate, amphotercin-B, heparin, and 2% FBS provided in a supplemental kit with the media from Cambrex (hereafter referred to as EGM-2 media). Prior to treatment, HUVECs were placed in EGM-2 media containing 2% dextran-coated charcoal stripped-FBS (CSS-FBS). Cells were serum-starved for 24 h prior to each experiment and treated with vehicle control (ethanol, EtOH), or other treatments in phenol-red free medium without serum for the time and concentration indicated in the Figs.
RNA Isolation, RT-PCR and Quantitative Real-Time-PCR (QRT-PCR)
RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA). The High Capacity cDNA archive kit (PE Applied Biosystems) was used to reverse transcribe total RNA using random hexamers. Taqman primers and probes for NRF-1 and 18S rRNA were purchased as Assays-on-Demand™ Gene Expression Products (PE Applied Biosystems) and QRT-PCR was performed in the ABI PRISM 7900 SDS 2.1 (PE Applied Biosystems). The expression of each target gene was determined in triplicate in 3–4 separate experiments and normalized using 18S. Analysis and fold differences were determined using the comparative CT method. Fold change was calculated from the ΔΔCT values with the formula 2−ΔΔCT and data are presented as relative to expression in EtOH-treated cells.
MTT assay
Cell viability was determined using the Cell Titer 96 AQueous One solution cell proliferation assay (Promega, Madison, WI) according to the manufacturer's protocol. Briefly, 2,000 cells were plated per well in 96-well plates. Twenty-four hours after plating the cells were treated with ethanol, E2, or DEPEs of various concentrations (see Fig. legends for details) for 5 days. Each treatment was performed in quadruplicate within each experiment. The absorbance of solubilized formazan product was measured at 490 nm. All values were compared with those in the wells treated with vehicle (EtOH) control, which was set to 1.0.
Bromodeoxyudridine (BrdU) Cell Proliferation
The BrdU ELISA kit (Roche, Indianapolis, IN, USA) was used for quantification of cell proliferation. HUVEC were seeded at a density of 6000 cells/ well in a 96-well plate. The cells were treated for 48 h with the indicated treatments. Absorbance was measured at 370 nm. Each treatment was performed in quadruplicate and the values were averaged. All values were compared with those in the wells treated with vehicle (EtOH) control, which was set to 1.0.
Complex IV Activity Assay
The MitoProfile Microplate Assay Kit for Human Complex IV Activity was purchased from MitoSciences (Eugene, OR, USA). In this assay, Complex IV was immunocaptured and its activity determined colorimetrically via the oxidation of reduced cytochrome c. This is measured as a decrease in absorbance at 550 nm. HUVEC were seeded at 60% confluency in 6-well plates. The cells were grown in EGM-2 media supplemented with 2% CCS-FBS for 24 h. The cells were treated in EGM-2 media with 2% CCS-FBS for 6 d. The treatment was reapplied every 2 d. Protein concentrations were determined using the Bio-Rad DC Protein Assay (Hercules, CA, USA). Absorbance was measured every 5 min for 75 min. The slope of the reaction leveled off after 75 min. A linear range between 20 min and 60 min was examined for all treatments. The rate of Complex IV activity was determined by calculating the slope of the line between these two points resulting in OD/min change. One experiment was completed and assayed in quadruplicate. The EtOH from this experiment was set to 1.
Statistical analysis
Statistical analyses were performed using Student's t-test or one-way ANOVA followed by Student-Newman-Keuls post-hoc test using MS Excel and or GraphPad Prism.
Results
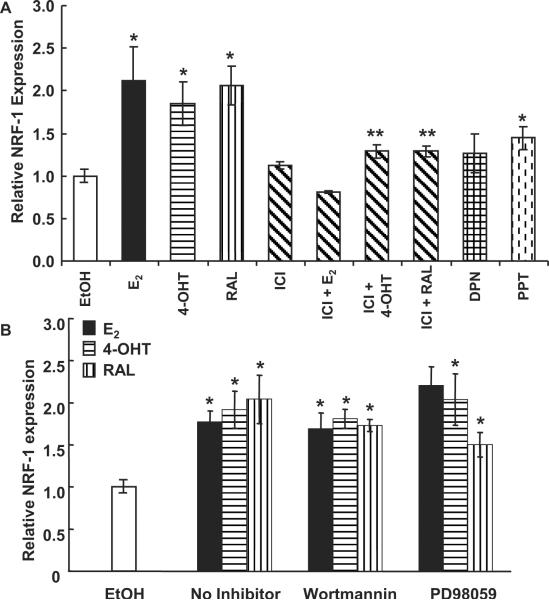
4-hydroxytamoxifen (4-OHT) and raloxifene (RAL) increase NRF-1 expression in HUVEC in an ER-dependent manner
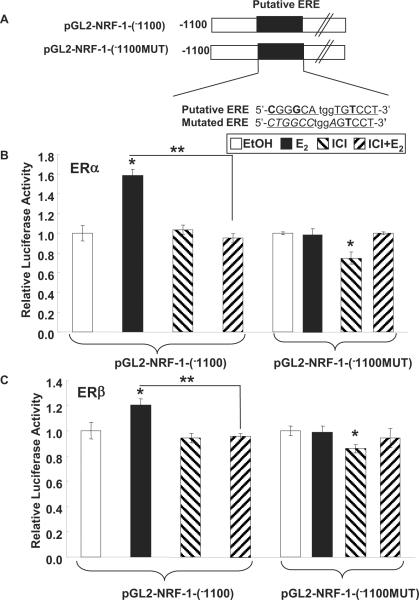
We previously reported that E2 and 4-OHT increased NRF-1 gene expression in MCF-7 breast cancer cells by activating ERα (Mattingly et al. 2008) and ERβ (Ivanova et al. 2011), respectively. Here we examined if E2 or the selective estrogen receptor modulators (SERMs) 4-OHT and RAL regulate NRF-1 transcription in HUVECs. E2, 4-OHT, and RAL increase NRF-1 transcription in HUVECs (Fig. 1A). HUVECs express both ERα and ERβ (Klinge et al. 2005). To further evaluate if ER is mediating the E2-, 4-OHT-, and RAL- induced increase in NRF-1 mRNA, HUVECs were pretreated with ICI 182,780, a pan ER antagonist (Wijayaratne et al. 1999), for 6 h prior to addition of E2, 4-OHT, and RAL. This pre-incubation time was selected because previous studies reported a ~80% decrease in ERα protein levels as early as 4 h post-treatment with ICI 182,780 (Wijayaratne and McDonnell 2001). ICI 182,780 inhibited the E2-, 4-OHT-, and RAL- induced increases in NRF-1 mRNA (Fig. 1A), indicating that ER mediates the induction responses. The ER subtype selective agonists DPN (ERβ) and PPT (ERα) increased NRF-1 transcription, but not as much as E2, 4-OHT, or RAL (Fig. 1A). These data suggest that both ERα and ERβ contribute to induction of NRF-1 transcription in HUVEC. This suggestion agrees with our results showing that transfection of ER-null HEK-283 cells with either ERα or ERβ activated luciferase reporter activity from the NRF-1 gene promoter containing the intact ERE site, but not when the ERE is mutated (Fig. 2). These data also show that ICI inhibited the E2-induced transcriptional activity of the transfected ERα and ERβ on the NRF-1-luciferase reporter.
Fig. 1. NRF-1 transcription is regulated by ER in HUVEC.
(A) HUVEC cells were treated with EtOH, 10 nM E2, 100 nM 4-OHT, 100 nM RAL, 10 nM DPN, or 10 nM PPT for 4 h; where indicated, cells were pretreated with 100 nM ICI 182,780 (ICI). (B) HUVEC cells were pre-treated with Wortmannin or PD98059 for 1 h +/− 100 nM 4-OHT and 100 nM RAL. *, p < 0.05 versus EtOH control; ** p < 0.05 versus same treatment without ICI.
Fig. 2. E2-ERα or E2-ERβ stimulates luciferase reporter activity from the NRF-1 promoter in transfected HEK-293 cells.
(A) The human NRF-1 promoter contains a nonconsensus ERE (half-sites) capitalized and underlined, nonconsensus nucleotides are in bold, and the 3 bp spacer in lower case italics (Mattingly et al. 2008). The mutation in the ERE to destroy its estrogenic activity is indicated by capitalized italics. HEK-293 cells were transfected with expression vectors for ERα (B) or ERβ (C), the indicated pGL2-NRF-1 promoter construct and Renilla luciferase. Transfected cells were treated with EtOH, 10 nM E2, 100 nM ICI 182,780, or the indicated combinations for 24 h. Dual luciferase reporter assays were performed. Values are relative luciferase activity compared to EtOH and are the mean ± SEM of 4 separate experiments. *, p < 0.05 versus EtOH control; ** p < 0.05 versus E2.
Signaling pathways including the MAPK (ER) and PI3K/AKT pathways are activated by nongenomic (membrane-initiated) E2 activity in HUVEC (Montiel et al. 2006; Sumanasekera et al. 2006). To determine if E2, 4-OHT, and RAL are increasing NRF-1 through these nongenomic pathways, HUVEC were pre-treated with the MEK kinase inhibitor PD98059 or the PI3K inhibitor Wortmannin, and then treated with E2, 4-OHT, and RAL. Neither PD98059 nor Wortmannin inhibited the E2-, 4-OHT- or RAL- induced increase in NRF-1 mRNA (Fig. 1B). We previously demonstrated that these inhibitors block E2-dependent increases in MAPK and AKT phosphorylation in HUVECs (Klinge et al. 2005; Klinge et al. 2008; Sumanasekera et al. 2007a). From these results, we conclude that E2, 4-OHT, and RAL are acting through classical genomic ER to increase NRF-1 transcription.
DEPEs inhibit basal NRF-1 mRNA expression
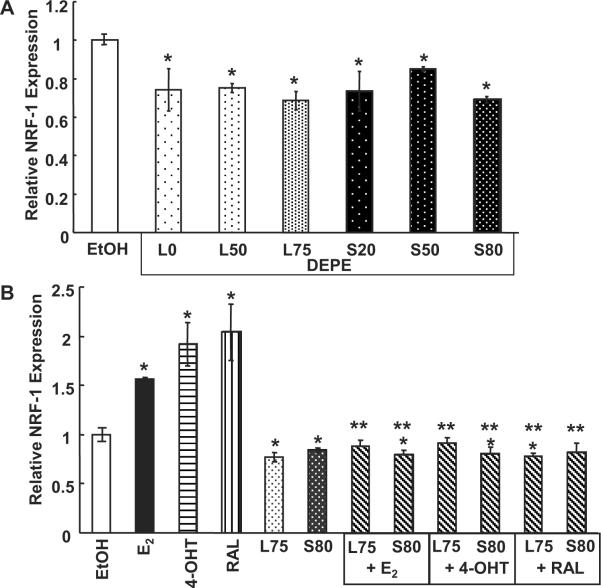
We have shown that DEPEs acted as antagonists of genomic E2 activity on an ERE-luciferase reporter in transiently transfected MCF-7 breast cancer cells (Okamura K 2002). Since NRF-1 is important to cardiovascular health through maintenance of mitochondrial function (Kelly and Scarpulla 2004), we examined if DEPEs interfered with the transcription of endogenous NRF-1. HUVEC cells were treated with 10 μg/mL of each DEPE and assayed for NRF-1 mRNA expression. All the DEPEs reduced basal NRF-1 expression by 10–20% (Fig. 3A). The reason for a lack of dose-response inhibition is unknown, but may result from the mixture of chemicals in each DEPE sample (Sumanasekera et al. 2007a).
Fig. 3. DEPEs reduces basal level expression of NRF-1 in HUVECs.
(A) HUVECs were treated with 10 μg/mL of each DEPE indicated. (B) Cells were treated with 10 nM E2, 100 nM 4-OHT, 100 nM RAL, or 10 mg/mL of L75 or S80 in the indicated combinations. All treatments for were 4 h. *, p < 0.05 versus EtOH control; ** p < 0.05 versus same treatment without DEPE.
DEPEs inhibit E2, 4-OHT, and RAL-induced NRF-1 mRNA expression
Two DEPE extracts, L75 and S80, were then used to treat HUVEC cells in combination with E2, 4-OHT and RAL to determine if the DEPEs could inhibit the induction in NRF-1 mRNA previously seen with each ER ligand (see Fig. 1A and 1B). L75 and S80 inhibited the E2, 4-OHT, and RAL mediated increase in NRF-1 mRNA (Fig. 3B). The precise mechanism for this inhibition is unknown. We did not detect any change in 18S mRNA expression, indicating a lack of general cell toxicity in response to DEPEs. The lack of apparent toxicity is in agreement with our previous studies showing no effect of DEPEs on MCF-7 viability (Okamura et al. 2002) and < 2% cytotoxicity as assessed by LDH release from HUVECs (Sumanasekera et al. 2007a).
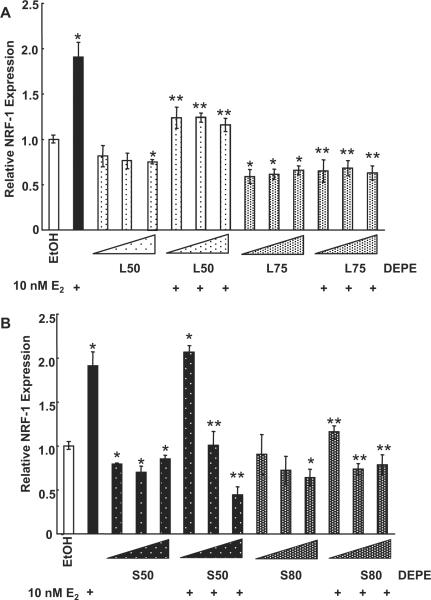
Next we tested the concentration-dependence of DEPEs L50, L75, S50, and S80 in combination with E2 to determine if only the higher concentrations of DEPEs (used in Fig. 3B) have antagonist properties. Fig. 4A shows that the DEPE L50 and L75 samples alone inhibited basal NRF-1 transcription regardless of concentration. There was no concentration-dependence in the observed inhibition of E2-induced NRF-1 transcription (Fig. 4A). However, L50 only partially inhibited the E2-induced increase in NRF-1, whereas L75 completely inhibited the E2-induced response at all three concentrations tested. The greatest chemical difference detected in the L versus S DEPE samples was the higher concentration of pyrene, benz[a]anthracene, chrysene, and benzo[b]fluoranthene in the L DEPEs (Sumanasekera et al. 2007a). The DEPE S50 sample inhibited basal expression of NRF-1 at all concentrations tested. Interestingly, S50 inhibited E2-induced increase in NRF-1 in a concentration-dependant manner, i.e., only the at the 1 μg/ml concentrations (Fig. 4B). S80 inhibited basal NRF-1 expression at the 10 μg/ml concentration. S80 inhibited the E2-induced increase in NRF-1 expression.
Fig. 4. DEPEs inhibit E2-induced NRF-1 transcription.
(A) HUVECs were treated for 6 h with 0.1, 1.0, or 10 μg/mL (indicated by size of triangle) of L50 or L75 DEPE in the presence or absence of 10 nM E2. (B) HUVECs were treated with 0.1, 1.0, or 10 μg/mL of S50 or S80 DEPE in the presence or absence of 10 nM E2. The total mRNA was isolated following treatment and QRT-PCR was performed as described in Material and Methods. Values are the average of 3 separate experiments ± SEM. * p < 0.05 versus EtOH; ** p < 0.05 versus E2.
Resveratrol induces an increase in NRF-1 through genomic ER
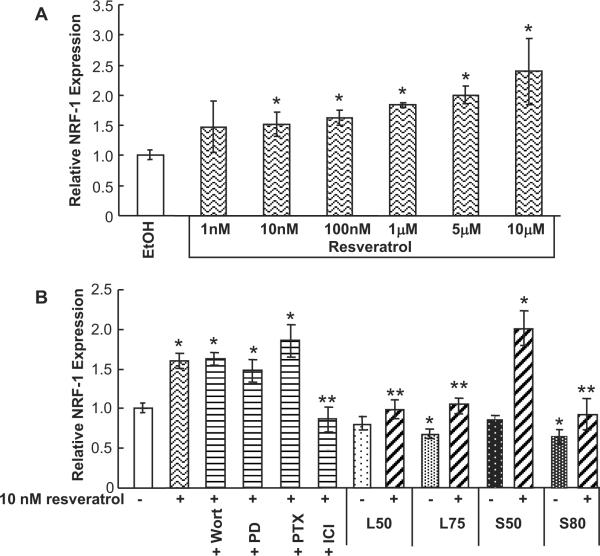
Resveratrol binds ERα and ERβ with low (59–130 μM) affinity (Bowers et al. 2000), but stimulates membrane-ER-initiated signaling in HUVECs at physiologically relevant concentrations of 50 nM (Klinge et al. 2008). Resveratrol increased NRF-1 mRNA expression in a concentration-dependant manner (Fig. 5A.) To determine if non-genomic signaling or genomic signaling was responsible for mediating this response, Wortmannin, PD 98059, pertussis toxin, and ICI 182,780 were used in combination with resveratrol. Only ICI 182,780 inhibited the resveratrol-induced NRF-1 transcription. These data indicate that genomic ER is responsible for resveratrol-induced NRF-1 expression in HUVECs (Fig. 5B).
Fig. 5. Resveratrol increases NRF-1 expression through genomic ER activity in HUVECs and ablates inhibition of basal NRF-1 expression by DEPEsL75 and S80.
(A) HUVECs were treated with EtOH or increasing concentrations of resveratrol. (B) Where indicated, HUVECs were pretreated with 100 nM ICI 182,780 for 6 h; 50 nM Wortmannin (Wort), 50 mM PD98059 (PD), or 50 nM pertussis toxin (PTX); or 10 μg/ml DEPE L59, L75, S50, or S80 for 1 h followed by the addition of 10 nM resveratrol for 4 h. Total mRNA was isolated following treatment and QRT-PCR was performed as described in Material and Methods. Values are the average of 3 separate experiments ± SEM. * p < 0.05 versus EtOH; ** p < 0.05 versus resveratrol.
Resveratrol ablates the suppression of basal NRF-1 transcription by DEPEs
We tested the ability of resveratrol to ablate the inhibitory effect of DEPEs on basal NRF-1 transcription. DEPEs L75 and S80 inhibited basal NRF-1 and resveratrol ablated this inhibitory effect. Further, DEPEs L75 and S80 inhibited resveratrol-induced NRF-1 transcription. S50 had lower antagonist activity in combination with resveratrol compared to the other DEPEs tested. S50 also had lower E2-antagonist activity (Fig. 4B).
NRF-1 regulated Tfam expression and Tfam-regulated expression of mitochondrial genes is regulated by E2, 4-OHT, and RAL in HUVEC
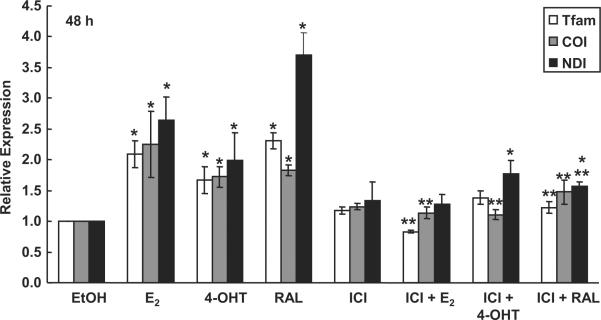
One nuclear encoded gene regulated by NRF-1 is the mitochondrial transcription factor Tfam which, in turn, regulates the transcription of the genes encoded in mtDNA (Scarpulla 2006). QRT-PCR was performed to determine the effect of E2, 4-OHT, or RAL in the presence or absence of ICI 182,780 on expression of Tfam in HUVECs. For these experiments, HUVECs were treated for 48 h of treatment because Tfam, as well as its mtDNA-encoded target genes cytochrome c oxidase subunit I (COI, MTCO1); and NADH dehydrogenase subunit I (NDI), were significantly increased in an E2-dependent manner in MCF-7 cells after 48 h of treatment (Mattingly et al. 2008). E2, 4-OHT, and RAL increased Tfam mRNA (Fig. 6). The observed E2- and RAL-mediated increase in the expression of each gene was inhibited by pretreatment with ICI 182,780. However, treatment with both 4-OHT and ICI 182,780 resulted in a decrease that was not significant compared to the control, but was also not significant when compared to the 4-OHT treatment alone. This result indicates that ER regulates the increase in Tfam and its mitochondrial targets COI and NDI in response to E2 and RAL. The lack of significant differences between 4-OHT and 4-OHT + ICI mRNA may be the result of the mixed agonist/antagonist activity of 4-OHT.
Fig. 6. Tfam and mtDNA-encoded target gene mRNA expression is increased in response to treatment with E2, 4-OHT, and RAL.
HUVECs were treated with 10 nM E2, 100 nM 4-OHT, or 100 nM RAL. Where indicated, cells were also pretreated for 6 h with 100 nM ICI 182,780 followed by addition of 10 nM E2, 100 nM 4-OHT, or 100 nM RAL for 48 h. Total mRNA was isolated following treatment and QRT-PCR was performed as described in Material and Methods. Values are the average of 3 separate experiments ± SEM. TFAM is nuclear-encoded and regulated by NRF-1. Tfam regulates mtDNA-encoded target genes cytochrome c oxidase subunit I (COI, MTCO1); and NADH dehydrogenase subunit I (NDI). *, p < 0.05 versus EtOH control; ** p < 0.05 versus the same treatment without ICI.
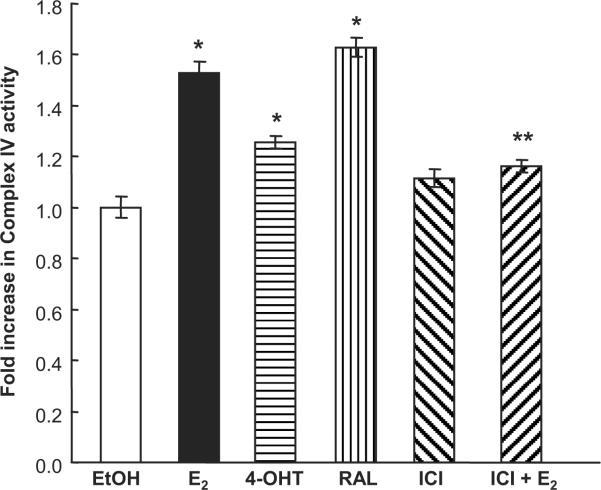
E2, 4-OHT, and RAL increase Complex IV activity in HUVECs
To determine if the observed increase in NRF-1 and Tfam expression in response to E2, 4-OHT, and RAL leads to increased mitochondrial activity, Complex IV activity was examined. E2, 4-OHT, and RAL increased Complex IV activity (Fig. 7). The E2-induced Complex IV activity was reduced by cotreatment with ICI 182,780, indicating in that the response is ER-mediated. These data agree with all previous data indicating that ER is required to induce NRF-1 and downstream gene expression in response to E2, 4-OHT, and RAL.
Fig. 7. E2, 4-OHT, and RAL stimulate Complex IV activity in HUVECs.
HUVECs were treated with 10 nM E2, 100 nM 4-OHT, 100 nM RAL, 100 nM ICI 182,780 or 10 nM E2 and 100 nM ICI 182,780 for 6 d. Complex IV activity was measured as described in Materials and Methods. The EtOH control was set to 1. The EtOH value was originally 1.13 ×10−4 OD/min. Values are the mean of quadruplicate determinations ± SEM. *, p < 0.05 versus EtOH control; ** p < 0.05 versus same treatment without ICI.
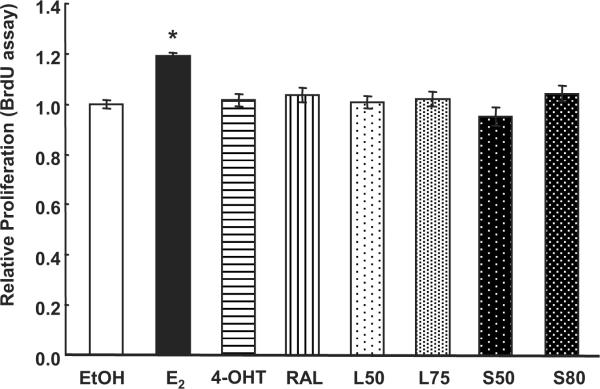
DEPEs do not affect HUVEC proliferation
BrdU assays were performed to determine the effect of DEPEs, E2, 4-OHT, and RAL on HUVEC proliferation. E2 increased BrdU incorporation (Fig. 8). In contrast, BrdU incorporation was unaffected by 4-OHT or RAL or by DEPEs L50, L75, S50, and S80. These data indicate that DEPEs do not inhibit DNA replication in HUVECs up to 48 h post-exposure.
Fig. 8. DEPE do not affect HUVEC proliferation.
HUVECs were treated with EtOH, 10 nM E2, 100 nM 4-OHT, 100 nM RAL, or DEPEs:10 μg/ml L50, L75, S50, or S80 for 48 h prior to performing a BrdU assay. Values are the average of 3 separate experiments ± SEM. * p < 0.05 versus EtOH
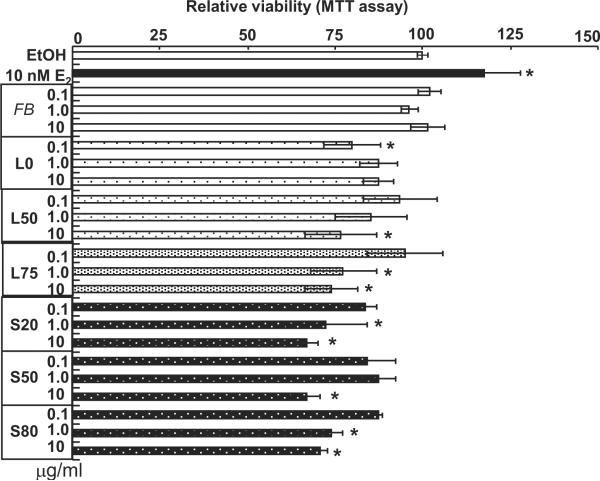
DEPEs reduce HUVEC viability
MTT assays were performed to determine the effects of DEPEs on HUVEC viability (Fig. 9). In contrast to the lack of proliferative effect of DEPEs revealed by BrdU incorporation (Fig. 8), DEPEs reduced cell viability, likely a reflection of reduced mitochondrial activity.
Fig. 9. DEPE reduce HUVEC viability.
HUVECs were treated with EtOH, 10 nM E2, or the indicated concentrations of filter blank (FB) or DEPEs for 48 h prior to performing an MTT assay. Values are the average of 3 separate experiments ± SEM. * p < 0.05 versus EtOH
Discussion
A recent study estimated that the economic value of the public health impact of traffic emissions, including 23,000 tons of PM < 2.5 μm, was $24 billion in 2007 (Levy et al. 2010). Thanks to significant reductions in vehicle emissions, the mortality risk associated with vehicle emissions is projected to decline from 2000 to 2020 (Levy et al. 2010). Elevated levels of ambient PM < 2.5 μm have been associated with an increase in emergency hospital visits for hypertension (Guo et al. 2010). The mechanisms by which ambient air pollution contributes to cardiovascular morbidity are poorly understood. The liberation of PAHs and other organic components of DEPs is thought to generate ROS and alter vascular and cardiac function in tested animal models of DEP exposure (Kodavanti et al. 2010; Simkhovich et al. 2008). Because in vivo DEP exposure in mice causes nuclear DNA damage and reduces mitochondrial potential in alveolar macrophages (Zhao et al. 2009), it is of interest to determine if DEPEs affect the expression of NRF-1, a nuclear transcription factor regulating mitochondrial activities (Scarpulla 2008). Here we demonstrated that DEPEs inhibit the basal transcription of NRF-1. Although the levels of DEPEs used in these experiments are likely higher than actual inhaled levels of DEPs, the in vivo concentrations of inhaled DEP reaching endothelial surfaces cannot be measured and an estimate would require detailed knowledge of inhalation capacity, rates and sites of DEP deposition, PM size distribution, measurements of clearance, and transmigration of the particles through the alveolar membranes to the capillary endothelial cells (Chao et al. 2011). The concentrations of DEPEs examined here is comparable (Chao et al. 2011) or less than ((Li et al. 2010b) used 25–50 μg/ml DEPE) those used in other in vitro studies demonstrating the impact of DEPEs on endothelial function.
DEPEs have antiestrogenic as well as antiandrogenic effects in cell culture models (Kizu et al. 2003a; Kizu et al. 2004; Okamura et al. 2004a; Okamura et al. 2002; Okamura et al. 2004b). Results from the present study allow us to speculate that one component of the observed increase in cardiovasculature disease and acute vascular effects seen in people exposed to diesel exhaust and PM may be a decrease in NRF-1 expression and a loss of the beneficial effects of E2 when the organic contents of these particles are liberated in circulation. The subtle decrease in mitochondrial activity that could result from this scenario, reflected in the reduced MTT assay activity seen here, may contribute to the observed increase in cardiovascular risk in urban areas (Anenberg et al. 2010). Examination of mitochondrial activities is a logical `next step' for this research.
Estrogens and SERMs have been proposed to have cardioprotective effects, however the mechanisms by which these effects are conveyed remain to be elucidated. In this study we demonstrated that E2, 4-OHT, and RAL act through classical genomic ERα and ERβ to increase NRF-1 transcription. ERα -selective agonist PPT and ERβ -selective agonist DPN also increased NRF-1 expression commensurate with their lower affinity for each ER subtype compared to E2 (Meyers et al. 2001; Sun et al. 2002). We propose that one of the vascular protective mechanisms of estrogens and SERMs may be an increase in NRF-1 leading to a subsequent increase in mitochondrial activity. The inhibition of E2, 4-OHT, and RAL induced NRF-1 by DEPEs indicates a potential dominant adverse effect of DEPEs. We reported that components of DEPEs activate the aryl hydrocarbon receptor (AHR) (Kizu et al. 2003a; Okamura et al. 2004b; Rouse et al. 2008). To our knowledge, there are no reports in the literature regarding the regulation of NRF-1 by AHR. NRF-1 transcription is not increased in MCF-7 cells in response to the AHR agonist β-napthoflavone (unpublished data, Klinge), but further studies in HUVECs are required to fully elucidate the mechanism by which DEPEs inhibit NRF-1 transcription.
We reported that resveratrol at a concentration of 50 nM rapidly activated MAPK, AKT, and eNOS and increased NO production in HUVECs (Klinge et al. 2008). Here we showed that resveratrol induced NRF-1 transcription in an ER-dependent manner and that 10 nM resveratrol significantly ablated the inhibition of NRF-1 expression by DEPEs. Resveratrol completely ablated the inhibitory activity of one of the DEPEs, i.e., S50. The precise reason for the lack of inhibitory activity of S50 with resveratrol is unknown but may relate to two previously described differences in chemical composition and bioactivity between the DEPEs prepared from the diesel truck run at different speeds (S) and engine loads (L) (Sumanasekera et al. 2007a). The L DEPEs did not inhibit E2-induced MAPK activity in HUVECs. While PD98059 did not ablate resveratrol-induced NRF-1 transcription, MAPK may affect proteins involved in NRF-1 transcription. Further, the S DEPEs contained lower concentration of pyrene, benz[a]anthracene, chrysene, and benzo[b]fluoranthene compared to the L DEPEs (Sumanasekera et al. 2007a). While it may be of interest to test individual components of the DEPEs on NRF-1 expression in HUVECs, people are environmentally exposed to complex mixtures of chemical compounds in air pollution and DEPs that include naphthalene, anthracene, pyrene, and benz(a)anthracene (Iwanari et al. 2002; Kawasaki et al. 2001; Landvik et al. 2007; Perera 1981; Yamazaki et al. 2000). Overall, one important result of the present study is that resveratrol ablated the ability of DEPEs to suppress basal NRF-1 transcription in HUVECs; thus, illustrating the potential of resveratrol, or other phytoalexins, to block the deleterious effects of DEP exposure in vascular endothelial cells.
Here we showed that DEPEs do not affect HUVEC proliferation as reflected in BrdU incorporation assays, but inhibited cell viability as seen in MTT assays. Because the MTT assay measures NADH-dependent dehydrogenase activity, the data in Fig. 9 indicate that DEPEs inhibit this activity. Further studies will be required to elucidate specific details of the mechanism involved. For example, one component of the DEPEs used in this study, i.e., 1-nitropyrene, was recently reported to rapidly (15 min) increase ROS production in HUVECs and increase protein, but not mRNA expression of the endoplasmic reticulum stress chaperone GRP78 (Andersson et al. 2009). Over the last decade, endoplasmic reticulum stress and the unfolded protein response (UPR) has emerged as a key mechanism in the pathogenesis of a number of factors that promote atherosclerosis in the vasculature (Tabas 2010). Another recent study demonstrated that individual PAHs had different effects on HUVEC cell viability (Li et al. 2010a). That study showed that chrysene inhibited HUVEC viability, but only at concentrations > 50 mM whereas pyrene, naphthalene, fluoranthene, anthracene, and benzo(a)pyrene had no effect on cell viability when assessed at 24 h at up to 0.5 mM concentrations (Li et al. 2010a). The DEPEs used in the present study include anthracene, pyrene, chrysene, and benzo(a)pyrene (Sumanasekera et al. 2007a). Clearly, further experiments will be required to elucidate the exact mechanisms by which exposure to complex chemical mixtures in air pollution including DEPs adversely affect human health including increasing the risk of cardiovascular disease.
Acknowledgements
This project was supported by NIH R01 DK53220 to C.M.K. and American Heart Association pre-doctoral fellowship 315087B to K.A.M. We thank Dr. Ryoichi Kizu for the DEPEs used in this study. We thank Dr. Barbara J. Clark for her suggestions for this manuscript.
References
- Andersson H, Piras E, Demma J, Hellman B, Brittebo E. Low levels of the air pollutant 1-nitropyrene induce DNA damage, increased levels of reactive oxygen species and endoplasmic reticulum stress in human endothelial cells. Toxicology. 2009;262:57–64. doi: 10.1016/j.tox.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Anenberg SC, Horowitz LW, Tong DQ, West JJ. An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ Health Perspect. 2010;118:1189–1195. doi: 10.1289/ehp.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal JF, Fontaine C, Billon-Gales A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air Pollution and Cardiovascular Disease: A Statement for Healthcare Professionals From the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Chao M-W, Kozlosky J, Po IP, Strickland PO, Svoboda KKH, Cooper K, Laumbach RJ, Gordon MK. Diesel exhaust particle exposure causes redistribution of endothelial tube VE-cadherin. Toxicology. 2011;279:73–84. doi: 10.1016/j.tox.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- Duckles SP, Krause DN. Mechanisms of Cerebrovascular Protection: Estrogen, Inflammation and Mitochondria. Acta Physiol (Oxf) 2010 doi: 10.1111/j.1748-1716.2010.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Tong S, Zhang Y, Barnett AG, Jia Y, Pan X. The relationship between particulate air pollution and emergency hospital visits for hypertension in Beijing, China. Science of The Total Environment. 2010;408:4446–4450. doi: 10.1016/j.scitotenv.2010.06.042. [DOI] [PubMed] [Google Scholar]
- Huo L, Scarpulla RC. Mitochondrial DNA Instability and Peri-Implantation Lethality Associated with Targeted Disruption of Nuclear Respiratory Factor 1 in Mice. Mol. Cell. Biol. 2001;21:644–654. doi: 10.1128/MCB.21.2.644-654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova MM, Luken KH, Zimmer AS, Lenzo FL, Smith RJ, Arteel MW, Kollenberg TJ, Mattingly KA, Klinge CM. Tamoxifen increases nuclear respiratory factor 1 transcription by activating estrogen receptor β and AP-1 recruitment to adjacent promoter binding sites. The FASEB Journal. 2011;25:1402–1416. doi: 10.1096/fj.10-169029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanari M, Nakajima M, Kizu R, Hayakawa K, Yokoi T. Induction of CYP1A1, CYP1A2, and CYP1B1 mRNAs by nitropolycyclic aromatic hydrocarbons in various human tissue-derived cells: chemical-, cytochrome P450 isoform-, and cell-specific differences. Arch Toxicol. 2002;76:287–298. doi: 10.1007/s00204-002-0340-z. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Takizawa H, Takami K, Desaki M, Okazaki H, Kasama T, Kobayashi K, Yamamoto K, Nakahara K, Tanaka M, Sagai M, Ohtoshi T. Benzene-extracted components are important for the major activity of diesel exhaust particles: effect on interleukin-8 gene expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;24:419–426. doi: 10.1165/ajrcmb.24.4.4085. [DOI] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- Kizu R, Okamura K, Toriba A, Mizokami A, Burnstein KL, Klinge CM, Hayakawa K. Antiandrogenic activities of diesel exhaust particle extracts in PC3/AR human prostate carcinoma cells. Toxicol Sci. 2003a;76:299–309. doi: 10.1093/toxsci/kfg230. [DOI] [PubMed] [Google Scholar]
- Kizu R, Okamura K, Toriba A, Mizokami A, Burnstein KL, Klinge CM, Hayakawa K, Thomas PB, Risinger KE. Antiandrogenic Activities of Diesel Exhaust Particle Extracts in PC3/AR Human Prostate Carcinoma Cells. Toxicol Sci. 2003b;76:299–309. doi: 10.1093/toxsci/kfg230. [DOI] [PubMed] [Google Scholar]
- Kizu R, Otsuki N, Kishida Y, Toriba A, Mizokami A, Burnstein KL, Klinge CM, Hayakawa K. A new luciferase reporter gene assay for the detection of androgenic and antiandrogenic effects based on a human prostate specific antigen promoter and PC3/AR human prostate cancer cells. Anal Sci. 2004;20:55–59. doi: 10.2116/analsci.20.55. [DOI] [PubMed] [Google Scholar]
- Klein GP, Hodge EM, Diamond ML, Yip A, Dann T, Stern G, Denison MS, Harper PA. Gas-phase ambient air contaminants exhibit significant dioxin-like and estrogen-like activity in vitro. Environ Health Perspect. 2006;114:697–703. doi: 10.1289/ehp.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280:7460–7468. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor {alpha}-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Thomas R, Ledbetter AD, Schladweiler MC, Shannahan JH, Wallenborn JG, Lund AK, Campen MJ, Butler EO, Gottipolu RR, Nyska A, Richards JE, Andrews D, Jaskot RH, McKee J, Kotha SR, Patel RB, Parianandi NL. Vascular and Cardiac Impairments in Rats Inhaling Ozone and Diesel Exhaust Particles. Environ Health Perspect. 2010 doi: 10.1289/ehp.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landvik NE, Gorria M, Arlt VM, Asare N, Solhaug A, Lagadic-Gossmann D, Holme JA. Effects of nitrated-polycyclic aromatic hydrocarbons and diesel exhaust particle extracts on cell signalling related to apoptosis: Possible implications for their mutagenic and carcinogenic effects. Toxicology. 2007;231:159–174. doi: 10.1016/j.tox.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Levy JI, Buonocore JJ, von Stackelberg K. Evaluation of the public health impacts of traffic congestion: a health risk assessment. Environ Health. 2010;9:65. doi: 10.1186/1476-069X-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-H, Cheng Y-W, Hsu Y-T, Hsu Y-J, Liao P-L, Kang J-J. Benzo[a]pyrene inhibits angiogenic factors-induced αvβ3 integrin expression, neovasculogenesis and angiogenesis in human umbilical vein endothelial cells. Toxicol Sci. 2010a doi: 10.1093/toxsci/kfq279. [DOI] [PubMed] [Google Scholar]
- Li R, Ning Z, Cui J, Yu F, Sioutas C, Hsiai T. Diesel exhaust particles modulate vascular endothelial cell permeability: Implication of ZO-1 expression. Toxicology Letters. 2010b;197:163–168. doi: 10.1016/j.toxlet.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, Klinge CM. Estradiol stimulates transcription of Nuclear Respiratory Factor-1 and increases mitochondrial biogenesis. Mol Endocrinol. 2008;22:609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Miller VM, Duckles SP. Vascular Actions of Estrogens: Functional Implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monforton C. Weight of the Evidence or Wait for the Evidence? Protecting Underground Miners From Diesel Particulate Matter. Am J Public Health. 2006;96:271–276. doi: 10.2105/AJPH.2005.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiel M, de la Blanca EP, Jimenez E. P2Y receptors activate MAPK/ERK through a pathway involving PI3K/PDK1/PKC-zeta in human vein endothelial cells. Cell Physiol Biochem. 2006;18:123–134. doi: 10.1159/000095180. [DOI] [PubMed] [Google Scholar]
- Okamura K, Kizu R, Hayakawa K, Toriba A, Mizokami A, Burnstein KL, Klinge CM, Kato S. Variation in the antiandrogenic activity of diesel exhaust particulates emitted under different engine loads. Polycycl Aromat Comp. 2004a;24:743–757. [Google Scholar]
- Okamura K, Kizu R, Toriba A, Klinge CM, Hayakawa K. Antiestrogenic activity of extracts of diesel exhaust particulate matter in MCF-7 human breast carcinoma cells. Polycycl Aromat Comp. 2002;22:747–759. [Google Scholar]
- Okamura K, Kizu R, Toriba A, Murahashi T, Mizokami A, Burnstein KL, Klinge CM, Hayakawa K. Antiandrogenic activity of extracts of diesel exhaust particles emitted from diesel-engine truck under different engine loads and speeds. Toxicology. 2004b;195:243–254. doi: 10.1016/j.tox.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Okamura KKR, Toriba A, Klinge CM, Hayakawa K. Antiestrogenic activity of extracts of diesel exhaust particulate matter in MCF-7 human breast carcinoma cells. Polycyclic Aromatic Compounds. 2002;22:747–759. [Google Scholar]
- Perera F. Carcinogenicity of airborne fine particulate benzo(a)pyrene: an appraisal of the evidence and the need for control. Environ Health Perspect. 1981;42:163–185. doi: 10.1289/ehp.8142163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, Procaccio V. Mitochondrial Effects of Estrogen Are Mediated by Estrogen Receptor {alpha} in Brain Endothelial Cells. J Pharmacol Exp Ther. 2008;325:782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha M, Apostolova N, Hernandez-Mijares A, Herance R, Victor VM. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr Med Chem. 2010;17:3827–3841. doi: 10.2174/092986710793205444. [DOI] [PubMed] [Google Scholar]
- Rouse RL, Murphy G, Boudreaux MJ, Paulsen DB, Penn AL. Soot Nanoparticles Promote Biotransformation, Oxidative Stress, and Inflammation in Murine Lungs. Am. J. Respir. Cell Mol. Biol. 2008;39:198–207. doi: 10.1165/rcmb.2008-0057OC. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta. 2002;1576:1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. Journal of Cellular Biochemistry. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear Control of Respiratory Chain Expression by Nuclear Respiratory Factors and PGC-1-Related Coactivator. Annals of the New York Academy of Sciences. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkhovich BZ, Kleinman MT, Kloner RA. Air Pollution and Cardiovascular Injury: Epidemiology, Toxicology, and Mechanisms. Journal of the American College of Cardiology. 2008;52:719–726. doi: 10.1016/j.jacc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen Increases Mitochondrial Efficiency and Reduces Oxidative Stress in Cerebral Blood Vessels. Mol Pharmacol. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Ivanova MM, Johnston BJ, Dougherty SM, Sumanasekera GU, Myers SR, Ali Y, Kizu R, Klinge CM. Rapid Effects of Diesel Exhaust Particulate Extracts on Intracellular Signaling in Human Endothelial Cells. Toxicology Letters. 2007a;174:61–73. doi: 10.1016/j.toxlet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Sumanasekera GU, Mattingly KA, Dougherty SM, Keynton RS, Klinge CM. Estradiol and dihydrotestosterone regulate endothelial cell barrier function after hypergravity-induced alterations in MAPK activity. Am J Physiol-Cell Ph. 2007b;293:C566–C573. doi: 10.1152/ajpcell.00418.2006. [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Zhao L, Ivanova M, Morgan DD, Noisin EL, Keynton RS, Klinge CM. Effect of estradiol and dihydrotestosterone on hypergravity-induced MAPK signaling and occludin expression in human umbilical vein endothelial cells. Cell Tissue Res. 2006;324:243–253. doi: 10.1007/s00441-005-0113-0. [DOI] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor alpha. Endocrinology. 2002;143:941–947. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- Tabas I. The Role of Endoplasmic Reticulum Stress in the Progression of Atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayaratne AL, McDonnell DP. The human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J. Biol. Chem. 2001;276:35684–35692. doi: 10.1074/jbc.M101097200. [DOI] [PubMed] [Google Scholar]
- Wijayaratne AL, Nagel SC, Paige LA, Christensen DJ, Norris JD, Fowlkes DM, McDonnell DP. Comparative analyses of mechanistic differences among antiestrogens. Endocrinology. 1999;140:5828–5840. doi: 10.1210/endo.140.12.7164. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Hatanaka N, Kizu R, Hayakawa K, Shimada N, Guengerich FP, Nakajima M, Yokoi T. Bioactivation of diesel exhaust particle extracts and their major nitrated polycyclic aromatic hydrocarbon components, 1-nitropyrene and dinitropyrenes, by human cytochromes P450 1A1, 1A2, and 1B1. Mutat Res. 2000;472:129–138. doi: 10.1016/s1383-5718(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ma JK, Barger MW, Mercer RR, Millecchia L, Schwegler-Berry D, Castranova V, Ma JY. Reactive oxygen species- and nitric oxide-mediated lung inflammation and mitochondrial dysfunction in wild-type and iNOS-deficient mice exposed to diesel exhaust particles. J Toxicol Environ Health A. 2009;72:560–570. doi: 10.1080/15287390802706330. [DOI] [PubMed] [Google Scholar]