Abstract
The Brown-Vialetto-Van Laere syndrome (BVVLS) is a rare neurological disease characterized by ponto-bulbar palsy, bilateral sensorineural deafness, and respiratory insufficiency. Recent genetic studies have identified mutations in the C20orf54 gene, which encodes the human riboflavin (RF) transporter -2 (hRFT-2) and suggested their link to the manifestation of BVVLS. However, there is nothing currently known about the effect of these mutations on functionality of hRFT-2, a protein that is expressed in a variety of tissues with high expression in the intestine. We addressed this issue using the human-derived intestinal epithelial Caco-2 cells. Our results showed significant (P < 0.01) impairment in RF uptake by Caco-2 cells transiently expressing W17R, P28T, E36K, E71K, and R132W (but not L350M) hRFT-2 mutants. This impairment in RF transport was not due to a decrease in transcription and/or translation of hRFT-2, since mRNA and protein levels of the carrier were similar in cells expressing the mutants and wild-type hRFT-2. Confocal images of live Caco-2 cells transiently transfected with hRFT-2 mutants (fused with green fluorescent protein) showed the P28T, E36K, E71K, and R132W mutants were retained within the endoplasmic reticulum, while the W17R and L350M mutants were expressed at the cell membrane; cell surface expression of the W17R mutant was further confirmed by direct determination of cell surface transporter density. These results show for the first time that some of the BVVLS associated mutations in hRFT-2 affect the transporter functionality and that this effect is mediated via alterations in membrane targeting and/or activity of the transporter.
Keywords: Riboflavin, intestine, hRFT-2, BVVLS, transporter, uptake
INTRODUCTION
The Brown-Vialetto-Van Laere syndrome (BVVLS) is a rare neurological disorder characterized by progressive ponto-bulbar palsy, bilateral sensorineural deafness, and respiratory insufficiency [reviewed in 1]. The age at onset ranges from infancy to the third decade of life. Severity and clinical course of BVVLS is variable with poor prognosis in the majority of cases. Recent studies have identified mutations in the C20orf54 gene in patients with BVVLS [2–5]. The C20orf54 gene encodes hRFT-2, a membrane protein of 469-amino acids with a predicted 11 transmembrane domains [6]. The hRFT-2 is expressed in a variety of tissues with high expression in the small intestine [7–9]. In the latter tissue, the hRFT-2 protein is exclusively expressed at the apical membrane domain of the polarized absorptive epithelial cells [6, 7, 9].
The water-soluble vitamin RF is essential for normal cellular functions, growth and development via its involvement (in the form of its biologically active compounds: flavin adenine dinucleotide, FAD, and flavin mononucleotide, FMN) in a variety of redox reactions that are critical for normal metabolism [10]. Thus, it is not surprising that deficiency of this essential micro-nutrient leads to a variety of clinical abnormalities that include degenerative changes in the nervous system, peripheral neuropathy, and growth retardation [10–12]. Deficiency of RF occurs in a variety of conditions [4, 5, 10–12], including patients with BVVLS and Fazio Londe syndromes (the same disease but without deafness; 13, 14). Patients with the latter conditions respond favorably to oral supplementation of RF leading to significant improvements in clinical symptoms and biochemical abnormalities [4, 5].
Humans cannot synthesize RF; rather, they obtain the vitamin from exogenous sources via intestinal absorption. The intestine is exposed to two sources of riboflavin: a dietary source and a bacterial source where the vitamin is synthesized by the normal microflora of the large intestine. Previous studies from our laboratory and others using a variety of human and animal intestinal preparations have shown that the process of RF absorption is specific and carriermediated in nature [reviewed in Ref. 15]. More recent studies have established a key role for hRFT-2 in intestinal absorption of RF [7, 9].
The mutations in C20orf54 gene (hRFT-2) identified in BVVLS patients comprise a wide spectrum of missense, nonsense, and frameshift mutations [2–5]. To date, nothing is known about the effect of these mutations on the functionality of hRFT-2, and thus, we addressed this issue in the current report. Investigating this issue is important for better understanding of molecular mechanism of the BVVLS disorder and in order to gain insight into the structure-function/biology of the hRFT-2 protein. Our results showed that some of the missense mutations in hRFT-2 identified in patients with BVVLS affect the functionality of the transporter via affecting its membrane targeting and/or activity.
MATERIALS AND METHODS
Materials
Human-derived intestinal epithelial Caco-2 and Madin-Darby canine kidney (MDCK) cells were purchased from American Type Culture Collection (Manassas, VA). [3H]-RF (specific activity: 21.2 Ci/mmol, radiochemical purity: >99%) was purchased from Moravek Biochemicals (Brea, CA). GFP-C3 and DsRed-ER fluorescent protein constructs were from Clontech (Palo Alto, CA). The full-length GFP-hRFT-2 cloned into GFP-C3 vector was from our laboratory and generated as described by us previously [6]. DNA oligonucleotide mutant primers were from Sigma Genosys. Anti-hRFT-2 polyclonal antibody was raised in rabbits against peptide (206-RPREENDLGPAGTVD-280) (Thermo Fisher Scientific, Huntsville, AL). Anti-β-actin monoclonal antibody was from (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Anti-rabbit IRDye-800 and anti-mouse IRDye-680 antibodies were from (LI-COR Bioscience, Lincoln, NE). All other chemicals and reagents used in this study were of analytical/molecular biology grade and were purchased from commercial sources.
Generation of GFP-hRFT-2 mutants
The Quick Change site-directed mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce point mutations into the open reading frame of hRFT-2. Overlapping primers containing the mutated nucleotides to the specified mutation sites (Table 1) and full-length GFP-hRFT-2 fused plasmid were used in PCR-based site-directed mutagenesis as previously described [6]. The nucleotide sequences of all constructs were confirmed by sequencing (Laragen, Los Angeles, CA).
Table 1.
Primers used for generating point mutations in hRFT-2.
| Mutation | Forward and Reverse Primers (5’-3’) |
|---|---|
| W17R | TTCGGAATGGGCTCCCGGGTGACCATCAATGGG |
| CCCATTGATGGTCACCCGGGAGCCCATTCCGAA | |
| P28T | GGGCTCTGGGTAGAGCTGACCCTGCTGGTGATGGAGCTGCCC |
| GGGCAGCTCCATCACCAGCAGGGTCAGCTCTACCCAGAGCCC | |
| E36K | GTGATGGAGCTGCCCAAGGGCTGGTACCTGCCCTCCTACC |
| GGTAGGAGGGCAGGTACCAGCCCTTGGGCAGCTCCATCACC | |
| E71K | CACTTCCGGCCCAGCTGCCTTTCCAAAGTGCCCATCATCTTCACCC |
| GGGTGAAGATGATGGGCACTTTGGAAAGGCAGCTGGGCCGGAAGTG | |
| R132W | CTGCCGTTCATGAGCTGGCTGCCCACCTACTACCTCACCACC |
| GGTGGTGAGGTAGTAGGTGGGCAGCCAGCTCATGAACGGCAG | |
| L350M | GTGGCCAACCCTCTTGCCTCGATGGTCTCCATGTTCCTGCC |
| GGCAGGAACATGGAGACCATCGAGGCAAGAGGGTTGGCCAC |
The mutated DNA codon is shown as underlined text.
Cell culture and transient transfection
Caco-2 and MDCK cells were maintained in MEM (ATCC) supplemented with 10% FBS, penicillin (100,000 U/l), and streptomycin (10 mg/l). For uptake studies, Caco-2 cells were grown on the 12-well tissue culture plates (Corning, NY). For imaging studies, Caco-2 and MDCK cells were grown on the sterile glass-bottomed Petri dishes (MatTek, Ashland, MA). At 80–90% confluence cells were transfected with 2–4 μg of plasmid DNA with the Lipofectamine 2000 (Invitrogen, Carlsbad, CA). 48 hours after transfection cells were used for uptake assays (followed by RNA and protein measurements) or imaged.
Uptake assay
[3H]-RF uptake measurements were performed for cells incubated in Krebs-Ringer buffer [containing (in mM) 133 NaCl, 4.93 KCl, 1.23 MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine, 10 HEPES, and 10 MES; pH 7.4] at 37°C for 2 min (the initial linear period; ref. 16) following established procedures [6, 9, 16]. [3H]-RF (14 nM) was added to the incubation medium at the onset of uptake experiment, and after incubation period the reaction was terminated by ice-cold buffer. Cells were then measured for radioactive content using a Beckman Coulter LS6500 multipurpose scintillation counter (Fullerton, CA). Protein was measured in parallel using a Bio-Rad DC Protein Assay kit (Bio-Rad).
Real-time PCR analysis
Total RNA isolated from Caco-2 cells 48 hours after transfection was treated with DNase I and subjected to reverse transcription using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The mRNA expression level was quantified in a CFX96 real-time PCR system (Bio-Rad), using iQ SYBR Green Supermix (Bio-Rad) and primers specific for hRFT-2 (forward: 5′-CCTTTCCGAAGTGCCCATC-3′ and reverse: 5′-_AGAAGGTGGTGAGGTAGTAGG-3′) and β-actin (forward: 5′-CATCCTGCGTCTGGACCT-3′ and reverse: 5′-TAATGTCACGCACGATTTCC-3′). Real-time PCR conditions were followed as previously described [17], and data normalized to β-actin were calculated using a relative relationship method supplied by the iCycler manufacture (Bio-Rad) [18].
Western blot analysis
Proteins (whole cell lysates or biotinylated proteins) were separated in NuPAGE 4–12% Bis-Tris gradient minigels (Invitrogen), transferred onto immobilon polyvinylidene difluoride membrane (Fisher Scientific), and analyzed by Western blotting as described previously [19]. The primary antibodies were anti-hRFT-2 polyclonal antibody (1:200 dilution) and anti-β-actin monoclonal antibody (1:3000 dilution). The secondary antibodies were anti-rabbit IRDye-800 and anti-mouse IRDye-680 (both at 1:30,000 dilution). Immunoreactive bands were detected using the Odyssey infrared imaging system (LI-COR Bioscience, Lincoln, NE) and their intensity was determined using LI-COR software for quantification.
Cell surface protein biotinylation
Biotinylation of cell surface proteins was performed using a cell surface protein isolation kit (Pierce Biotechnology, Rockford, IL) as described previously [20]. Briefly, 48 hours after transfection Caco-2 cells were labeled with EZ-Link Sulfo-NHS-SS-Biotin, lysed, and an equal amount of cell lysates (0.5 mg of total soluble protein) was used for the isolation of the biotinylated proteins with NeutrAvidin agarose. The bound proteins were released with sample loading buffer for SDS-PAGE and analyzed by Western blotting using anti-hRFT-2 antibody. Data were normalized to the total amount of cellular hRFT-2 protein.
Live cell confocal imaging
Confluent Caco-2 or MDCK cell monolayers were imaged using an inverted Nikon C-1 confocal microscopy 48 hours posttransfection of wild-type and mutant constructs. The green fluorescent protein (GFP) was excited with the 488nm line from an argon ion laser and emitted fluorescence was monitored at 515±30nm short pass filter and the red fluorescent protein (DsRed) was excited with the 543nm line from a HeNe ion laser and emitted fluorescence was monitored at 570±50nm long pass filter. Using Nikon C-1 software (Nikon Instruments Inc, NY) images were captured.
Data presentation and statistical analysis
Uptake data are the means ± SE of 3–5 independent experiments expressed in terms of femtomoles per milligram of protein per 2 min. Uptake data were analyzed by performing the Student's t-test with statistical significance set at P < 0.01. All Western blot analyses, biotinylation assays, real-time PCR assays, and imaging studies were performed on three or more separate occasions with similar results.
RESULTS
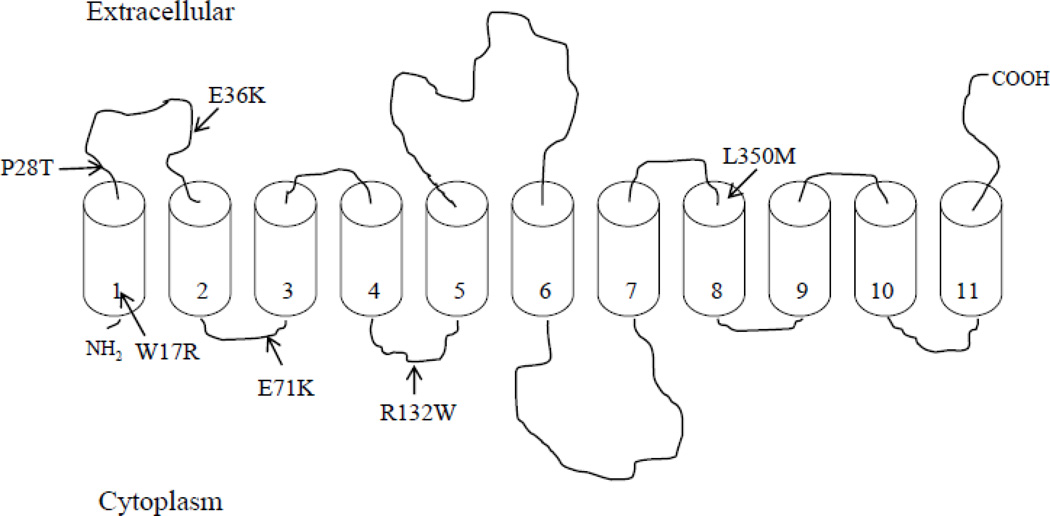
To date, mutations in the C20orf54 gene (which encodes hRFT-2) identified in patients with BVVL syndrome comprise a wide spectrum of missense, nonsense, and frame-shift mutations [2–5]. Our focus in this report was to study the effect of the missense clinical mutations in C20orf54 gene on functionality of hRFT-2. Figure 1 represents a schematic representation of the predicted membrane topology of hRFT-2 [6] and shows the sites of the missense mutations that were examined in this study.
Figure 1. Hypothetical membrane topology of hRFT-2 and location of BVVLS missense mutations.
hRFT-2 is predicted to have 11 potential transmembrane domains and an extracellular oriented COOH-terminus [6]. Arrows indicate the location of BVVLS missense mutations that were examined in this study.
Effect of missense mutations in hRFT-2 found in BVVLS on RF uptake
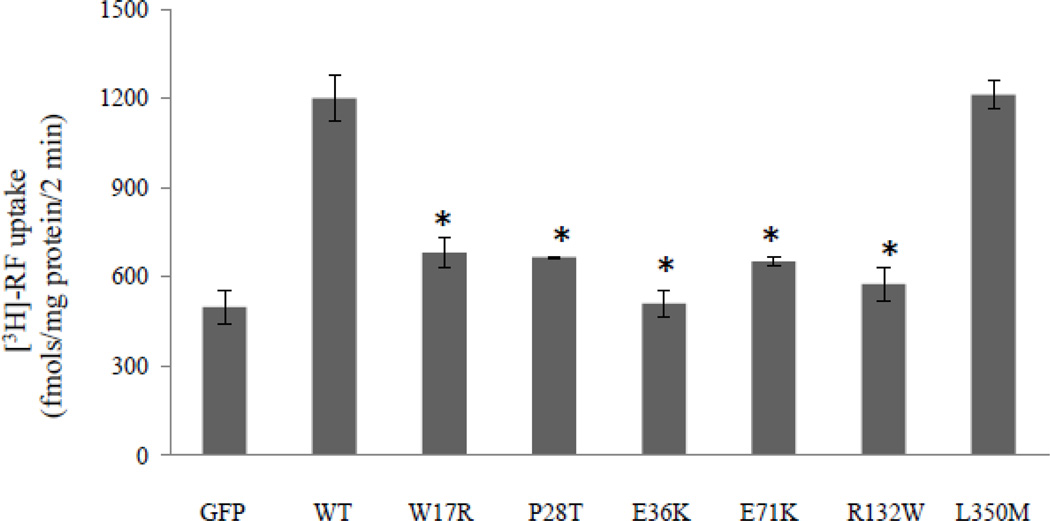
We introduced point mutations into the hRFT-2 and examined the effect of the individual mutation on functionality of the mutated hRFT-2, i. e., on RF (14 nM) uptake, in transiently transfected human intestinal epithelial Caco-2 cells. The results showed a significant (P < 0.01) inhibition in riboflavin uptake in Caco-2 cells expressing W17R, P28T, E36K, E71K, and R132W mutants when compared with those expressing the wild-type hRFT-2 (Figure 2). The L350M mutation (which is one of the two mutations found in some patients with BVVLS) on the other hand, showed no effect on riboflavin uptake by Caco-2 cells (Figure 2). The L350M mutation is thought to have no pathogenic consequences since it is tolerated by the ‘Sorting Intolerant from Tolerant’ (SIFT) algorithm (program that predicts whether an amino acid substitution affects protein function; SIFT is available at http://sift.jcvi.org) and involves an amino acid that is not conserved in mammalian RFT-2 (2).
Figure 2. Effect of BVVLS missense mutations on RF uptake.
RF uptake by human intestinal epithelial Caco-2 cells transiently transfected with wild-type (WT) and mutated hRFT-2. Uptake of 3H-RF (14 nM) was measured 48 hours post transfection in Krebs-Ringer buffer (pH 7.4) as described in MATERIALS AND METHODS and expressed in fmol·mg protein−1·2 min−1. Data are means ± SE of 3–5 independent experiments. GFP - cells transiently transfected with the empty GFP-C3 vector. *P < 0.01.
Effect of missense mutations in hRFT-2 found in BVVLS on level of expression of the transporter
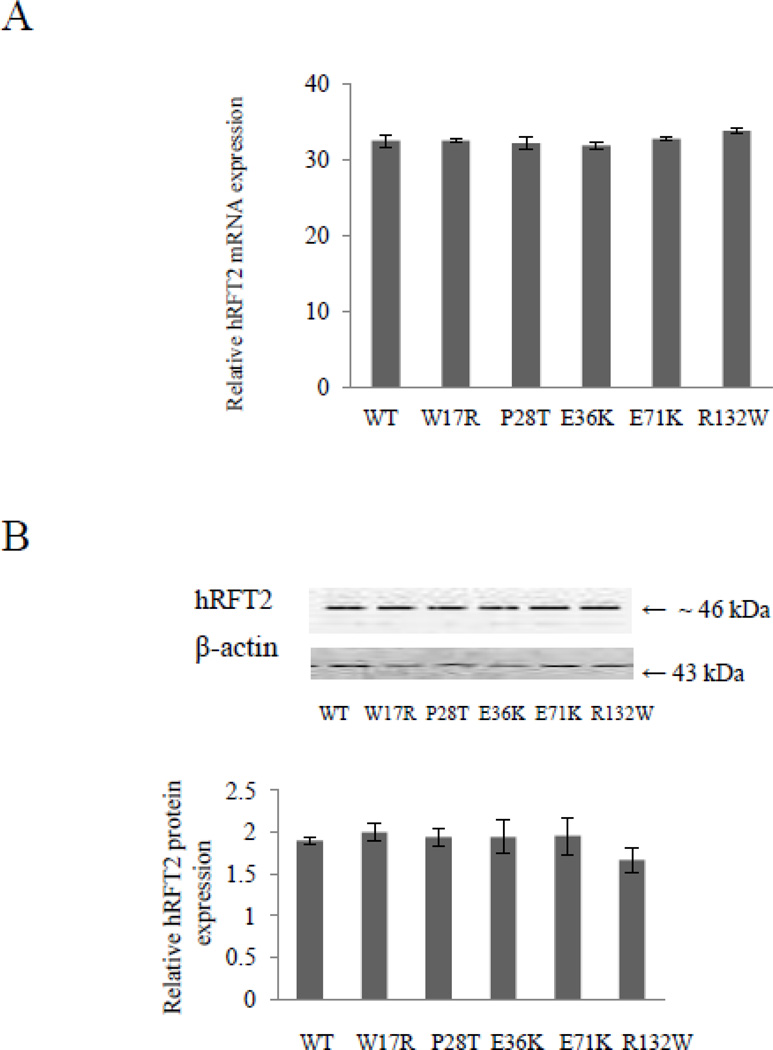
To determine whether the impairment of RF uptake observed with the W17R, P28T, E36K, E71K, and R132W hRFT-2 mutants is due to a decrease in transcription and/or translation of these mutants, we examined expression of these mutants at the mRNA and protein levels as compared to level of expression of the wild-type hRFT-2. Steady-state mRNA level of hRFT-2 was measured by mean of real-time quantitative PCR in Caco-2 cells transfected with the mutated and wild-type hRFT-2. The results (Figure 3A) showed similar mean levels of mRNA expression in cells expressing the W17R, P28T, E36K, E71K, and R132W hRFT-2 mutants and those expressing the wild-type protein. Protein expression levels of the mutated and wild-type hRFT-2 protein were determined by mean of Western blot analysis using specific polyclonal anti-hRFT-2 antibodies. The results showed levels of expression of the wild-type and mutated hRFT-2 proteins were similar (Figure 3B). These data suggest that the W17R, P28T, E36K, E71K, and R132W mutations in hRFT-2 do not affect the steady state mRNA levels or protein levels of hRFT-2.
Figure 3. Effect of BVVLS missense mutations on level of expression of hRFT-2.
Caco-2 cells were transiently transfected with wild-type (WT) and mutated hRFT-2 followed by determination of hRFT-2 level of expression 48 hours posttransfection. A: Total RNA was isolated and quantitative real-time PCR was performed as described in MATERIALS AND METHODS. Data (means ± SE) are from at least three independent experiments, normalized relative to β-actin, and given in folds over the hRFT-2 RNA level in cells transiently transfected with the empty GFP-C3 vector, which was set at 1. Note that some statistical SEs of the mean bars are not visible on the scale of the graph presented. B: Western blot analysis was performed using equal amounts (20 μg) of total protein from the cell lysates as described in MATERIALS AND METHODS. Blot was probed with the anti-hRFT-2 polyclonal antibodies. Data (means ± SE) from three independent sets of experiment are expressed in intensity units normalized to the amount of β-actin. Inset is the image of a representative gel.
Confocal imaging of the hRFT-2 missense mutants in living cells
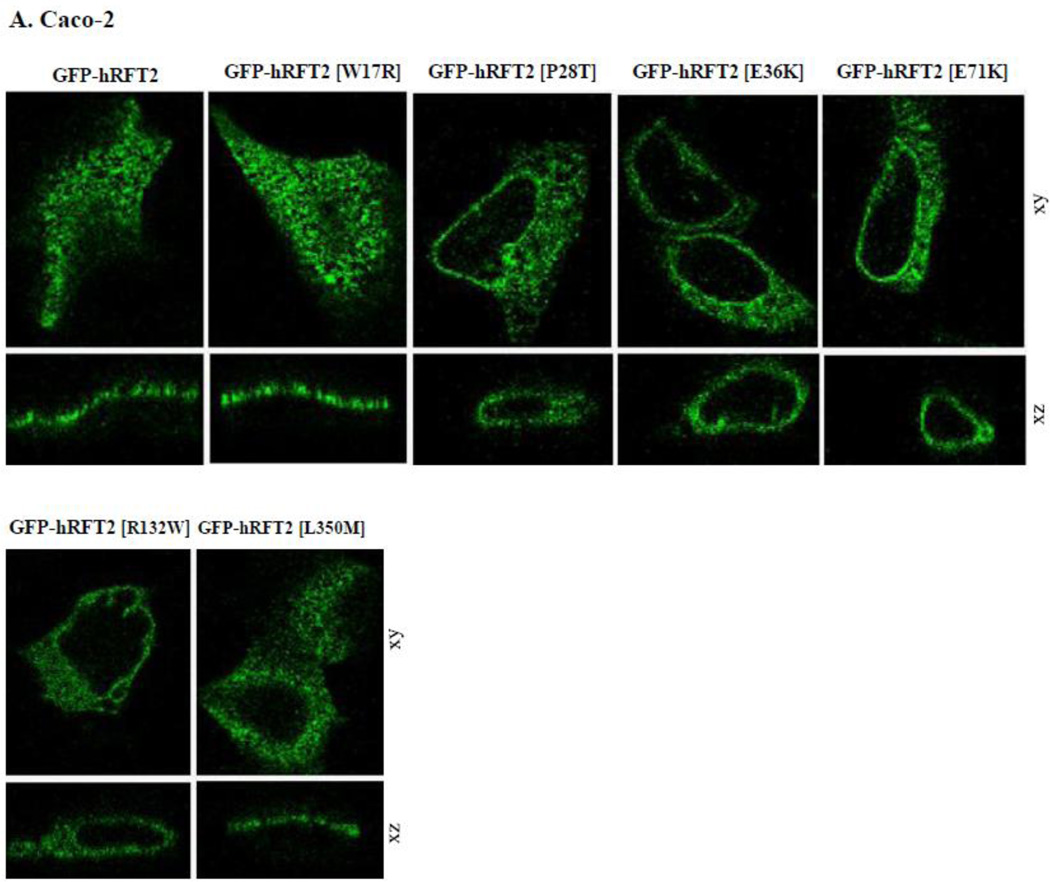
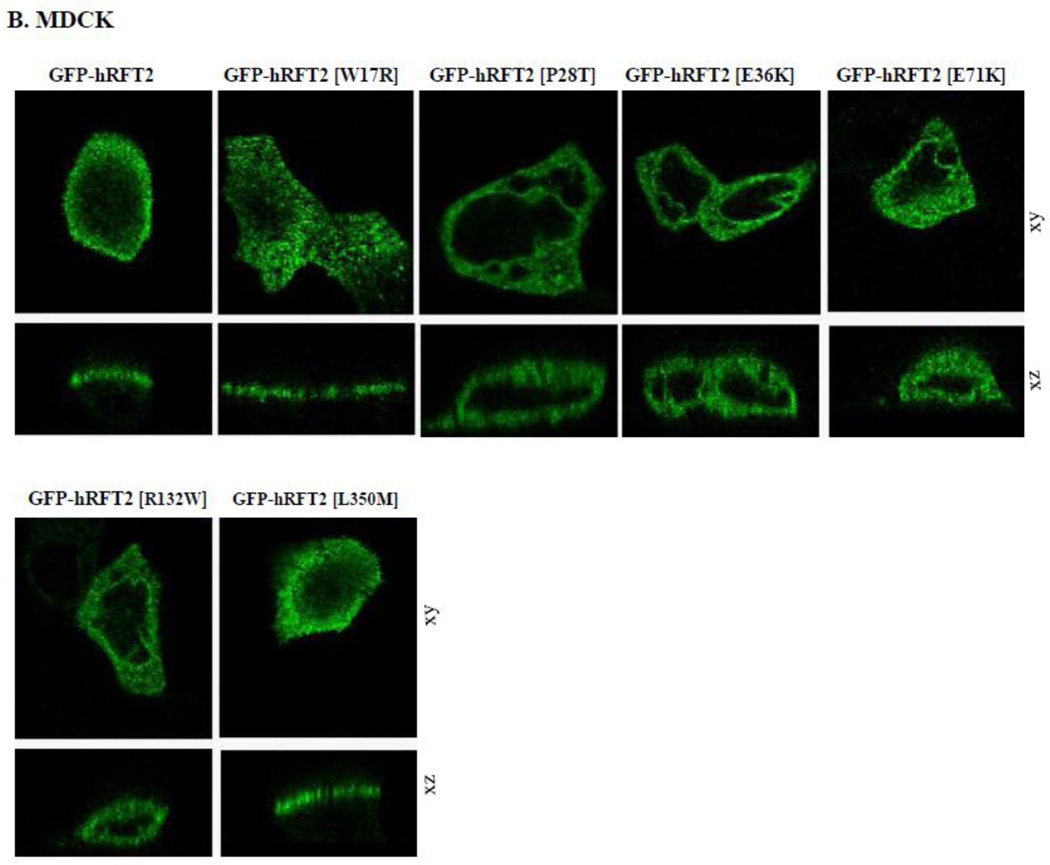
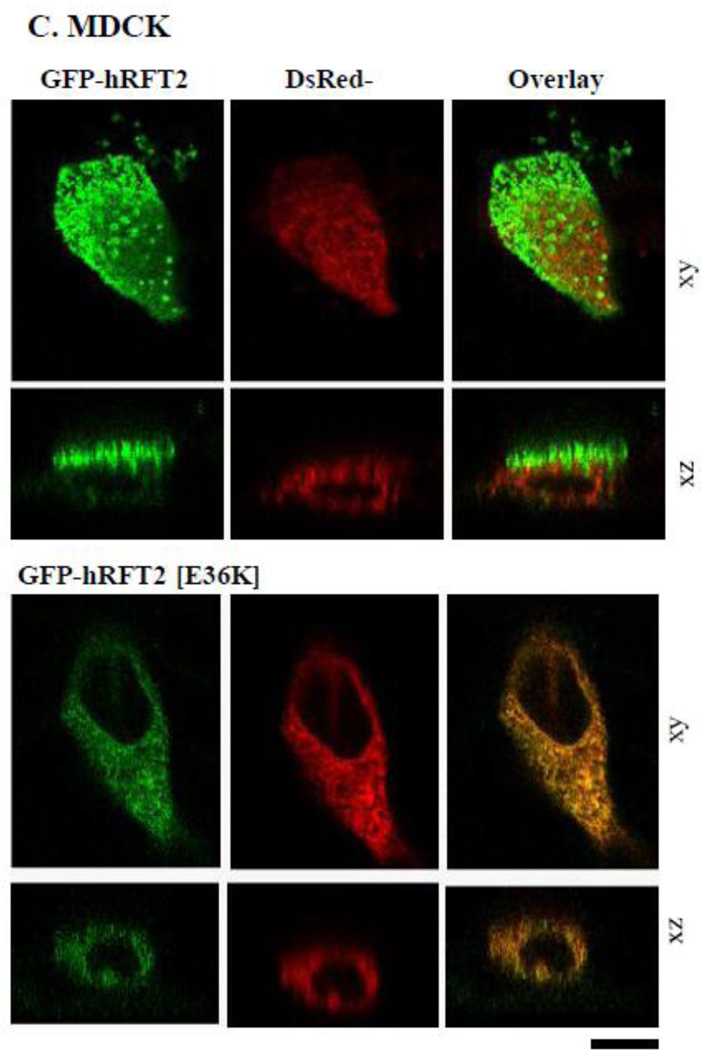
The hRFT-2 protein is predominantly expressed at the apical membrane domain of intestinal epithelial Caco-2 cells and renal epithelial MDCK cells [6, 9]. We used MDCK cells in this study to complement the findings with Caco-2 cells since the MDCK cell line is a commonly used cellular model to study polarized targeting of membrane proteins. We transiently expressed GFP-hRFT-2 (wild-type) and six missense hRFT-2 mutants in Caco-2 and MDCK cells and imaged the living cells using confocal microscope. Four mutants [GFP-hRFT-2[P28T], GFP-hRFT-2[E36K], GFP-hRFT-2[E71K] and GFP-hRFT-2[R132W] showed no cell surface expression, rather they were retained intracellularly in both cell types (Figure 4A and 4B). In contrast, two mutants, the GFP-hRFT-2 [W17R] and the GFP-hRFT-2 [L350M], were targeted to the cell membrane (Figure 4A and 4B). Co-transfecting one of the intracellularly retained construct (the GFP-hRFT-2 [E36K]), with an endoplasmic reticulum marker (the ER-targeted red fluorescent protein construct, DsRed-ER) displayed significant fluorescence overlap, a situation that was not seen when the co-transfection was done with the wild-type GFP-hRFT-2 construct (Figure 4C).
Figure 4. Cellular distribution of hRFT-2 mutants in Caco-2 and MDCK cells.
A and B: distribution of indicated hRFT-2 mutant constructs in Caco-2 and MDCK cells in lateral (xy, top) and axial (xz, bottom) section. C: a construct retained intracellularly in MDCK cells; cotransfection of GFP-hRFT-2 (top) or GFP-hRFT-2[E36K] (bottom) in lateral 9xy) and axial (xz) section together with DsRed-ER (middle), shown as overlay (right). Scale bar is 10 μM.
Confirmation of cell surface expression of the W17R hRFT-2 mutant
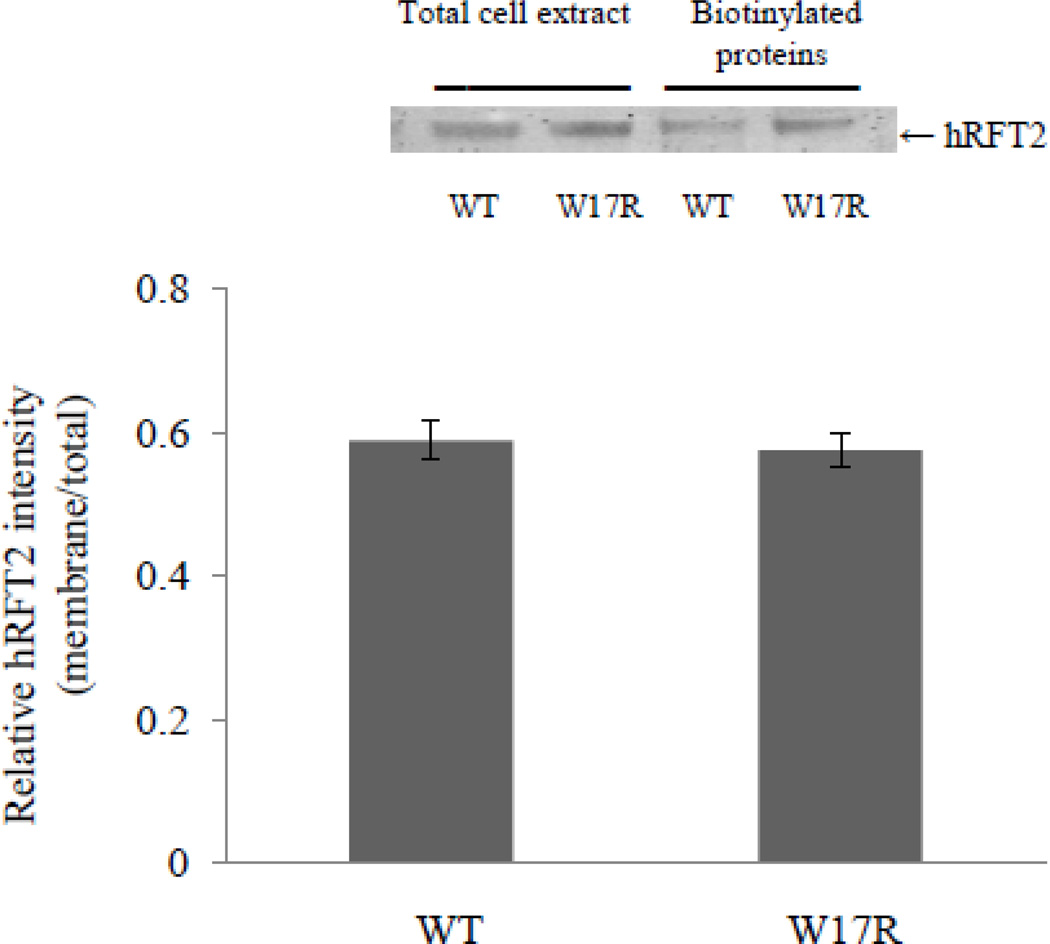
To further confirm cell surface expression of the W17R hRFT-2 mutant in Caco-2 cells, we performed a biotinylation assay followed by Western blot analysis. Cells were transiently transfected with equal amount of wild-type and mutated (W17R) hRFT-2, and the level of biotinylated hRFT-2 was then determined and compared between these cells. The results showed similar level of biotinylated hRFT-2 in cells expressing mutated and wild-type transporter (Figure 5), further confirming that the expression of the W17R mutant is at the cell membrane.
Figure 5. Surface expression of W17R hRFT-2 mutant.
Caco-2 cells were transfected with wild-type (WT) and mutated hRFT-2 (W17R). Forty eight hours post transfection cells were labeled with EZ-Link Sulfo-NHS-SS-Biotin, and biotinylated proteins were isolated on NeutrAvidin agarose. Proteins (whole cell lysates and biotinylated proteins) were analyzed by Western blotting using anti-hRFT-2 antibodies. Level of biotinylated cell surface hRFT-2 was normalized relative to total amount of cellular hRFT-2. Data shown are the means ± SE of 3 independent experiments. Inset: Western blot showing level of total and cell surface expression of wild-type and mutant hRFT-2 construct.
DISCUSSION
A spectrum of mutations in the C20orf54 gene (which encodes hRFT-2) has been identified in patients with BVVLS [2–5]. The hRFT-2 is expressed in a variety of tissues with high expression in the small intestine [7–9]. In the gut, the protein is localized exclusively at the apical membrane domain of the polarized enterocytes [7, 9], and is believed to play an important role in intestinal RF absorption [6, 9]. Patients with BVVLS/Fazio Londe syndrome are riboflavin deficient despite normal dietary intake of the vitamin suggesting impairment in normal riboflavin assimilation [4, 5]; RF supplementation improves the clinical symptoms and the biochemical abnormalities in the affected patients [4, 5]. Based on this knowledge, we designed a study to examine the effect of hRFT-2 mutations identified in patients with BVVLS on functionality of the carrier protein in transporting riboflavin. The human intestinal epithelial Caco-2 cells were chosen for the study because of their suitability to study riboflavin transport [16] and because intestinal epithelial cells express high levels of hRFT-2 [9]. We generated six clinically relevant missense hRFT-2 mutants carrying single amino acid substitutions and examined the effect of the individual mutation on riboflavin uptake and expression profile of the mutated proteins in living cells.
Results of our study showed that five hRFT-2 mutants (W17R, P28T, E36K, E71K, and R132W) to cause a significant (P < 0.01) inhibition in RF uptake by Caco-2 cells. It is interesting that three of the mutated sites (W17, P28 and E36) are highly conserved in RFT-2 of different species, whereas the other two residues (E71 and R132) are not highly conserved (E71 can also be a D and T, and R132 can also be a Q). The mutated residues important for RF uptake are predicted to be located in the transmembrane domain 1 (W17), extracellular loop (P28 and E36K), and intracellular loops (E71K and R132W) of the hRFT-2 polypeptide (Figure 1). The decrease in riboflavin uptake found in the five hRFT-2 missense mutants were not due to decrease in their transcriptional or translational efficiencies as judged by the observation that expression level of their mRNA and protein were similar to that of wild-type. The finding that the L350M mutation in hRFT-2 does not affect riboflavin uptake when expressed in Caco-2 cells is consistent with the previous suggestion that this mutation does not have pathogenic phenotype [2]. This mutation, which is found in some BVVLS patients with compound homozygous mutations, is tolerated by SIFT algorithm and the amino acid involved is not conserved in mammalian RFT-2 [2].
The hRFT-2 mutants P28T, E36K, E71K, and R132W were found by confocal imaging to be retained intracellular, most probably in the endoplasmic reticulum. This retention was uniformly associated with impairment in riboflavin uptake. This finding suggests an important role for these amino acid residues in the movement of the hRFT-2 protein to the cell membrane. Similar observations of impaired delivery to cell surface of clinically relevant mutants have been seen with other membrane transporters including the D-glucose transporter SGLT-1 in patients with glucose-galactose malabsorption and the thiamine transporter hTHTR-1 in patients with thiamine-responsive megaloblastic anaemia (TRMA) [21, 22]. A different situation was found for the W17R mutant where confocal imaging showed the protein to be expressed at the cell surface at the same level as that of the wild-type hRFT-2; nevertheless the mutated protein is functionality impaired. Confirmation of cell surface expression the W17R hRFT-2 mutant came from the results of the biotinylation assay, which again showed a similar level of membrane expression of the mutant and wild-type hRFT. These findings suggest that the tryptophan affected at position 17 of the hRFT-2 polypeptide is important for the transport function of hRFT-2, but not for its membrane targeting. Further studies at the cellular/molecular levels are needed to determine how this single mutation affects the functionality of hRFT-2.
In summary, our data demonstrate for the first time that missense mutations in hRFT-2 found in patients with BVVLS affect the functionality of the transporter in human intestinal epithelial cells and that this effect is mediated via alteration in membrane targeting and/or transporter activity.
HIGHLIGHTS.
This study provides evidence that some of the Brown-Vialetto-Van Laere Syndrome (BVVLS) causing mutations in human riboflavin transporter -2 (hRFT-2) affect the transporter functionality in intestinal epithelial cells.
This study also provides evidence that the BVVLS associated impairment in hRFT-2 functionality is mediated via alterations in membrane targeting and/or activity of the transporter.
This study is important for better understanding of molecular mechanism of the BVVLS disorder and in order to gain insight into the structure-function/biology of the hRFT-2 protein.
Acknowledgments
FUNDING
This work was supported by the Dept. of Veterans Affairs, NIH grants DK-56061, DK-58057 AA-18071(H.M.S) and DK-84094 (V.S.S).
Abbreviations
- BVVLS
Brown-Vialetto-Van Laere syndrome
- hRFT-2
human riboflavin transporter-2
- GFP
green fluorescent protein
- DsRed2
red fluorescent protein
- DsRed2-ER
red fluorescent protein-endoplasmic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
S.M.N., V.S.S., and H.M.S. designed research, analyzed data and wrote the paper; S.M.N. and V.S.S. performed the experiments.
REFERENCES
- 1.Sathasivam S. Brown-Vialetto-Van Laere syndrome. Orphanet J. Rare Dis. 2008;3:9. doi: 10.1186/1750-1172-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green P, Wiseman M, Crow YJ, Houlden H, Riphagen S, Lin JP, Raymond FL, Childs AM, Sheridan E, Edwards S, Josifova DJ. Brown-Vialetto-Van Laere syndrome, a ponto-bulbar palsy with deafness, is caused by mutations in c20orf54. Am. J. Hum. Genet. 2010;86:485–489. doi: 10.1016/j.ajhg.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson JO, Gibbs JR, Van Maldergem L, Houlden H, Singleton AB. Exome sequencing in Brown-Vialetto-van Laere syndrome. Am. J. Hum. Genet. 2010;87:567–569. doi: 10.1016/j.ajhg.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch AM, Abeling NG, Ijlst L, Knoester H, van der Pol WL, Stroomer AE, Wanders RJ, Visser G, Wijburg FA, Duran M, Waterham HR. Brown-Vialetto-Van Laere and Fazio Londe syndrome is associated with a riboflavin transporter defect mimicking mild MADD: a new inborn error of metabolism with potential treatment. J. Inherit. Metab. Dis. 2011;34:159–164. doi: 10.1007/s10545-010-9242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand G, Hasan N, Jayapal S, Huma Z, Ali T, Hull J, Blair E, McShane T, Jayawant S. Early use of high-dose riboflavin in a case of Brown-Vialetto-Van Laere syndrome. Dev. Med. Child Neurol. 2011 doi: 10.1111/j.1469-8749.2011.04142.x. [DOI] [PubMed] [Google Scholar]
- 6.Subramanian VS, Rapp L, Marchant JS, Said HM. Role of cysteine residues in cell surface expression of the human riboflavin transporter-2 (hRFT-2) in intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G100–G109. doi: 10.1152/ajpgi.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimura M, Yamamoto S, Murata T, Yasujima T, Inoue K, Ohta KY, Yuasa H. Functional characteristics of the human ortholog of riboflavin transporter 2 and riboflavin-responsive expression of its rat ortholog in the small intestine indicate its involvement in riboflavin absorption. J. Nutr. 2010;140:1722–1727. doi: 10.3945/jn.110.128330. [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Yonezawa A, Yoshimatsu H, Masuda S, Katsura T, Inui K-I. Identification and comparative functional characterization of a new human riboflavin transporter hRFT-3 expressed in the Brain. J. Nutr. 2010;140:1220–1226. doi: 10.3945/jn.110.122911. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian VS, Subramanya SB, Rapp L, Marchant JS, Ma TY, Said HM. Differential expression of human riboflavin transporters -1, 2, and -3 in polarized epithelia: A key role for hRFT-2 in intestinal riboflavin uptake. Biochim Biophys Acta. 2011;1808:3016–3021. doi: 10.1016/j.bbamem.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooperman JM, Lopez R. Riboflavin. In: Machlin LJ, editor. Handbook of Vitamins: Nutritional, Biochemical and Clinical Aspects. New York: Dekker; 1984. pp. 299–327. [Google Scholar]
- 11.Powers HJ. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003;77:1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 12.Leshner RT. Riboflavin deficiency -a reversible neurodegenerative disease. Ann. Neurol. 1981;10:294–295. [Google Scholar]
- 13.Dipti S, Childs AM, Livingston JH, Aggarwal AK, Miller M, Williams C, Crow YJ. Brown-Vialetto-Van Laere syndrome; variability in age at onset and disease progression highlighting the phenotypic overlap with Fazio-Londe disease. Brain Dev. 2005;27:443–446. doi: 10.1016/j.braindev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 14.McShane MA, Boyd S, Harding B, Brett EM, Wilson J. Progressive bulbar paralysis of childhood. A reappraisal of Fazio-Londe disease. Brain. 1992;115:1889–1900. doi: 10.1093/brain/115.6.1889. [DOI] [PubMed] [Google Scholar]
- 15.Said HM. Recent advances in carrier-mediated intestinal absorption of water-soluble vitamins. Annu. Rev. Physiol. 2004;66:419–446. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- 16.Said HM, Ma TY. Mechanism of riboflavine uptake by Caco-2 human intestinal epithelial cells. Am. J. Physiol. 1994;266:G15–G21. doi: 10.1152/ajpgi.1994.266.1.G15. [DOI] [PubMed] [Google Scholar]
- 17.Reidling J, Nabokina SM, Said HM. Molecular mechanisms involved in the adaptive regulation of human intestinal biotin uptake: A study of the hSMVT system. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G275–G281. doi: 10.1152/ajpgi.00327.2006. [DOI] [PubMed] [Google Scholar]
- 18.Reidling JC, Nabokina SM, Balamurugan K, Said HM. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and -2 promoters. J. Cell Physiol. 2006;206:371–377. doi: 10.1002/jcp.20492. [DOI] [PubMed] [Google Scholar]
- 19.Subramanya SB, Subramanian VS, Sekar VT, Said HM. Thiamin uptake by pancreatic acinar cells: Effect of chronic alcohol feeding/exposure. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G896–G904. doi: 10.1152/ajpgi.00308.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabokina SM, Subramanian VS, Said HM. Association of PDZ containing protein PDZD11 with the human sodium-dependent multivitamin transporter. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G561–G567. doi: 10.1152/ajpgi.00530.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martín MG, Lostao MP, Turk E, Lam J, Kreman M, Wright EM. Compound missense mutations in the sodium/D-glucose cotransporter result in trafficking defects. Gastroenterology. 1997;112:1206–1212. doi: 10.1016/s0016-5085(97)70132-x. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian VS, Marchant JS, Said HM. Targeting and intracellular trafficking of clinically relevant hTHTR1 mutations in human cell lines. Clin. Sci. (Lond.) 2007;113:93–102. doi: 10.1042/CS20060331. [DOI] [PubMed] [Google Scholar]