Abstract
Objective
To describe the methodology of the first NIH-funded clinical trial for seniors with comorbid depression and chronic low back pain.
Methods
Randomized controlled effectiveness trial using stepped care methodology. Participants are ≥ 60 years old. Phase 1 (6 weeks) is open treatment with venlafaxine xr 150 mg/day and supportive management (SM). Response is two weeks of PHQ-9 ≤ 5 and at least 30% improvement in the average numeric rating scale for pain. Non-responders progress to phase 2 (14 weeks) in which they are randomized to high-dose venlafaxine xr (up to 300 mg/day) with Problem Solving Therapy for Depression and Pain (PST-DP) or high-dose venlafaxine xr and continued SM. Primary outcomes are the univariate pain and depression response and both observed and self-report disability. Survival analytic techniques will be used, and the clinical effect size will be estimated with NNT. We hypothesize that self-efficacy for pain management will mediate response for subjects randomized to venlafaxine xr and PST-DP.
Results
Not applicable.
Conclusions
The results of this trial will inform the care of these complex patients and further understanding of comorbid pain and depression in late-life.
Keywords: clinical trial, geriatrics, depression, back pain, survival analysis
Introduction
The links between chronic low back pain (CLBP) and depression are well-established. Both conditions: 1) are risk factors for the other’s onset (1–3), 2) interfere with each others’ treatment response (4), 3) worsen the negative experience or “bothersomeness” of the other disorder (5), 4) mutually slow rates of remission (6–8), and 5) may increase the risk of the others’ recurrence. In late-life, these conditions increase the risk of suicide (9, 10), polypharmacy (11), physical deconditioning and disability (12, 13), cognitive impairment (14, 15), and worsened caregiver burden (16). A shared biology (e.g., similar neurotransmitter perturbation (17)), and psychology (learned helplessness, low self-efficacy (18)) support a unified approach to treatment. Rationally developed and tested age-specific interventions targeting these linked conditions are sorely needed.
Most older adults seek treatment for mood disorders and pain in primary care. Twelve percent of primary care elderly are depressed (19). The prevalence of CLBP in late-life is similarly estimated to be 12% (20). In our own work, we have observed that CLBP is the most common pain problem for which older adults are referred from primary care to a pain clinic (14). In addition, we have recently published a report describing that older adults with CLBP have higher rates of mood disorders than older adults with knee osteoarthritis (21), further supporting our decision to focus on low back pain in late-life. Treating these chronic conditions as linked may minimize the stigma of depression treatment and improve treatment acceptability.
The ADAPT Study (Addressing Depression and Pain Together) is a clinical trial testing a stepped care approach for these linked conditions in late-life. The primary aim of the study is to test the effectiveness of combination high-dose venlafaxine with Problem Solving Therapy for Depression and Pain (PST-DP) compared to high-dose venlafaxine with supportive management. The dependent variables are reduction in low back pain and depression and physical and psychosocial disability. The secondary aims of the study are to 1) explore the mediating role of self-efficacy, and 2) compare rates of recurrence over the course of 12 month follow-up between the two conditions.
While the Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) trial (22), another NIH-funded study of combined pharmacological and behavioral intervention for depression and pain in primary care patients, described how optimized antidepressant therapy followed by a pain self-management program resulted in substantial improvement in depression as well as moderate reductions in pain severity and disability, ADAPT is unique because of 1) the specific focus on low back pain, one of the most therapeutically challenging pain problems in late-life; 2) the stepped-care use of two dosing ranges of venlafaxine; 3) the use of a consistent problem solving therapy approach, which has established efficacy in late-life mood disorders comorbid with medical illness; 4) the use of an objective measure of physical disability; 5) use of the electronic medical record as a primary method of recruitment; and 6) collection of data from caregivers. In addition, while the SCAMP study tested the sequential treatment of depression and then musculoskeletal pain, the ADAPT trial treats these conditions as linked, addressing both conditions simultaneously.
Selecting an appropriate study design
In addition to reflecting the primary care approach to therapeutically complex conditions, a stepped-care approach is consistent with the spirit of effectiveness research (enhanced external validity, relaxation of exclusionary criteria, inclusion of participants with multiple morbidities, delivery of the intervention in real-world locations, and a broadened scope of outcome measures) (23). We considered (but did not adopt) a 2 x 2 factorial design to compare four different interventions. While this would have allowed us to simultaneously test two different hypotheses (24), it did not reflect “real-world” clinical practice.
Combination pharmacotherapy and psychotherapy (versus a monotherapy) is usually offered for patients living with depression and a major psychosocial and/or physical stressor (such as CLBP) (43, 44). Likewise, an approach that combines both pharmacologic analgesia and a psychosocial treatment is often indicated for CLBP complicated by passive coping styles and high emotional distress (frequently observed in pain patients living with depression) (45). With this in mind, the goal of the ADAPT study is to test the effectiveness of combination treatment with an antidepressant medication that has analgesic properties and a learning-based and behaviorally-activating psychotherapy for those patients who do not respond to traditional and/or less intensive interventions.
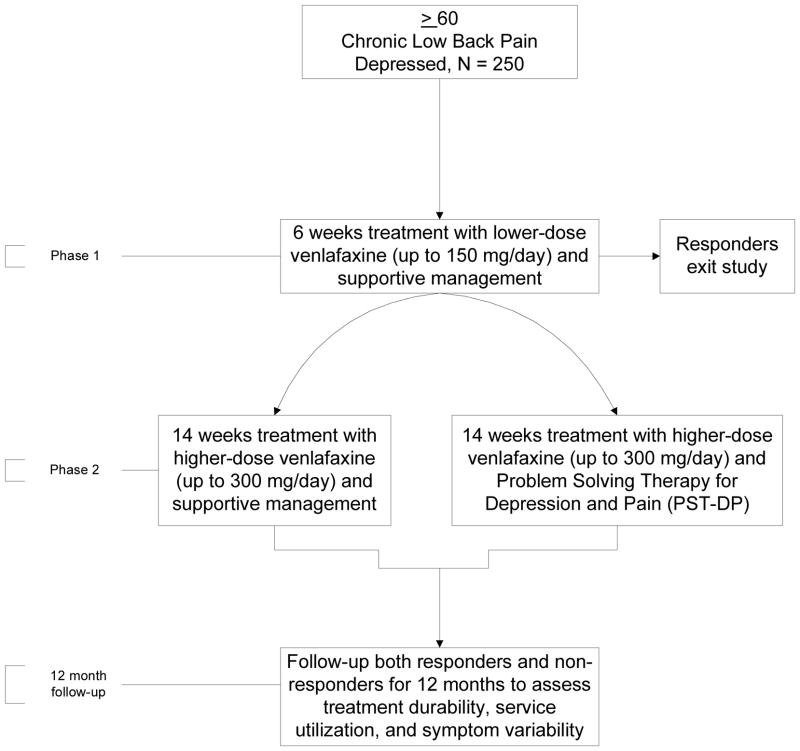
During phase 1 of ADAPT, for 6 weeks all subjects receive open-label treatment with venlafaxine xr up to 150 mg/day and supportive management (SM). Six weeks is the average response time for an antidepressant effect (25). In addition, both our group and others have observed an improvement in low back pain with antidepressant pharmacotherapy by week 4 (26, 27). Participants whose depression and/or pain does not improve then progress to phase 2. During the 14 weeks of phase 2, participants are randomized to either higher-dose venlafaxine xr (up to 300 mg/day) and Problem Solving Therapy for Depression and Pain (PST-DP) or to higher-dose venlafaxine xr and continued SM. Fourteen weeks was chosen to accommodate 10 visits without undue participant burden. Graduates of phase 2 are followed for 12 months to assess treatment durability and symptom variability. The study design is shown in Figure 1.
Figure 1.
Study Design
Subject Characteristics
The inclusion and exclusion criteria are listed in Table 1. We selected age 60 rather than the traditional age 65 to define older adults because individuals living with depression and/or pain have higher levels of medical comorbidity (28), more brain changes consistent with the aging process and exposure to a high stress state (29, 30), and are prescribed a greater number of medications than euthymic or pain-free individuals (11, 31). The 3MS is used as a screening instrument, so that subjects with dementia are excluded, but subjects who are cognitively normal or who have MCI will be included. It is our goal to include subjects with MCI to reflect the range of cognitive functioning in late-life. Depressed older adults with comorbid MCI (in particular, executive dysfunction) have been shown to both learn and benefit from PST (32).
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
| 60 and older |
| PHQ-9 score ≥ 10 (consistent with at least moderate depression severity). |
| PRIME-MD diagnosis of major depression, minor depression, or dysthymia. |
| Low back pain more days than not, of at least moderate severity, for at least the past 3 months. Must endorse at least an 8 on the 20-item NRS for average low back pain within the past week. |
| Must have tried without continued success any of the following: 1) prescription or OTC analgesics, 2) physical therapy, 3) acupuncture, 4) injection therapy, 5) back surgery, 6) multidisciplinary pain program, 7) psychological treatment for chronic pain, or 8) any other physician-prescribed treatment for chronic low back pain. |
| If subjects endorse other pain problems, low back pain is their primary pain problem. |
| Must score ≥ 80 on the Modified Mini-Mental State (3MS) exam. |
| No alcohol/substance mis-abuse for the past six months. |
| Willingness to stop current antidepressant pharmacotherapy. |
| Exclusion Criteria |
| Low back pain “red flag” suggesting medically emergent condition (e.g., vertebral fracture, infection, cauda equina syndrome, disk herniation, cancer). |
| Psychotic spectrum disorder or bipolar disorder. |
| Medically unstable, delirious, or terminally ill; or medical contraindication to use of venlafaxine therapy, including hypersensitivity, history of venlafaxine-induced SIADH, uncontrolled narrow angle glaucoma, AST or ALT > 1.5x upper limit of normal, on dialysis). |
| Wheelchair-bound as this level of disability does not represent most older adults living with CLBP. |
| Receiving or involved with workers compensation or other legal action related to back pain/injury. |
| Uncontrolled narrow angle glaucoma. |
Given the high prevalence of other painful conditions in late life (e.g., arthritis, neuropathic pain, fibromyalgia), if subjects endorse other painful conditions, low back pain must be identified as the primary pain problem, and all pain ratings are focused on low back pain. Depression and psychiatric diagnoses are agreed upon during weekly consensus meetings attended by at least two geriatric psychiatrists and a multidisciplinary team of clinical researchers. In addition to a score of ≥ 10 on the PHQ-9, all subjects must meet PRIME-MD criteria for a depressive syndrome (dysthymia, minor depression, major depression). This means that all patients endorse at least one of the cardinal symptoms of depression: low mood or anhedonia. This criteria for minimal depressive severity has been used in the recently published Collaborative Care for Chronic Pain in Primary Care project (33), further establishing precedent for using this criteria. A depression of this severity is considered the low-end of the moderate range yet worthy of pharmacological treatment (see: http://www.depression-rimarycare.org/clinicians/toolkits/materials/forms/phq9/score_table/). We chose to not use a clinician administered scale of depression because of the high overlap with neurovegetative symptoms such as with the Hamilton Rating Scale for Depression (34) and also for ease of administration in primary care. We view the use of the PHQ-9 as a scalable approach for use in primary care, as in addition to its excellent psychometrics (35), it is a measure with which primary care physicians are familiar (36, 37).
The quality and severity of low back pain are assessed at baseline. Subjects must endorse pain severity of at least 8 on a numeric rating scale for low back pain (ranging from 0–20) (38) (personal communication, K Herr, 2009), indicating at least moderately severe low back pain. In addition to the NRS, the McGill Pain Questionnaire – Short Form (39), and a pain map, subjects are surveyed about: 1) other pain conditions, 2) treatments sought for low back pain including surgical history, 3) radiating pain below the knee in one or both legs, 4) use of an ambulatory assistive device, and 5) if pain interferes with sleep. While restricting study entry to those only living with low back pain (and no other pain complaints) would have been ideal, this would not have been practical since many older adults with low back pain often live with osteoarthritis, which is often generalized. Indeed, about one third of individuals older than 65 years experience symptomatic osteoarthritis of the knee, and almost 80% of persons have degenerative joint disease after age 70 years (40, 41). Requiring older patients to not have any other pain conditions would not be consistent with our effectiveness approach to research and would make it challenging to generalize the results to the general population of older adults with CLBP. To assure the focus of response is the low back, upon entering the clinical trial, participants must identify low back pain as their primary pain problem and are asked to think about their low back when answering all questions about pain severity. Information about other pain diagnoses and pain extensity (i.e., the number of different pain sites throughout the body) will be added to the statistical models as covariates.
Participants are requested to remain on the same type and dose of concurrently prescribed medications during their participation. Standing analgesic medication use, prn analgesic medication use, and change in analgesic use are assessed and documented at every visit. Excluding subjects who took concurrently prescribed analgesics would be inconsistent with an effectiveness research approach. Any changes in analgesic use are documented. The daily dose of regularly scheduled opioid analgesics will be calculated and converted to oral morphine equivalents (42). We will also calculate a standardized average daily dose for non-opioid analgesics using established methods (43), and this information will be entered into analytic models as covariates.
The Cumulative Illness Rating Scale for Geriatrics (CIRS-G) (44) is administered by an advanced practice nurse (this involves medical chart and medication review) in which further information about CLBP and medical comorbidity are captured (e.g., imaging data, specific surgical interventions) and recorded as ICD-9 diagnostic codes.
Selecting the interventions
Venlafaxine is a serotonin norepinephrine reuptake inhibitor (SNRI) which at lower doses has greater affinity for serotonin than norepinephrine transporters. Thus, it acts as a serotonin specific reuptake inhibitor (SSRI) at lower doses (≤150mg/d) and a SNRI at higher doses (45, 46). While venlafaxine is not approved for the treatment of any pain conditions, it has been shown to have analgesic properties in several studies (47–51), and when prescribed in the 150–225 mg/day range for activity-limiting osteoarthritis pain, significantly reduced pain intensity and marginally improved self-reported function (52). Reuptake inhibition of norepinephrine at higher doses may lead to further antidepressant effect as well as enhanced analgesia. We did not select duloxetine or minalcipran because 1) these agents are not generic and 2) there is no change in pharmacodynamics with increased dosing. Tricyclic antidepressants were not selected to avoid concerns about cardiotoxicity and anticholinergic burden, both issues of significance for older adults (53).
Problem Solving Therapy for Depression and Pain (PST-DP)
PST is increasingly being used in primary care with older adults (54, 55) and patients with medical comorbidity (56–58) because of its proven efficacy in large clinical trials (54, 59, 60), its equivalence to cognitive behavioral therapy (CBT) (61), easy integration into primary care (62), and efficacy for patients with mild cognitive impairment (63). In addition, there is evidence that PST is suitable for patients with MCI (63). PST-DP is a practical treatment for primary care because of its 1) duration of treatment (usually no more than 10 sessions (62)), 2) session duration (30–45 minutes), and 3) the relative ease of training of medical nurses and other non-mental health specialists in its reliable delivery (62). PST also has been shown to reduce pain and disability in middle aged and older adults with CLBP (64). Through consistent implementation of a step-wise approach to managing problems related to pain and depression and with behavioral activation, PST is hypothesized to work by reducing learned helplessness and improving self-efficacy (65, 66).
PST-DP differs from traditional PST for primary care (62) as follows: 1) “The Back Book” (67) is provided during the first session, 2) greater focus is spent on activity and movement scheduling, 3) clinicians are trained to provide brief behavioral treatment of insomnia (BBTI) (68) and progressive muscle relaxation and diaphragmatic breathing if indicated, and 4) more effective use of analgesics and communication with PCPs about pain management may be offered. The problem solving style of patients is assessed with the Social Problem Solving Inventory (69). This information about how patients approach problem solving, along with hypothesized links between pain, depression, and disability specific to each patient, are provided early in treatment. This case conceptualization provides a rationale for why both conditions are being addressed, serves as a road-map for problem solving, and is frequently revisited during the intervention. Thus, PST-DP has similarities with Cognitive Behavioral Therapy (CBT), an established intervention for both pain and depression, but modules (e.g., BBTI, relaxation) are only delivered if these problems are identified by the participant and decided upon using a problem solving approach. This approach is consistent with the goal of improving self-efficacy by having participants choose the problems upon which they will work.
As we have found that caregivers of depressed patients are often burdened (16), when possible we encourage caregivers to be an active participant in PST-DP. Our goals for including caregivers are to: 1) improve compliance with the intervention by enhancing caregiver “buy-in,” 2) reduce caregiver burden to reduce their risk of conversion to (or worsen current symptoms of) depression, and 3) reinforce the durability of PST-DP since the caregiver may “coach” the subject to utilize these skills.
The limited improvement in pain-related outcomes in SCAMP may be due to the heterogenous group of participants with a variety of pain conditions. While chronic low back pain, especially in late-life, is often multifactorial in etiology, we expect our sample will be more homogenous in terms of type of pain, age, and absence of secondary gain. While PST-DP focuses on whatever problems the patient identifies, usually pain is a focus early in treatment, and is encouraged to be addressed by the clinician early during phase 2. This systematic, learning-based, and behaviorally activating intervention guided by an individual therapist is unique from the pain self-management intervention provided in SCAMP. In addition, motivational interviewing (70) is an integral component of PST-DP and utilized to enhance engagement and participation, and minimize attrition, especially given the relatively high frequency of scheduled visits.
Supportive management
During our 20 years experience in conducting late-life antidepressant pharmacotherapy clinical trials, we have learned that non-specific and frequently beneficial clinician-subject interactions occur when subjects receive a “pharmacotherapy-only” condition. We refer to these non-specific interactions as supportive management. The characteristics of supportive management are described in Table 2.
Table 2.
Description of Psychosocial Interactions Allowed and Forbidden in Supportive Management Condition
| Permitted Interactions |
|
| Forbidden Interactions |
|
Maintaining Adherence to Treatment Assignment and the Study Blind
The same clinicians provide supportive management and PST-DP. To maintain equipoise and minimize allegiance bias, we routinely remind clinicians during weekly group supervision that we do not know which intervention is superior. Clinicians have all been certified in Problem Solving Therapy by a certified PST-trainer (JQM). All sessions are audiotaped and a random selection of 20% of sessions (PST-DP and SM) are reviewed for adherence (by JQM) to the assigned intervention and to monitor for therapist “drift.” Treatment fidelity is assured by rating the tapes with an assessment of technical compliance. Clinicians who score below average for the entire measure or are deficient in delivering a particular component (e.g., case conceptualization, setting an agenda, maintaining the problem solving or supportive management format, delivering the optional modules according to the manual) receive immediate corrective feedback.
After the screening and baseline visits, all of the assessments are self-report except for the Short Physical Performance Battery. For this assessment only, we utilize an independent assessor. Participants are instructed to not divulge to which condition they are assigned. During weekly clinical and safety management meetings, assignment to PST-DP is not disclosed, further assuring the blind. The investigators are not blind to assignment during phase 2 because of their involvement in weekly supervision.
Selecting Criteria for Response
We used the MacArthur Initiative scoring protocol in developing our criteria for response. While a PHQ-9 score of ≤ 5 is ambitious for response, in our own work with depressed patients with cardiac disease, we have successfully used a PHQ-9 score ≤ 5 as criteria for response (71). In the SCAMP trial, the target PHQ-9 score was also ≤ 5 lending further credence to this depression-response criteria (22, 72).
Our selection of ≥ 30% improvement on the numeric rating scale for pain is based on work by Farar et al (73–75) who noted that an improvement of this magnitude represents a clinically significant difference. As stated earlier, linking the depression and pain criterion for response into a unified response criteria is novel and we predict will lead to a more robust and durable response.
While the planned analytic approach explores response as a categorical variable, we will also conduct sensitivity analyses using different PHQ-9 scores as criteria for depression response, a range of percent improvement in pain intensity, and explore improvement as a continuous (not categorical) measure.
Recruitment procedures
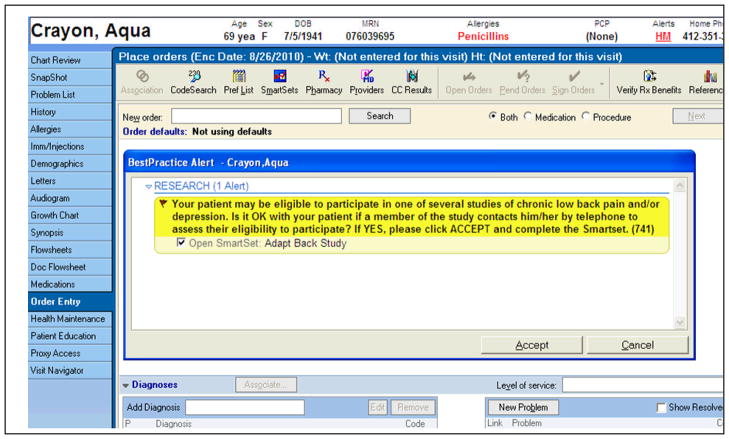
The bulk of our recruitment strategies are based in primary care. We are using the electronic medical record (EMR) as our primary recruitment strategy. This approach has been found to be more efficient than deploying research assistants in primary care waiting rooms and also resulted in higher than estimated recruitment of African Americans into a study of primary care based treatment of anxiety (76). The EMR prompt exposes the PCP to a reminder about our study at the time of the clinical encounter for patients aged 60 and older with 1) an ICD-9 code for low back pain in the electronic problem list or as the encounter diagnosis (Fig. 2).
Figure 2.
Electronic Medical Record Recruitment Reminder for Physicians
We designed our alert to be Health Insurance Portability and Accountability Act (HIPAA)-compliant, instructing PCPs to seek patient permission before forwarding contact information to a study recruiter (Fig. 2). We routinely remind PCPs about the EMR prompt and study progress with regular visits and study-specific marketing materials (e.g., pens, mugs, and newsletters). During all detailing, we find that highlighting the principles outlined in Table 3 enhances physician “buy-in.” We also recruit participants via radio advertisements, the internet (www.ADAPTstudy.com), and posters placed at local pharmacies and community centers.
Table 3.
Principles Highlighted to PCPs During Academic Detailing of the Study to Encourage Referrals of Potential Subjects
| Principle | Language Used |
|---|---|
| Funding Source | “We are conducting a NIH-funded clinical trial to improve care for older adults with low back pain and depression.” |
| Benefit to Patients | “All participants receive active treatment.” |
| Benefit to Patients | “Participants may be compensated up to $130 for participating.” |
| Benefit to Patients | “Participants receive study medication and all interventions at no cost.” |
| Benefit to Physicians | “We will keep you updated throughout your patient’s participation in ADAPT about their progress.” |
| Benefit to Physicians | “Participation may decrease between visit calls about pain.” |
| Benefit to Physicians | “Participation may decrease opiate use – we are tracking this closely.” |
Study Procedures
Phase 1
250 subjects will receive six-weeks of open treatment with lower-dose venlafaxine and supportive management (VEN/SM). The titration schedule of venlafaxine is described in Table 4. Prior to beginning venlafaxine, if subjects are taking another antidepressant, we taper the ineffective antidepressant. To minimize subject discomfort from worsening symptoms, to reduce the risk of emerging suicidality, and to reflect usual care, a wash-out period is not required. Sessions during step 1 are weekly, and last 30 minutes. The location (in-person, telephone, subject’s home) is always documented. The list and schedule of assessments are included in Table 5.
Table 4.
Venlafaxine xr Dosing Schedule for Phase 1 and Phase 2
| Phase 1 | |
| Day 1 – Day 3 | Venlafaxine xr 37.5 mg daily |
| Day 4 – Day 6 | Venlafaxine xr 75 mg daily |
| Day 7 – Day 9 | Venlafaxine xr 112.5 mg daily |
| Day 10 – 42 (through end of week 6) | Venlafaxine xr 150 mg daily |
| Phase 2 | |
| The dose of Venlafaxine xr is increased in increments of 37.5 mg to 75 mg separated by at least 3 days based on response to the medication and as tolerated. | |
Table 5.
Phase 1 and Phase 2 Schedule of Assessments
| Phase 1 | Phase 2 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VISIT/WEEK# | Screen | B* | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Depression | ||||||||||||||||||||||
| Structured Diagnosis (PRIME MD/MINI) | X | |||||||||||||||||||||
| PHQ-9 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| ATHF | X | |||||||||||||||||||||
| Pain | ||||||||||||||||||||||
| Low Back Pain Hx. | X | |||||||||||||||||||||
| McGill Pain Quest. | X | X | X | X | X | X | X | |||||||||||||||
| NRS for pain | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| P-GIC | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| FABQ | X | X | X | X | X | X | ||||||||||||||||
| Disability & HRQOL | ||||||||||||||||||||||
| Lee Fatigue Quest. | X | X | X | X | X | |||||||||||||||||
| Roland Morris Quest. | X | X | X | X | X | |||||||||||||||||
| SPPB | X | X | X | X | ||||||||||||||||||
| RAND-12 | X | X | X | X | ||||||||||||||||||
| PASE | X | X | X | |||||||||||||||||||
| Neuropsychological Functioning | ||||||||||||||||||||||
| 3MS | X | |||||||||||||||||||||
| EXIT | X | |||||||||||||||||||||
| HVLT | X | |||||||||||||||||||||
| Psychological | ||||||||||||||||||||||
| SPSI | X | X | ||||||||||||||||||||
| Pain Self-Efficacy | X | X | X | X | ||||||||||||||||||
| BSI Anxiety subscale | X | X | X | X | X | X | X | |||||||||||||||
| CSQ-catastrophizing | X | X | X | X | ||||||||||||||||||
| Medical | ||||||||||||||||||||||
| CIRS-G and ICD-9 | X | |||||||||||||||||||||
| PG and PK | X | |||||||||||||||||||||
| Vitals | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| UKU Side Effects | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Fibromyalgia Exam | X | |||||||||||||||||||||
| Current Medications | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Safety | ||||||||||||||||||||||
| EKG and laboratory | X | |||||||||||||||||||||
| Adherence & Health | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Service use | ||||||||||||||||||||||
| Suicide Ideation Scale | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Time in hours | 2.5 | 2.5 | .5 | .5 | .5 | .5 | .5 | .5 | .1 | .3 | .3 | .3 | .3 | .5 | .3 | .5 | .3 | .5 | ||||
Based on pilot work and to reflect standard practice, we have allowed subjects to remain on stable doses of concurrently prescribed analgesics (27). Participants agree to not begin a new analgesic or other pain treatment. We are tracking the use of all prescribed and over the counter analgesics (including PRN doses) at every visit.
Randomization and stratification
After 6 weeks, subjects who have not responded (response is univariably defined as PHQ-9 ≤ 5 and ≥ 30% improvement on the 20-item pain NRS for 2 sequential weeks) have the dose of venlafaxine increased to 300 mg/day or maintained at the highest tolerable dose. The algorithm used to promote a stable response is listed in Table 6. Non-responders are randomized to: 1) high-dose venlafaxine xr and Problem Solving Therapy for Depression and Pain (VEN/PST-DP) or 2) high-dose venlafaxine xr and supportive management (VEN/SM). We are using a stratified permuted block design for randomization. Stratification is guided by both 1) primary care referral versus other referral source (e.g., media, mental health referral) and 2) diagnosis of fibromyalgia. Subjects referred from primary care may be different from those self-referred (i.e., more comorbid medical conditions). Fibromyalgia is a stratification variable (diagnosed by history and physical exam by JK) due to the 1) relatively high rates of fibromyalgia in late-life, 2) fact that the pain of fibromyalgia is often concentrated axially, and 3) possibility of different treatment response to venlafaxine when fibromyalgia is comorbid (77). We considered using radiculopathy (defined as pain radiating below one or both knees) as a stratification variable to control for the possible effect on treatment response of neurologic injury. However, given the multifactorial causes of low back pain in late-life (e.g., myofascial disorder, spinal degeneration, hip joint arthritis, fibromyalgia, piriformis syndrome, sacroiliac joint syndrome) (78), and that conditions other than spinal stenosis can lead to leg pain (e.g., tensor fascia lata strain, sacroiliac joint syndrome, hip and knee arthritis (79)), we did not include radiculopathy as a stratification variable. Using only two variables reflects our parsimonious approach to stratification (80) and our faith in the randomization procedure. Randomization lists are generated by the statistician and maintained by the data manager. When a participant is eligible, the study coordinator contacts the data manager who provides the randomization assignment.
Table 6.
Phase 1 Response Criteria
| For both week 5 and week 6, if PHQ-9 is ≤ 5 AND NRS is ≥ 30% improved from baseline, subjects are responders and exit the study. |
| For both week 5 and week 6, if the PHQ-9 is > 5 and the NRS is < 30% improved from baseline, subjects are non-responders and proceed to phase 2. |
| If subjects are responders at week 5 followed by non-response at week 6, they are considered non-responders and proceed to phase 2. |
| If subjects are non-responders at week 5 followed by response at week 6, phase 1 is extended for one more week. If at this additional visit subjects are non-responders, they proceed to phase 2. If at this additional visit they are responders, they exit the study. |
Note: These response criteria were established so participants without a stable response are those who progress to phase 2.
Phase 2
Based on pilot work, we estimate 150 non-responders will progress to Phase 2 and be randomized to either: 1) higher-dose VEN/PST-DP or 2) higher-dose VEN/SM. Sessions for VEN/PST-DP will last about 45 minutes and sessions for VEN/SM will last about 30 minutes. This differential in face-to-face time may have an indirect effect on outcome, but is consistent with our effectiveness approach to the trial. We are tracking the duration of every session during phase 2.
The schedule of assessments is shown in Table 5. If there is a delay in completing the 10 sessions of phase 2, every attempt will be made to complete 10 sessions, but all active interventions of phase 2 will end by week 24. Criteria for response is 2 consecutive scores in which the PHQ-9 is ≤ 5 and the NRS for pain is ≥ 30% improved from baseline (75).
Twelve Month Follow-up
To assess and compare treatment durability between VEN/SM and VEN/PST-DP, we will follow phase 2 participants quarterly over the course of one year. To learn about incident treatments and the fluctuating nature of symptoms, non-responders will also be followed. In addition to assessing low back pain and depression, we will assess disability associated with low back pain (81), chronic pain self-efficacy (82), and other treatments for mood and pain prescribed in the interval. For subjects randomized to VEN/PST-DP, we will assess whether they continue to implement the skills previously acquired.
Power calculations
Based on our own and others’ experience (83–86), we expect that approximately 20–25% of subjects randomized to VEN/SM will respond and that approximately 50–55% of the VEN/PST-DP group will respond. We would have sufficient power of 80% to detect a 30% difference assuming 20% attrition with a randomized sample of 104. A between group difference of 30% is based on several depression and pain treatment studies. For example, an effect size of 0.73 was observed in a comparison of a behavioral activation intervention versus supportive management among a group of psychiatric inpatients (87). Although the Hopko paper describes pilot research (n=25), there appears to be a signal that a behaviorally activating intervention may be effective for severely depressed patients. In another study of primary care patients with depression, those assigned to receive a structured depression treatment program which included behavioral treatmentto increase use of adaptive coping strategies had significantly more improvement in depression severity than those randomized to receive treatment as usual from their primary care physician (88). In a study of 128 chronic low back pain patients randomized to cognitive behavioral therapy(described as a “package” of respondent, operative, and cognitive interventions) or a wait-list control condition, those receiving the CBT intervention had significantly more improvement in pain with a moderate effect size of 0.60 (89). Because of the established benefit of venlafaxine on both mood and pain, we predict a more conservative but clinically significant effect size of about 0.3 between the groups.
We estimate 10% attrition during step 1, 20% attrition during step 2, and predict that 30% of subjects will respond during step 1. Thus, we expect 40% of the recruited sample will not progress to step 2 (10% attrition + 30% response). Based on attrition rates described above, the number needed to recruit to randomize 104 subjects is (104/0.60) = 173. However, in case attrition or treatment response during step 1 is higher than anticipated, we will “over-recruit” by 77 subjects for a total sample size of 250. Recruiting 250 subjects into step 1 will assure that, even with rates of attrition and treatment response up to 60% during step 1, we will have enough subjects for hypothesis testing during step 2. Of note, if the attrition and response rates are consistent with our projections (i.e., 60% of subjects progress to step 2) we will still be powered to detect a between group difference of .25. Increasing the sample to 150 also increases our power to 85% to detect a mediation effect.
Planned analysis
We will use survival analyses to compare time to response between the two conditions. We are using a univariate outcome comprised of improvement for both depression and low back pain. Participants must have two sequential visits meeting this criterion to be determined responders. We will use Kaplan-Meier survival curves to illustrate the proportion yet to respond over time; the curves will be tested with the Wilcoxon χ2 test which weights more favorable earlier observations. If we observe any covariate strongly associated with the main outcome and the two treatment arms, we will adjust for it using standard techniques such as logistic regression or proportional hazard models with covariates. We will estimate the clinical effect size by calculating the number needed to treat (NNT, with 95% confidence intervals) between the two conditions.
A bivariate repeated measures mixed-effect analyses will model self-reported and observed physical disability between the two interventions across time. By using a bivariate mixed effects model, we will be able to examine the two outcome measures jointly. This bivariate mixed model will allow us to examine how these two outcomes are associated over time. The modeling procedure will provide information about the lag structure of the correlations. For example, it will allow us to estimate correlations between the two disability outcome assessments when they are measured at the same point of time as well as when they are measured at different time points. Accounting for these correlations, if they exist, will reduce the standard error of the difference between the treatment effects resulting in sharper test statistics for the comparison of the two treatment arms. The model will be from baseline to study end or termination. The bivariate model will have random intercepts for subjects. Fixed effects of treatment and time will be included. A significant treatment by time interaction will indicate a differential pattern of the outcome variables over time between the treatments.
To investigate whether self-efficacy will mediate treatment response, we will use latent growth curve modeling (90). The mediator and the outcome measured across multiple time points will be viewed as two parallel processes. These two parallel processes will be modeled by standard longitudinal data modeling techniques as latent growth curves, and the mediation effect will be defined as the change in the treatment response influenced by the change in the mediator which was influenced by the treatment. We hypothesize that self-efficacy will mediate the response for participants exposed to PST-DP.
Subgroup and moderator exploratory analysis
To take advantage of this rich and unique dataset, we will perform exploratory subgroup and moderator analyses (91). These analyses will help us profile groups with varying rates of and times to response, and will assist in guiding the next stage in our work with this population. Some planned analyses include comparisons of subjects 1) whose depression preceded their low back pain and vice versa; 2) with high versus low levels of anxiety (e.g., fear avoidance); 3) with radiculopathy versus those without; 4) who score high on the patient global impression of change versus those who score low; and 5) with varying degrees of health related quality of life and types and level of medical comorbidity. We will also explore a moderating effect of executive functioning and memory on PST-DP using the EXIT (92) and HVLT (93) tests of these specific cognitive domains.
As we will attempt to collect data from subjects who drop out of active participation, we will perform our intent to treat analysis with these additional data using both “on-treatment” and “off-treatment” data with a mixture model. Collection of this information will allow our model to account for drop-out times that may be different between drop-outs and those who remain in the protocol. We will perform this analysis for each of the outcomes separately for a full utilization of data using a well developed statistical modeling approach (94).
Conclusion
ADAPT is the first primary care-based randomized clinical trial to test the effectiveness of combination SNRI pharmacotherapy plus a behaviorally-activating, learning-based psychotherapy for older adults living with CLBP and depression. If found to be effective, this combination approach may be implemented in primary care with depression and pain care managers. This work will influence other scalable approaches such as telephone or web-based depression and pain care management. Indeed, the mood disorder care management literature supports the importance of managing pain in addition to depression in mid and late-life (8, 27, 95, 96). Establishing the effectiveness of higher-dose venlafaxine combined with PST-DP for these linked conditions is preferable than 1) chronic exposure to opioids (with risks of constipation, fracture, neurocognitive effects, diversion, and more recently identified cardiovascular effects (97), 2) chronic exposure to NSAIDs (with risks of gastrointestinal bleeding, cardiovascular events, and renal impairment), 3) more invasive interventions such as surgery, or 4) a polypharmacy approach to depression and pain treatment. ADAPT expands the findings of the SCAMP study, a stepped-care trial for mixed-age adults living with musculoskeletal pain and depression in the following ways: 1) assuring the intervention is effective for older adults; 2) improving knowledge of how to treat these linked conditions with a unified, as opposed to a sequential, approach; and 3) advancing understanding of how observed disability improves when both depression and low back pain are treated simultaneously. Clinically relevant findings from the SCAMP trial were that 1) optimized antidepressant therapy followed by a pain self-management program produced substantial reductions in depression severity as well as enhanced response and remission rates; 2) the intervention resulted in moderate reductions in both pain severity and pain-related disability; and 3) the benefits on both depression and pain outcomes were sustained over the 12 months of the trial. We anticipate that the ADAPT results will build upon these findings and provide clinicians with additional treatment options, as the design is consistent with suggestions for future work described in the SCAMP trial that additional interventions may be needed to produce larger improvements in pain and higher depression response and remission rates such as optimized analgesic management and cognitive behavioral therapy approaches.
Acknowledgments
Supported by grant AG033575 (Karp) and by grant KL2 RR024154 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH. Medications were provided by Pfizer for this investigator initiated trial.
Acronyms used in text
- MINI
MINI International Neuropsychiatric Interview
- PHQ-9
Patient Health Questionnaire – 9 item
- ATHF
Antidepressant Treatment History Form
- NRS
Numeric Rating Scale
- FABQ
Fear Avoidance Behavior Questionnaire
- P-GIC
Patient Global Impression of Change
- SPPB
Short Physical Performance Battery
- RAND-12
RAND 12-Item Health Survey
- PASE
Physical Activity Scale for Elderly
- 3MS
Modified Mini Mental State Examination
- EXIT
Executive Functioning Interview
- HVLT
Hopkins Verbal Learning Test
- SPSI
Social Problem Solving Inventory
- BSI
Brief Symptom Inventory
- CSQ
Coping Strategies Questionnaire
- CIRS-G
Cumulative Illness Rating Scale for Geriatrics
- ICD-9
International Statistical Classification of Diseases and Related Health Problems – 9th Edition
- UKU Side Effects
Udvalg for Kliniske Undersøgelser Side Effect Rating Scale
- EKG
electrocardiogram
- OTC
Over the counter
Footnotes
Clinicaltrials.gov Trial Registration Identifier: NCT01124188
Potential Conflicts of Interest: Dr. Karp served on advisory boards for Eli Lilly and Theravance greater than one year ago. He has received medication supplies for investigator initiated research from Pfizer, Eli Lilly, and Reckitt Bensicker. He owns stock in Corcept. Dr. Reynolds has received medication supplies for investigator initiated research from Eli Lilly, Pfizer, Forest, and Bristol Myers. The other authors do not declare any potential conflict of interest.
References
- 1.Reid MC, Williams CS, Concato J, Tinetti ME, Gill TM. Depressive symptoms as a risk factor for disabling back pain in community-dwelling older persons. Journal of the American Geriatrics Society. 2003;51:1710–7. doi: 10.1046/j.1532-5415.2003.51554.x. [DOI] [PubMed] [Google Scholar]
- 2.Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. Clinical Journal of Pain. 1997;13:116–37. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Rudy TE, Kerns RD, Turk DC. Chronic pain and depression: toward a cognitive-behavioral mediation model. Pain. 1988;35:129–40. doi: 10.1016/0304-3959(88)90220-5. [DOI] [PubMed] [Google Scholar]
- 4.Lin EH, Katon W, Von Korff M, Tang L, Williams JW, Jr, Kroenke K, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial.[see comment][comment] JAMA. 2003;290:2428–9. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 5.Dunn KM, Croft PR. Classification of low back pain in primary care: using “bothersomeness” to identify the most severe cases. Spine. 2005;30:1887–92. doi: 10.1097/01.brs.0000173900.46863.02. [DOI] [PubMed] [Google Scholar]
- 6.Karp J, Scott J, Houck P, Reynolds C, Kupfer D, Frank E. Pain predicts longer time to remission during treatment of recurrent depression. Journal of Clinical Psychiatry. 2005;66:591–7. doi: 10.4088/jcp.v66n0508. [DOI] [PubMed] [Google Scholar]
- 7.Karp J, Weiner D, Seligman K, Butters M, Miller M, Frank E, et al. Body Pain and Treatment Response in Late-Life Depression. Am J Geriatr Psychiatry. 2005;13:188–94. doi: 10.1176/appi.ajgp.13.3.188. [DOI] [PubMed] [Google Scholar]
- 8.Mavandadi S, Ten Have TR, Katz IR, Durai UN, Krahn DD, Llorente MD, et al. Effect of depression treatment on depressive symptoms in older adulthood: the moderating role of pain. Journal of the American Geriatrics Society. 2007;55:202–11. doi: 10.1111/j.1532-5415.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- 9.Fishbain DA, Bruns D, Disorbio JM, Lewis JE. Risk for five forms of suicidality in acute pain patients and chronic pain patients vs pain-free community controls. Pain Medicine. 2009;10:1095–105. doi: 10.1111/j.1526-4637.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 10.Szanto K, Mulsant BH, Houck PR, Dew MA, Dombrovski A, Pollock BG, et al. Emergence, persistence, and resolution of suicidal ideation during treatment of depression in old age. Journal of Affective Disorders. 2007;98:153–61. doi: 10.1016/j.jad.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. American Journal of Physical Medicine & Rehabilitation. 2005;84:S56–63. [PubMed] [Google Scholar]
- 12.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Medicine. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 13.Karp J, Skidmore E, Lotz M, Lenze E, Dew M, Reynolds C. Use of the Late-Life Function and Disability Instrument to Assess Disability in Major Depression. Journal of the American Geriatrics Society. 2009;57:1612–19. doi: 10.1111/j.1532-5415.2009.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karp JF, Reynolds CF, Butters MA, Dew MA, Mazumdar S, Begley AE, et al. The Relationship Between Pain and Mental Flexibility in Older Adult Pain Clinic Patients. Pain Medicine. 2006;7:444–52. doi: 10.1111/j.1526-4637.2006.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, et al. Persistence of Neuropsychologic Deficits in the Remitted State of Late-Life Depression. Am J Geriatr Psychiatry. 2006;14:419–27. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 16.Martire L, Schulz R, Karp J, Gildengers A, Whyte E, Reynolds C. Treatment of Late-Life Depression Alleviates Caregiver Burden. Journal of the American Geriatrics Society. doi: 10.1111/j.1532-5415.2009.02610.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moskowitz M, Fishman S. The neurobiological and therapeutic intersection of pain and affective disorders. Focus. 2006;IV:465–71. [Google Scholar]
- 18.Seligman ME. Learned helplessness. Annual Review of Medicine. 1972;23:407–12. doi: 10.1146/annurev.me.23.020172.002203. [DOI] [PubMed] [Google Scholar]
- 19.Lyness JM, Caine ED, King DA, Cox C, Yoediono Z. Psychiatric disorders in older primary care patients. Journal of General Internal Medicine. 1999;14:249–54. doi: 10.1046/j.1525-1497.1999.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freburger JK, Holmes GM, Agans RP, Jackman AM, Darter JD, Wallace AS, et al. The Rising Prevalence of Chronic Low Back Pain. Arch Intern Med. 2009;169:251–58. doi: 10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morone N, Karp J, Lynch C, Bost J, El Khoudary S, Weiner D. Impact of chronic musculoskeletal pathology on older adults: A study of differences between knee OA and low back pain. Pain Medicine. 2009;10:693–701. doi: 10.1111/j.1526-4637.2009.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Bair MJ, Damush TM, Wu J, Hoke S, Sutherland J, et al. Optimized Antidepressant Therapy and Pain Self-management in Primary Care Patients With Depression and Musculoskeletal Pain: A Randomized Controlled Trial. JAMA. 2009;301:2099–110. doi: 10.1001/jama.2009.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summerfelt WT, Meltzer HY. Efficacy vs. Effectiveness in Psychiatric Research. Psychiatr Serv. 1998;49:834. doi: 10.1176/ps.49.6.834. [DOI] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Buring JE, Willett W, Rosner B, Eberlein K, Hennekens CH. The 2 x 2 factorial design: its application to a randomized trial of aspirin and carotene in U.S. physicians. Statistics in Medicine. 1985;4:111–6. doi: 10.1002/sim.4780040202. [DOI] [PubMed] [Google Scholar]
- 25.Nierenberg AA, Farabaugh AH, Alpert JE, Gordon J, Worthington JJ, Rosenbaum JF, et al. Timing of Onset of Antidepressant Response With Fluoxetine Treatment. Am J Psychiatry. 2000;157:1423–28. doi: 10.1176/appi.ajp.157.9.1423. [DOI] [PubMed] [Google Scholar]
- 26.Skljarevski V, Desaiah D, Liu-Seifert H, Zhang Q, Chappell AS, Detke MJ, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine. 2010;35:E578–85. doi: 10.1097/BRS.0b013e3181d3cef6. [DOI] [PubMed] [Google Scholar]
- 27.Karp J, Weiner D, Dew M, Begley A, Reynolds C., III Duloxetine and Care Management Treatment of Older Adults with Comorbid Major Depressive Disorder and Chronic Low Back Pain: Results of an Open-Label Trial. International Journal of Geriatric Psychiatry. doi: 10.1002/gps.2386. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyness JM, Niculescu A, Tu X, Reynolds CF, III, Caine ED. The Relationship of Medical Comorbidity and Depression in Older, Primary Care Patients. Psychosomatics. 2006;47:435–39. doi: 10.1176/appi.psy.47.5.435. [DOI] [PubMed] [Google Scholar]
- 29.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. Journal of Neuroscience. 2004;24:10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19:5034–43. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montamat SC, Cusack BJ, Vestal RE. Management of drug therapy in the elderly. New England Journal of Medicine. 1989;321:303–9. doi: 10.1056/NEJM198908033210507. [DOI] [PubMed] [Google Scholar]
- 32.Arean PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. American Journal of Psychiatry. 2010;167:1391–8. doi: 10.1176/appi.ajp.2010.09091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobscha SK, Corson K, Perrin NA, Hanson GC, Leibowitz RQ, Doak MN, et al. Collaborative Care for Chronic Pain in Primary Care: A Cluster Randomized Trial. JAMA. 2009;301:1242–52. doi: 10.1001/jama.2009.377. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Löwe B, Kroenke K, Herzog W, Gräfe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) Journal of Affective Disorders. 2004;81:61–66. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 37.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 38.Herr KA, Garand L. Assessment and measurement of pain in older adults. Clinics in Geriatric Medicine. 2001;17:457–78. doi: 10.1016/s0749-0690(05)70080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 40.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis & Rheumatism. 1987;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 41.Creamer P, Flores R, Hochberg MC. Management of osteoarthritis in older adults. Clinics in Geriatric Medicine. 1998;14:435–54. [PubMed] [Google Scholar]
- 42.Hanks G, Cherny N. Opioid analgesic therapy. In: Doyle D, Hanks G, MacDonald N, editors. Oxford Textbook of Palliative Medicine. Oxford: Oxford University Press; 1998. pp. 331–55. [Google Scholar]
- 43.Pahor M, Guralnik JM, Wan JY, Ferrucci L, Penninx BW, Lyles A, et al. Lower body osteoarticular pain and dose of analgesic medications in older disabled women: the Women’s Health and Aging Study. American Journal of Public Health. 1999;89:930–4. doi: 10.2105/ajph.89.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Research. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 45.Harvey AT, Rudolph RL, Preskorn SH. Evidence of the dual mechanisms of action of venlafaxine. Archives of General Psychiatry. 2000;57:503–9. doi: 10.1001/archpsyc.57.5.503. [DOI] [PubMed] [Google Scholar]
- 46.Debonnel G, Saint-Andre E, Hebert C, de Montigny C, Lavoie N, Blier P. Differential physiological effects of a low dose and high doses of venlafaxine in major depression. International Journal of Neuropsychopharmacology. 2007;10:51–61. doi: 10.1017/S1461145705006413. [DOI] [PubMed] [Google Scholar]
- 47.Bradley RH, Barkin RL, Jerome J, DeYoung K, Dodge CW. Efficacy of venlafaxine for the long term treatment of chronic pain with associated major depressive disorder. American Journal of Therapeutics. 2003;10:318–23. doi: 10.1097/00045391-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Begre S, Traber M, Gerber M, von Kanel R. Change in pain severity with open label venlafaxine use in patients with a depressive symptomatology: an observational study in primary care. European Psychiatry: the Journal of the Association of European Psychiatrists. 2008;23:178–86. doi: 10.1016/j.eurpsy.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Kadiroglu AK, Sit D, Kayabasi H, Tuzcu AK, Tasdemir N, Yilmaz ME. The effect of venlafaxine HCl on painful peripheral diabetic neuropathy in patients with type 2 diabetes mellitus. Journal of Diabetes & its Complications. 2008;22:241–5. doi: 10.1016/j.jdiacomp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Marchand F, Alloui A, Chapuy E, Hernandez A, Pelissier T, Ardid D, et al. The antihyperalgesic effect of venlafaxine in diabetic rats does not involve the opioid system. Neuroscience Letters. 2003;342:105–8. doi: 10.1016/s0304-3940(03)00270-2. [DOI] [PubMed] [Google Scholar]
- 51.Marchand F, Alloui A, Pelissier T, Hernandez A, Authier N, Alvarez P, et al. Evidence for an antihyperalgesic effect of venlafaxine in vincristine-induced neuropathy in rat. Brain Research. 2003;980:117–20. doi: 10.1016/s0006-8993(03)02946-9. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan M, Bentley S, Fan MY, Gardner G. A single-blind placebo run-in study of venlafaxine XR for activity-limiting osteoarthritis pain. Pain Medicine. 2009;10:806–12. doi: 10.1111/j.1526-4637.2009.00637.x. [DOI] [PubMed] [Google Scholar]
- 53.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Archives of Internal Medicine. 2003;163:2716–24. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 54.Areán P, Hegel M, Vannoy S, Fan M-Y, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. Gerontologist. 2008;48:311–23. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 55.Arean PA, Cook BL. Psychotherapy and combined psychotherapy/pharmacotherapy for late life depression. Biological Psychiatry. 2002;52:293–303. doi: 10.1016/s0006-3223(02)01371-9. [DOI] [PubMed] [Google Scholar]
- 56.Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. Journal of Consulting & Clinical Psychology. 2003;71:1036–48. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 57.Nezu AM, Nezu CM, Jain D, Xanthopoulos MS, Cos TA, Friedman J, et al. Social Problem Solving and Noncardiac Chest Pain. Psychosom Med. 2007 doi: 10.1097/PSY.0b013e31815a995a. :PSY.0b013e31815a995a. [DOI] [PubMed] [Google Scholar]
- 58.Teri L, Logsdon RG, Uomoto J, McCurry SM. Behavioral treatment of depression in dementia patients: a controlled clinical trial. Journals of Gerontology Series B-Psychological Sciences & Social Sciences. 1997;52:P159–66. doi: 10.1093/geronb/52b.4.p159. [DOI] [PubMed] [Google Scholar]
- 59.Mynors-Wallis LM, Gath DH, Day A, Baker F. Randomised controlled trial of problem solving treatment, antidepressant medication, and combined treatment for major depression in primary care.[see comment] BMJ. 2000;320:26–30. doi: 10.1136/bmj.320.7226.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlinson D. Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care.[see comment] BMJ. 1995;310:441–5. doi: 10.1136/bmj.310.6977.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warmerdam L, van Straten A, Twisk J, Riper H, Cuijpers P. Internet-Based Treatment for Adults with Depressive Symptoms: Randomized Controlled Trial. Journal of Medical Internet Research. 2008;10:e44. doi: 10.2196/jmir.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hegel M, Arean PA. Problem-solving Treatment for Primary Care (PST-PC): A Treatment Manual for Depression. Hanover, NH: Dartmouth University; 2003. [Google Scholar]
- 63.Alexopoulos GS, Raue P, Arean P. Problem-Solving Therapy Versus Supportive Therapy in Geriatric Major Depression With Executive Dysfunction. Am J Geriatr Psychiatry. 2003;11:46–52. [PubMed] [Google Scholar]
- 64.van den Hout JHC, Vlaeyen JWS, Heuts PHTG, Zijlema JHL, Wijnen JAG. Secondary prevention of work-related disability in nonspecific low back pain: does problem-solving therapy help? A randomized clinical trial. Clinical Journal of Pain. 2003;19:87–96. doi: 10.1097/00002508-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 65.D’Zurilla T, Nezu A. Problem-solving therapies. In: Dobson K, editor. Handbook of cognitive-behavioral therapies. New York: Guilford Press; 2001. pp. 211–45. [Google Scholar]
- 66.Bandura A. The exercise of control. New York: Freeman; 1997. [Google Scholar]
- 67.Burton K. The Back Book. England: The Stationary Office Books; 2002. [Google Scholar]
- 68.Germain A, Moul DE, Franzen PL, Miewald JM, Reynolds CF, 3rd, Monk TH, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. Journal of Clinical Sleep Medicine. 2006;2:403–6. [PubMed] [Google Scholar]
- 69.D’Zurilla T, Nezu A, Maydeu-Olivares A. Social Problem Solving Inventory-revised (SPSI-R) North Tonawanda NY: Multi-health Systems Inc; 2002. [Google Scholar]
- 70.Heapy AA, Stroud MW, Higgins DM, Sellinger JJ. Tailoring cognitive-behavioral therapy for chronic pain: a case example. Journal of Clinical Psychology. 2006;62:1345–54. doi: 10.1002/jclp.20314. [DOI] [PubMed] [Google Scholar]
- 71.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Schulberg HC, Reynolds CF., III The Bypassing the Blues Treatment Protocol: Stepped Collaborative Care for Treating Post-CABG Depression. Psychosom Med. 2009 doi: 10.1097/PSY.0b013e3181970c1c. PSY.0b013e3181970c1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroenke K, Spitzer R. The PHQ-9: A new depression and diagnostic severity measure. Psychiatric Annals. 2002;32:509–21. [Google Scholar]
- 73.Farrar JT. What is clinically meaningful: outcome measures in pain clinical trials. Clinical Journal of Pain. 2000;16:S106–12. doi: 10.1097/00002508-200006001-00018. [DOI] [PubMed] [Google Scholar]
- 74.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2001;88:287–94. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 75.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale.[see comment] Pain. 2001;94:149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 76.Rollman B, Fischer G, Zhu F, Belnap B. Comparison of Electronic Physician Prompts versus Waitroom Case-Finding on Clinical Trial Enrollment. Journal of General Internal Medicine. 2008;23:447–50. doi: 10.1007/s11606-007-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sayar K, Aksu G, Ak I, Tosun M. Venlafaxine treatment of fibromyalgia. Annals of Pharmacotherapy. 2003;37:1561–5. doi: 10.1345/aph.1D112. [DOI] [PubMed] [Google Scholar]
- 78.Weiner DK, Sakamoto S, Perera S, Breuer P. Chronic low back pain in older adults: prevalence, reliability, and validity of physical examination findings. Journal of the American Geriatrics Society. 2006;54:11–20. doi: 10.1111/j.1532-5415.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- 79.Kirkaldy-Willis WH, Hill RJ. A more precise diagnosis for low-back pain. Spine. 1979;4:102–9. doi: 10.1097/00007632-197903000-00003. [DOI] [PubMed] [Google Scholar]
- 80.Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. Journal of Clinical Epidemiology. 1999;52:19–26. doi: 10.1016/s0895-4356(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 81.Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–4. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63:77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 83.Georgotas A, McCue RE, Cooper TB. A placebo-controlled comparison of nortriptyline and phenelzine in maintenance therapy of elderly depressed patients. Archives of General Psychiatry. 1989;46:783–6. doi: 10.1001/archpsyc.1989.01810090025004. [DOI] [PubMed] [Google Scholar]
- 84.Karp J, Whyte E, Lenze E, Dew M, Begley A, Miller M, et al. Rescue pharmacotherapy with duloxetine for SSRI non-responders in late-life depression: Outcome and tolerability. Journal of Clinical Psychiatry. 2008;69:457–63. doi: 10.4088/jcp.v69n0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whyte EM, Basinski J, Farhi P, Dew MA, Begley A, Mulsant BH, et al. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. Journal of Clinical Psychiatry. 2004;65:1634–41. doi: 10.4088/jcp.v65n1208. [DOI] [PubMed] [Google Scholar]
- 86.Raskin J, Wiltse CG, Siegal A, Sheikh J, Xu J, Dinkel JJ, et al. Efficacy of Duloxetine on Cognition, Depression, and Pain in Elderly Patients With Major Depressive Disorder: An 8-Week, Double-Blind, Placebo-Controlled Trial. Am J Psychiatry. 2007;164:900–09. doi: 10.1176/ajp.2007.164.6.900. [DOI] [PubMed] [Google Scholar]
- 87.Hopko DR, Lejuez CW, LePage JP, Hopko SD, McNeil DW. A brief behavioral activation treatment for depression. A randomized pilot trial within an inpatient psychiatric hospital. Behavior Modification. 2003;27:458–69. doi: 10.1177/0145445503255489. [DOI] [PubMed] [Google Scholar]
- 88.Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, et al. A multifaceted intervention to improve treatment of depression in primary care. Archives of General Psychiatry. 1996;53:924–32. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- 89.Glombiewski JA, Hartwich-Tersek J, Rief W. Two psychological interventions are effective in severely disabled, chronic back pain patients: a randomised controlled trial. International Journal of Behavioral Medicine. 2010;17:97–107. doi: 10.1007/s12529-009-9070-4. [DOI] [PubMed] [Google Scholar]
- 90.Cheong J, MacKinnon D, Khoo S. Investigation of Mediational Processes using Parallel Process Latent Growth Curve modeling. Structural Equation Modeling. 2003;10:238–62. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–56. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 92.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. Journal of the American Geriatrics Society. 1992;40:1221–6. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 93.Vanderploeg RD, Schinka JA, Jones T, Small BJ, Graves AB, Mortimer JA. Elderly norms for the Hopkins Verbal Learning Test-Revised. Clinical Neuropsychologist. 2000;14:318–24. doi: 10.1076/1385-4046(200008)14:3;1-P;FT318. [DOI] [PubMed] [Google Scholar]
- 94.Mazumdar S, Houck PR, Liu KS, Mulsant BH, Pollock BG, Dew MA, et al. Intent-to-treat analysis for clinical trials: use of data collected after termination of treatment protocol. Journal of Psychiatric Research. 2002;36:153–64. doi: 10.1016/s0022-3956(01)00057-7. [DOI] [PubMed] [Google Scholar]
- 95.Morone NE, Weiner DK, Belnap BH, Karp JF, Mazumdar S, Houck PR, et al. The impact of pain and depression on recovery after coronary artery bypass grafting. Psychosomatic Medicine. 2010;72:620–5. doi: 10.1097/PSY.0b013e3181e6df90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thielke S, Fan M, Sullivan M, Unutzer J. Pain limits the effectiveness of collaborative care for depression. American Journal of Geriatric Psychiatry. 2007;15:699–707. doi: 10.1097/JGP.0b013e3180325a2d. [DOI] [PubMed] [Google Scholar]
- 97.Solomon DH, Rassen JA, Glynn RJ, Garneau K, Levin R, Lee J, et al. The Comparative Safety of Opioids for Nonmalignant Pain in Older Adults. Arch Intern Med. 170:1979–86. doi: 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]