Abstract
Background
Some experimental evidence suggests that uric acid impairs endothelial function. It is controversial if high uric acid levels and impaired endothelial function are related in healthy adults. In addition, the effect of uric acid on endothelial cells (ECs) of humans is unexplored.
Methods
Data of 107 healthy adult volunteers were analyzed. The association between serum uric acid and endothelial-dependant dilation (EDD) and endothelial-independent dilation (EID) was evaluated by linear regression models. We also examined the relations between uric acid and systemic and cellular markers of inflammation and oxidative stress in all or subsets of participants.
Results
Uric acid levels and EDD were not related in unadjusted or adjusted models. There was a significant negative correlation between uric acid and EID in the pooled sample (r = −0.34, P = 0.005). This correlation remained significant after adjusting for demographics (P = 0.04) and was attenuated after adjusting for other cardiac risk factors (P = 0.12). Higher serum uric acid levels were found to correlate significantly with C-reactive protein (CRP) (r = 0.31, P = 0.002). Serum uric acid levels were not associated with brachial artery EC nuclear factor-κB (NF-κB) p65 or NADPH oxidase p47phox expression or with nitrotyrosine staining, but were inversely associated with EC manganese superoxide dismutase (MnSOD) expression (r = −0.5, P = 0.01, n = 25).
Conclusion
Elevated serum uric acid is not associated with endothelial dysfunction among healthy adults, but is inversely related to EID and EC MnSOD, and positively related to systemic inflammation. These findings may have implications for cardiovascular risk in healthy adults.
Keywords: blood pressure, endothelium, hypertension, inflammation, uric acid
Endothelial dysfunction predicts cardiovascular disease1 and is central to the development and progression of atherosclerosis.2 Similarly, oxidative stress and inflammation are believed to play a role in atherosclerosis and have been shown to modulate the nitric oxide (NO) system.3 Considering the growing health burden of cardiovascular disease, risk factors that could influence endothelial function, inflammation, and oxidative stress are of great interest, such as uric acid. Uric acid is generated by the liver and other peripheral tissues as a normal byproduct of purine metabolism. Recent studies have highlighted a potential role for elevated serum uric acid levels in cardiovascular disease.4
Although the association between serum uric acid levels and ischemic cardiovascular disease is well-established in several large epidemiological studies,5,6,7 controversy exists to whether this association is causal.8,9 On one hand, under physiological concentrations, urate (the soluble form of uric acid) is a powerful antioxidant that can scavenge superoxide, hydroxyl radicals, and singlet oxygen.10 In addition, xanthine oxidase, the enzyme that converts hypoxanthine to xanthine, and xanthine to uric acid, also generates reactive oxidative species and may play a role in vascular disease11 suggesting that uric acid is merely a marker for disease. Alternatively, uric acid can react with NO irreversibly in vitro leading NO depletion12 and may cause oxidative stress once internalized by the cells.13 In vascular smooth muscle cells, uric acid induces inflammatory mediators such as C-reactive protein (CRP) and activates nuclear factor-κB (NF-κB).14,15 In some animal models, even mild elevation in uric acid levels has been reported to induce endothelial dysfunction16 and inflammatory cytokines,17 while other animal studies have shown the opposite.18 Similarly, it remains unclear if circulating uric acid concentrations are related to endothelial function among healthy adults.19,20 Importantly, the relation between uric acid and markers of inflammation and oxidative stress in endothelial cells (ECs) obtained in vivo from humans is unknown.
Hence, we hypothesized that increased serum uric acid levels are associated with impaired endothelial function and with increased systemic and cellular inflammation and oxidative stress in healthy adults. We used a community based cohort in Boulder, Colorado to test our hypothesis. Endothelial-dependant dilation (EDD) was assessed by brachial artery flow-mediated dilation and endothelial-independent dilation (EID) was assessed by change in diameter after nitroglycerin (NTG) administration. ECs were collected from the brachial arteries of the participants and immunofluorescence was performed for markers of inflammation and oxidative stress.
Methods
Study population. Data for this analysis were collected as part of a community-based study in Boulder, Colorado. The analysis included 107 adult participants (20–78 years) who had complete data on EDD and serum uric acid levels. Subjects were healthy with no history of diabetes, hypertension, or known cardiovascular disease, as assessed by history, physical examination, biochemical tests, and electrocardiogram. None of the subjects were smokers or taking medications (prescription or over the counter medications), hormone replacement therapy, or dietary supplements (including those with antioxidant properties). All procedures were approved by the Human Research Committee of the University of Colorado at Boulder. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation.
Predictor and outcome. The independent variable of interest was serum uric acid levels (mg/dl). Serum uric acid levels were measured on stored baseline samples via the Clinical Analyzer utilizing an uricase-based commercial kit as previously described.21
The dependant variable of interest was EDD estimated via brachial artery flow-mediated dilation with upper forearm cuff position. In addition, we examined the association between uric acid and EID assessed by measurement of brachial artery dilation in response to sublingual NTG given at a dose of 0.4 mg. Baseline shear rate, peak shear rate during flow-mediated dilation (FMD), and EID were determined using duplex ultrasonography (Power Vision 6000; Toshiba, Tochigi, Japan) with a linear array transducer as described previously.22 Responses are expressed as absolute (mm) and percent change from baseline diameter as per recent guidelines (see Supplementary Materials and Methods and Supplementary Tables S1 and S2 online).23
Other outcomes. To examine the association between serum uric acid levels and systemic inflammation and oxidative stress, we elected CRP and oxidized low-density lipoprotein (oxLDL), respectively. CRP was measured by an Olympus AU400e Chemistry Analyzer (Olympus, Melville, NY) and is reported in mg/l. oxLDL was determined with an ELISA plate assay (Alpco Diagnostics, Salem, NH) and is reported as U/l.
We then sought to understand the relationship between serum uric acid levels and markers of inflammation and oxidative stress in ECs of the participants. NF-κB p65 subunit (n = 35) and NADPH oxidase enzyme subunit p47phox (n = 32) were evaluated due to prior literature suggesting they mediate uric acid-induced injury.15,24 To obtain a more comprehensive understanding of cellular oxidative stress, in addition to staining for pro-oxidant NADPH oxidase p47phox, we stained for the antioxidant enzyme manganese superoxide dismutase (MnSOD) (n = 25). Nitrotyrosine (n = 43) was also evaluated as a marker of oxidative damage. The ECs were collected as previously described,25 and the detailed methods are included in the Supplementary Materials and Methods and Supplementary Tables S1 and S2 online. The small number of participants with immunostaining is due to the difficulty of obtaining molecular data in humans.
Other covariates. Covariates were selected for inclusion in the multivariate model-based on their physiological relevance and the literature. Demographics included were: age (years), gender (male vs. female), and ethnicity (Caucasian, Hispanic, Asian, and African American). Body mass index (BMI) was calculated from height and weight to the nearest 0.1 kg. To account for central obesity, waist circumference (cm) was included. Arterial blood pressure was measured over the brachial artery during seated rest using a semiautomated device (Dynamap XL; Johnson & Johnson, New Brunswick, NJ).26 Plasma low-density lipoprotein cholesterol and glucose were determined using standard assays and are reported in mg/dl. All measurements were performed at the University of Colorado at Boulder Clinical and Translational Research Center after an overnight fast (water only) and a 24-h abstention from alcohol and vigorous physical activity. Chronic alcohol intake was not correlated with EDD or with serum uric acid levels and as such was not included in the final model.
Statistical analysis. ANOVA with a Tukey–Kramer P value adjustment was used to compare covariates among quartiles of uric acid. χ2-test of independence was used to compare categorical variables among quartiles of uric acid. In order to examine the association of serum uric acid levels and vascular function, serum uric acid levels were modeled as a continuous variable in univariate and multivariate models. For n = 107, we calculated that we had 80% power to detect a correlation r = 0.267 with a two-sided α = 0.05. Multiple linear regression was used to test for a linear relationship of uric acid with FMD % Δ and NTG dilation % Δ. Two sequential sets of covariates were considered. In model 1, the covariates included age, gender, and ethnicity. In model 2, the covariates included those in model 1 in addition to BMI, waist circumference, systolic blood pressure, low-density lipoprotein cholesterol, fasting blood glucose, and baseline diameter. A detailed description of the number of participants in each model is included in the Supplementary Materials and Methods and Supplementary Tables S1 and S2 online. Scatter plots and Pearson correlation coefficients were used to show the unadjusted relationship of uric acid with EDD (FMD % Δ) and EID (NTG % Δ). The correlation between uric and CRP and oxLDL was examined using unadjusted and adjusted linear regression. Similarly, linear regression was employed to examine the relation between uric acid levels and the immunostains designated above. All analyses were conducted using SAS 9.2 (Research Triangle Institute, Research Triangle Park, NC). P < 0.05 was considered statistically significant.
Results
Characteristics of participants by serum uric acid quartile
Characteristics of the participants according to quartiles of uric acid are shown in Table 1. Individuals with the highest quartile of uric acid were older and more likely to be male when compared to the lowest quartile of uric acid. Higher serum uric acid levels were associated with significantly higher BMI and fasting blood glucose levels. There were no significant differences in EDD (FMD % Δ) across increasing uric acid levels. However, there was a tendency for EID (NTG % Δ) to decrease across increasing quartiles of uric acid. This tendency did not achieve statistical significance (P = 0.09). Serum CRP levels increased significantly with increased uric acid quartiles (P < 0.005). There was a trend towards increased oxLDL levels with increasing serum uric acid levels but these differences were not found to be statistically significant.
Table 1. Subject characteristics according to serum uric acid quartiles (mg/dl).
Association between serum uric acid levels and EDD (FMD % Δ)
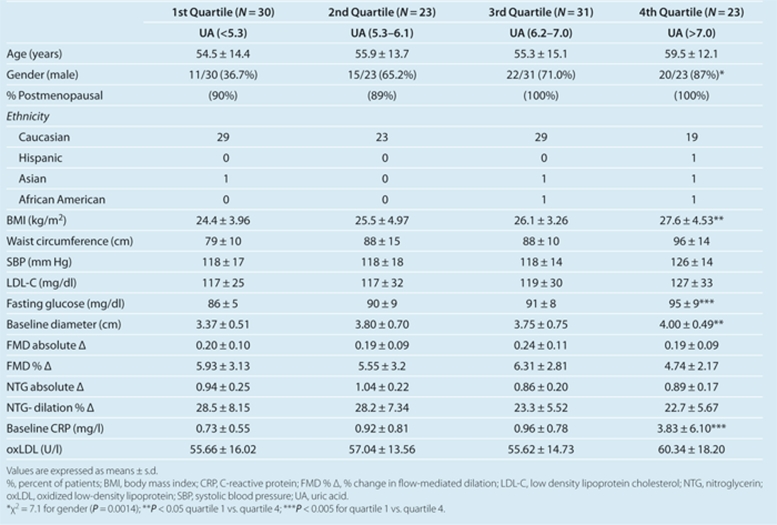
There was no correlation between serum uric acid levels and FMD as depicted in Figure 1a; the unadjusted Pearson correlation was r = 0.07, P = 0.46. Similarly, multiple linear regression adjusting for age, gender, and ethnicity (model 1) demonstrated no correlation between uric acid and FMD % Δ (β = –0.03 ± 0.25, P = 0.89), and linear regression adjusting for age, gender, ethnicity, BMI, waist circumference, systolic blood pressure, low-density lipoprotein cholesterol, and fasting blood glucose (model 2) demonstrated no correlation between uric acid and FMD % Δ (β = 0.05 ± 0.27, P = 0.86).
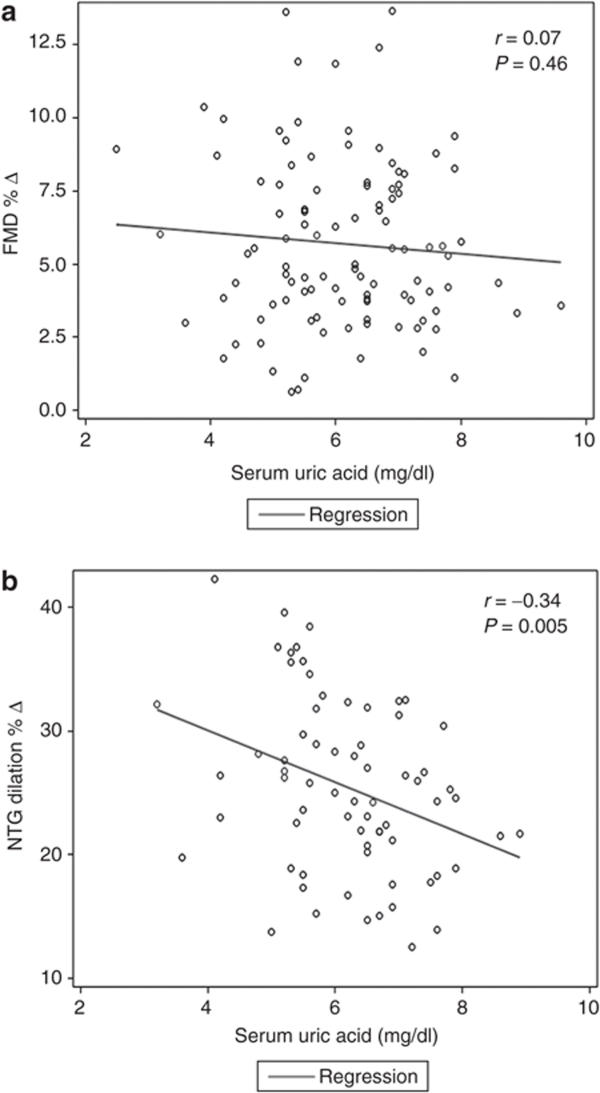
Figure 1.
Scatter plots for vascular function measurements against uric acid levels. (a) Scatter plot of FMD % Δ against serum uric acid levels (mg/dl). (b) Scatter plot of NTG % Δ against serum uric acid levels (mg/dl). FMD % Δ = % change in flow-mediated dilation; NTG, nitroglycerin
Association between serum uric acid levels and EID (NTG % Δ)
Seventy subjects had no contraindication to NTG administration and underwent EID measurement. Results of the unadjusted analysis are shown in Figure 1b. The unadjusted correlation between uric acid and NTG dilation % was r = –0.34, P = 0.005. The association between serum uric acid levels and EID remained significant after adjusting for age, gender, and ethnicity (β = –1.57 ± 0.75, P = 0.04; model 1). There was a negative correlation between uric acid and NTG dilation % after adjusting for all other covariates, but this did not achieve statistical significance (β = –1.25 ± 0.80, P = 0.12; model 2).
Relationship between serum uric acid levels and markers of systemic inflammation and oxidative stress
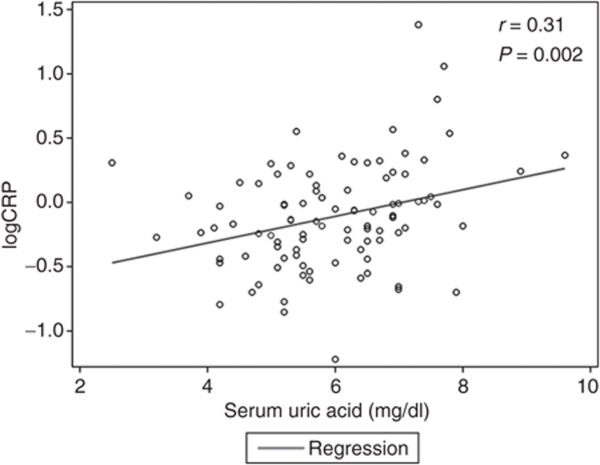
Linear regression was initially performed between uric acid and CPR levels both as continuous variables, and on initial analysis there were some highly influential points. This was confirmed by influence diagnostics. Hence, a log 10 transformation was applied and a better fit was obtained. In Figure 2, we show that there is a significant relationship between uric acid and CRP with a correlation coefficient of 0.31 (P = 0.002) in unadjusted analysis. This relation was attenuated after adjusting for age, gender, and ethnicity (P = 0.28), and remained not significant after adjusting for all the covariates included in model 2 (P = 0.43). Consistent with our findings in Table 1, there was no correlation between serum uric acid levels and oxLDL when they were both examined as continuous variables (P = 0.43 in unadjusted analysis, and P = 0.82 in the fully-adjusted model).
Figure 2.
Scatter plot of C-reactive protein (CRP) against serum uric acid levels (mg/dl).
Relation between serum uric acid levels and ECs markers of inflammation and oxidative stress
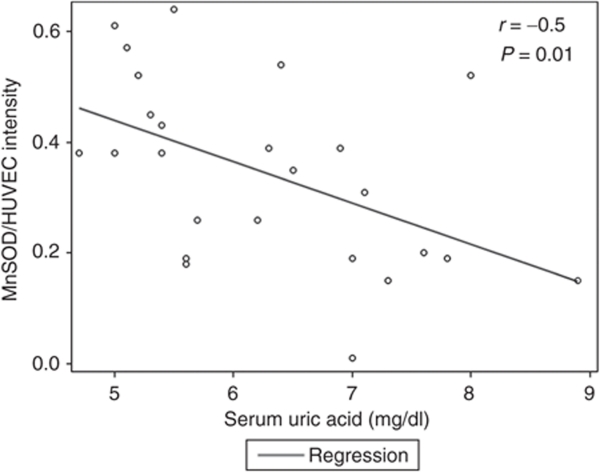
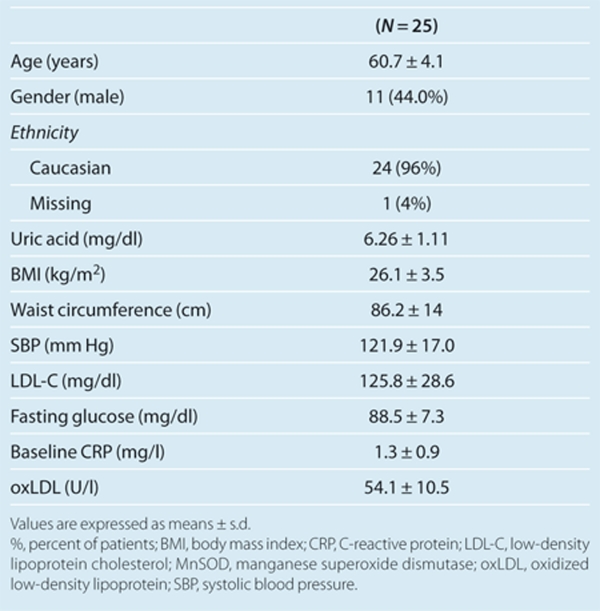
There was no correlation between serum uric acid levels and the expression of NF-κB p65 or NADPH oxidase p47phox or nitrotyrosine staining in brachial artery ECs. In contrast, we found that higher uric acid levels were inversely related to the expression of MnSOD in our participants with a correlation coefficient of –0.50 and a P value of 0.01. The scatter plot is shown in Figure 3. To further illustrate our findings, mean values and representative images of the immunostains are shown according to serum uric acid quartiles in Figure 4. Descriptive data for the subgroup with available MnSOD staining (n = 25) are shown in Table 2.
Figure 3.
Scatter plot of manganese superoxide dismutase (MnSOD) protein expression against serum uric acid levels (mg/dl)
Figure 4.
Expression of markers of inflammation and oxidative stress in brachial artery endothelial cells according to serum uric acid quartiles. Values represent ratios of subject endothelial protein expression to HUVEC control, and are shown as mean ± s.d. Unpaired t-test with Welch's correction was used to compare the expression between the 1st quartile and the 2nd, 3rd, or 4th quartiles. The number of available stains is depicted on each figure. (a) NF-κB expression (n = 35), (b) nitrotyrosine abundance (n = 43), (c) NADPH oxidase protein expression (n = 32), and (d) manganese superoxide dismutase (MnSOD) protein expression (n = 25). *P = 0.04 and #P = 0.006 both in comparison to the lowest uric acid quartile (<5.3 mg/dl). UA, uric acid.
Table 2. Characteristics of the subjects with available staining for MnSOD.
Discussion
In the current analysis, we explored the potential association between uric acid and vascular function in healthy adults. Our study is the first attempt to correlate uric acid levels with molecular changes in ECs of healthy adults. We found that serum uric acid levels are not correlated with EDD in unadjusted or adjusted analysis, despite a significant positive correlation between serum uric acid levels and CRP levels (in unadjusted linear regression). On the cellular level we found no correlation between uric acid levels and NF-κB p65, NADPH oxidase p47phox, or nitrotyrosine. However, there was a significant inverse relation between serum uric acid and the expression of the antioxidant enzyme MnSOD.
The controversy surrounding the relation between uric acid and endothelial dysfunction has been fueled to some extent by the negative findings of some studies in healthy controls. For example, induced hyperuricemia did not impair endothelial function in a group of healthy controls.20 Similarly, a prior study by our group found that xanthine oxidase inhibition by allopurinol lowered uric acid levels but did not improve EDD in healthy subjects.27 Both of these studies assessed the effects of uric acid and allopurinol on endothelial function acutely rather than chronically. But our current observations are in agreement with these prior negative studies and suggest that hyperuricemia alone is not an effective mediator of endothelial dysfunction. Consistent with this conclusion, no correlation was found between uric acid levels and cellular NF-κB p65, NADPH oxidase p47phox, or nitrotyrosine (markers of endothelial inflammation and oxidative stress).
The dissociation between the expression of MnSOD and EDD in our study is intriguing. MnSOD is a member of the superoxide dismutase family of enzymes that play an important role in the regulation of local superoxide levels and have a protective effect. Homozygous MnSOD-deficient mice die in the neonatal period, suggesting that MnSOD is a key regulator of oxidative stress.28 Consistent with such a role, heterozygous MnSOD-deficient mice show greater endothelial dysfunction than wild-type mice in models of aging29 and heart failure.30 Yet, heterozygous MnSOD-deficient mice exhibit similar vasomotor responses to wild type mice under normal conditions,31 suggesting that MnSOD deficiency alone is insufficient to induce vascular dysfunction. As such, reduced MnSOD activity may impair the host's ability to cope with increased oxidative stress associated with certain conditions. Our study population is a healthy population (with no evidence of hypertension or diabetes). This could explain why the effect of uric acid on MnSOD expression was unaccompanied by significant changes in endothelial function in this analysis. After all uric acid appears to be a stronger predictor of risk in high risk groups such as African Americans,32 patients with underlying hypertension,33 congestive heart failure,34 and chronic kidney disease.35 Along the same lines, these findings could reconcile the different findings of studies on the topic in healthy subjects. In settings in which uric acid is related to endothelial dysfunction,19 other factors that modify the oxidative state may be involved such as sedentary lifestyle or higher dietary intake of pro-oxidants.
Another interesting observation in our study is the dissociation between systemic and cellular inflammation. Considering the in vitro evidence that CRP induces ECs dysfunction,36 these finding seem counterintuitive. However, there is evidence to indicate that certain biochemical characters of ECs are induced by CRP only in the presence of an additional adverse factors such as aldosterone,37 suggesting that high risk populations are more likely to see the ill effects of any one mediator. Another explanation for our data may be that systemic inflammation does not always reflect cell-, tissue- or organ-specific inflammation.
Rather than EDD, we found an association between uric acid and EID. This association remained significant after adjusting for demographics, and was attenuated after adjusting for potential mechanisms such as systolic blood pressure, serum glucose, and LDL cholesterol. These findings are consistent with previous studies in which uric acid has been shown to induce inflammation (including CRP expression) in cultured vascular smooth muscle cells by activating the mitogen-activated protein kinase pathway.14 In turn, activation of the mitogen-activated protein kinase pathway has been shown to play a role in vascular smooth muscle proliferation.38 Differential associations between cardiovascular risk factors and EDD and EID have been reported in the literature. Notably, lower EID as opposed to EDD has been reported in association with various other elements of the metabolic syndrome such as insulin resistance, increased triglycerides, and higher BMI.39,40 Our findings are consistent with these previous reports, and suggest that uric acid correlates with reduced vascular smooth muscle sensitivity to NO. One potential explanation for the lack of correlation between uric acid and EDD despite the in vitro evidence for uric acid suppressing NO bioavailability, is that individuals with mild hyperuricemia may have greater compensatory flow-mediated dilation in response to non-NO endothelium-synthesized dilator molecules such as vasodilatory prostaglandins and/or endothelium-dependent hyperpolarizing factors.41 Alternatively, uric acid may modulate the response to NO differently in endothelial vs. vascular smooth muscle cells.
Our study has several limitations. First this is a cross-sectional observational study rather than an interventional study, and cause and effect between uric acid and inflammation and oxidative stress or EID cannot be established based on such an analysis. Second, the majority of participants with elevated uric acid in our study were men, and uric acid may be a stronger risk factor for cardiovascular disease in women.42 Of note, an interaction term for gender was not significant. In addition, the majority of our participants had mild hyperuricemia and we cannot exclude a relation between severe hyperuricemia and endothelial dysfunction in healthy adults based on our results.43 Finally, the number of participants with immunostaining of ECs was small and although the relationship between uric acid levels and MnSOD is striking, other studies are needed to verify our findings.
In conclusion, uric acid is not associated with vascular endothelial dysfunction in healthy adults. Although serum uric acid levels correlated with circulating CRP in our population, there was no correlation between serum uric acid and the expression of cellular markers of inflammation. Increased serum uric acid levels, however, were associated with reduced ECs expression of the antioxidant enzyme, MnSOD. In addition increased uric acid levels were associated with reduced vascular smooth muscle sensitivity to NO. These findings may help explain the inconsistent association between uric acid and endothelial dysfunction in different studies.
Acknowledgments
This study was funded by the following grants: AG013038, AG006537, RR025780, AG033994, 1K23DK088833–01, R01 DK081473-01A1, R01DK078112-01A2, as well by the Veteran's Administration Eastern Colorado Health Care System. We acknowledge Eric Chung for his help with obtaining the immunofluorescence images.
The authors declared no conflict of interest.
Footnotes
Supplementary material is linked to the online version of the paper at http://www.nature.com/ajh
Supplementary Material
References
- Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32S–39S. doi: 10.1016/s0002-9343(98)00209-5. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson C, Lapidus L, Stendahl C, Waldenström J. Hyperuricaemia and risk of cardiovascular disease and overall death. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Acta Med Scand. 1988;224:549–555. [PubMed] [Google Scholar]
- Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationship to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med. 1973;132:401–410. doi: 10.1001/archinte.132.3.401. [DOI] [PubMed] [Google Scholar]
- Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- Esen AM, Akcakoyun M, Esen O, Acar G, Emiroglu Y, Pala S, Kargin R, Karapinar H, Ozcan O, Barutcu I. Uric acid as a marker of oxidative stress in dilatation of the ascending aorta. Am J Hypertens. 2011;24:149–54. doi: 10.1038/ajh.2010.219. [DOI] [PubMed] [Google Scholar]
- Coutinho Tde A, Turner ST, Peyser PA, Bielak LF, Sheedy PF 2nd, Kullo IJ. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am J Hypertens. 2007;20:83–89. doi: 10.1016/j.amjhyper.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boueiz A, Damarla M, Hassoun PM. Xanthine oxidoreductase in respiratory and cardiovascular disorders. Am J Physiol Lung Cell Mol Physiol. 2008;294:L830–L840. doi: 10.1152/ajplung.00007.2008. [DOI] [PubMed] [Google Scholar]
- Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27:967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, Patel J. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol, Cell Physiol. 2008;295:C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Netea MG, Kullberg BJ, Blok WL, Netea RT, van der Meer JW. The role of hyperuricemia in the increased cytokine production after lipopolysaccharide challenge in neutropenic mice. Blood. 1997;89:577–582. [PubMed] [Google Scholar]
- Kurra V, Eräranta A, Jolma P, Vehmas TI, Riutta A, Moilanen E, Tahvanainen A, Kalliovalkama J, Niemelä O, Myllymäki J, Mustonen J, Pörsti I. Hyperuricemia, oxidative stress, and carotid artery tone in experimental renal insufficiency. Am J Hypertens. 2009;22:964–970. doi: 10.1038/ajh.2009.109. [DOI] [PubMed] [Google Scholar]
- Erdogan D, Gullu H, Caliskan M, Yildirim E, Bilgi M, Ulus T, Sezgin N, Muderrisoglu H. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005;59:1276–1282. doi: 10.1111/j.1742-1241.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- Waring WS, Adwani SH, Breukels O, Webb DJ, Maxwell SR. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart. 2004;90:155–159. doi: 10.1136/hrt.2003.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal DI, Rivard CJ, Johnson RJ, Maahs DM, McFann K, Rewers M, Snell-Bergeon JK. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study. Nephrol Dial Transplant. 2010;25:1865–1869. doi: 10.1093/ndt/gfp740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension. 2008;52:72–79. doi: 10.1161/HYPERTENSIONAHA.108.111427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Sánchez-Lozada LG, Soto V, Tapia E, Avila-Casado C, Sautin YY, Nakagawa T, Franco M, Rodríguez-Iturbe B, Johnson RJ. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295:F1134–F1141. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor- kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol (Lond) 2006;571:661–668. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann K, Törnig J, Flechtenmacher C, Nabokov A, Mall G, Ritz E. Blood-pressure-independent wall thickening of intramyocardial arterioles in experimental uraemia: evidence for a permissive action of PTH. Nephrol Dial Transplant. 1995;10:2043–2048. [PubMed] [Google Scholar]
- Brown AJ, Slatopolsky E. Drug insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nat Clin Pract Endocrinol Metab. 2007;3:134–144. doi: 10.1038/ncpendmet0394. [DOI] [PubMed] [Google Scholar]
- Miller JD, Peotta VA, Chu Y, Weiss RM, Zimmerman K, Brooks RM, Heistad DD. MnSOD protects against COX1-mediated endothelial dysfunction in chronic heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1600–H1607. doi: 10.1152/ajpheart.01108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andresen JJ, Faraci FM, Heistad DD. Vasomotor responses in MnSOD-deficient mice. Am J Physiol Heart Circ Physiol. 2004;287:H1141–H1148. doi: 10.1152/ajpheart.01215.2003. [DOI] [PubMed] [Google Scholar]
- Dyer AR, Liu K, Walsh M, Kiefe C, Jacobs DR, Jr, Bild DE. Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21. doi: 10.1038/sj.jhh.1000740. [DOI] [PubMed] [Google Scholar]
- Alderman MH, Cohen H, Madhavan S, Kivlighn S. Serum uric acid and cardiovascular events in successfully treated hypertensive patients. Hypertension. 1999;34:144–150. doi: 10.1161/01.hyp.34.1.144. [DOI] [PubMed] [Google Scholar]
- Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V. Uric acid and long-term outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–1441. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- Kusche-Vihrog K, Urbanova K, Blanqué A, Wilhelmi M, Schillers H, Kliche K, Pavenstädt H, Brand E, Oberleithner H. C-reactive protein makes human endothelium stiff and tight. Hypertension. 2011;57:231–237. doi: 10.1161/HYPERTENSIONAHA.110.163444. [DOI] [PubMed] [Google Scholar]
- Gennaro G, Ménard C, Michaud SE, Deblois D, Rivard A. Inhibition of vascular smooth muscle cell proliferation and neointimal formation in injured arteries by a novel, oral mitogen-activated protein kinase/extracellular signal-regulated kinase inhibitor. Circulation. 2004;110:3367–3371. doi: 10.1161/01.CIR.0000147773.86866.CD. [DOI] [PubMed] [Google Scholar]
- Dengel DR, Jacobs DR, Steinberger J, Moran AM, Sinaiko AR. Gender differences in vascular function and insulin sensitivity in young adults. Clin Sci. 2011;120:153–160. doi: 10.1042/CS20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med. 2001;250:29–36. doi: 10.1046/j.1365-2796.2001.00843.x. [DOI] [PubMed] [Google Scholar]
- Parker BA, Tschakovsky ME, Augeri AL, Polk DM, Thompson PD, Kiernan FJ. Heterogenous vasodilator pathways underlie flow-mediated dilation in men and women. Am J Physiol Heart Circ Physiol. 2011;301:H1118–H1126. doi: 10.1152/ajpheart.00400.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboa Eboule AC, De Smet P, Dramaix M, De Backer G, Kornitzer M. [Relation between uricemia and total, cardiovascular and coronary mortality in both genders of non-selected sample of the Belgium population] Rev Epidemiol Sante Publique. 2001;49:531–539. [PubMed] [Google Scholar]
- Tomiyama H, Higashi Y, Takase B, Node K, Sata M, Inoue T, Ishibashi Y, Ueda S, Shimada K, Yamashina A. Relationships among hyperuricemia, metabolic syndrome, and endothelial function. Am J Hypertens. 2011;24:770–774. doi: 10.1038/ajh.2011.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.