Abstract
BACKGROUND
The androgen receptor (AR) plays a critical role in prostate cancer development and progression. Therefore, the inhibition of AR function is an established therapeutic intervention. Since the expression of the AR is retained and often increased in progressive disease, AR protein down-regulation is a promising therapeutic approach against prostate cancer. We show here that the curcumin analog 27 (ca27) down-regulates AR expression in several prostate cancer cell lines.
METHODS
ca27 at low micromolar concentrations was tested for its effect on AR expression, AR activation, and induction of oxidative stress in human LNCaP, C4-2, and LAPC-4 prostate cancer cells.
RESULTS
ca27 induced the down-regulation of AR protein expression in LNCaP, C4-2, and LAPC-4 cells within 12 hr. Further, ca27 led to the rapid induction of reactive oxygen species (ROS). To further support this finding, ca27 treatment led to the activation of the cellular redox sensor NF-E2-related factor 2 (Nrf2) and the induction of the Nrf2-regulated genes NAD(P)H quinone oxidoreductase 1 and aldoketoreductase 1C1. We show that ROS production preceded AR protein loss and that ca27-mediated down-regulation of the AR was attenuated by the antioxidant, N-acetyl cysteine.
CONCLUSIONS
ca27 induces ROS and mediates AR protein down-regulation through an oxidative stress mechanism of action. Our results suggest that ca27 represents a novel agent for the elucidation of mechanisms of AR down-regulation, which could lead to effective new anti-androgenic strategies for the treatment of advanced prostate cancer.
Keywords: prostate cancer, androgen receptor, curcumin analog, oxidative stress
INTRODUCTION
The androgen receptor (AR) is a ligand activated steroid hormone receptor and a key regulator of both normal prostate development and function [1]. The AR plays a critical role in both prostate cancer development and progression [2]. Consequently, the current therapeutic strategies for prostate cancer intervention, such as androgen ablation therapy [3] target the inhibition of AR function. Such treatment, in its most aggressive form is based on combinations of androgen synthesis suppression and AR inhibition [4]. Fortunately, the majority of men undergoing androgen ablation therapy successfully respond to this therapy. However, the median response to androgen ablation is typically <2 years, and patients recur with progressive disease within 12–18 months, developing androgen ablation resistant cancer [5]. This advanced stage is characterized by the continuous expression and function of the AR in the presence of low concentrations of androgens [6,7]. Under these conditions, the AR supports prostate cancer cell survival, as the down-regulation of AR protein in androgen ablation resistant prostate cancer cells and animal models leads to cell growth inhibition and death [8,9]. These findings emphasize the importance of the AR and its signaling axis for all stages of prostate cancer, thus rendering it a prominent and promising target [2,4,10,11]. Therefore, the identification of chemical agents that down-regulate AR expression by known or novel mechanisms warrant further investigation for development as a novel prostate cancer therapeutic approach.
We have previously reported the synthesis of an enone analog chemical library of the natural diphenolic product curcumin (diferuloylmethane, or 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione) [12–14]. In the present study, we report on a compound from this library, curcumin analog 27 (ca27) [14]. ca27 belongs to a series of symmetrical diphenolic analogs which in contrast to curcumin feature a shorter 5-carbon unsaturated linker with a single carbonyl group (Fig. 1A) [14]. The two phenolic rings of ca27 feature symmetrical ortho-hydroxyl groups. The carbon linker retains the character of an α,β-unsaturated ketone, which has properties of a Michael acceptor for strong nucleophilic groups [15]. Structure analysis relationship studies reported by several other groups indicate that this property is responsible for conferring the anti-proliferative abilities of curcumin analogs [15,16].
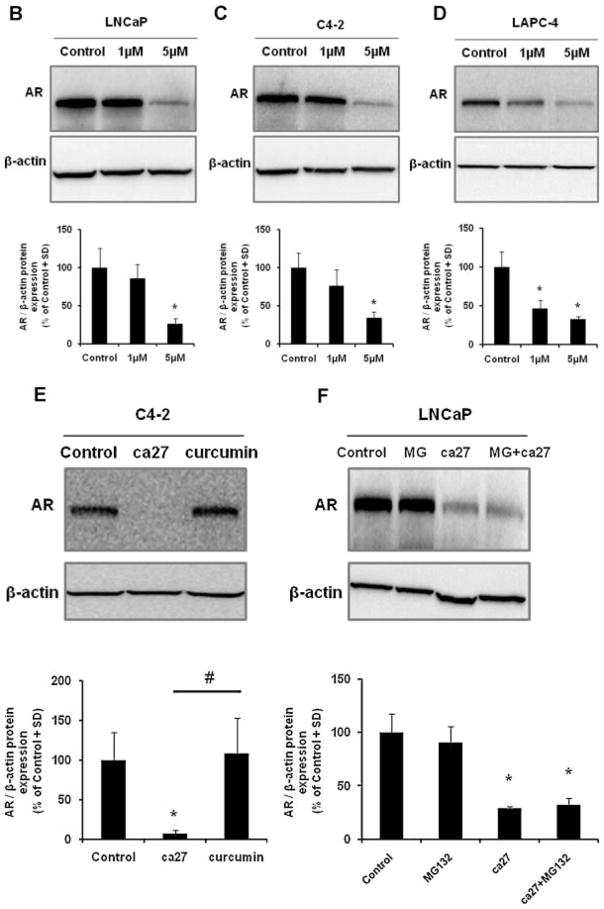
Fig. 1.
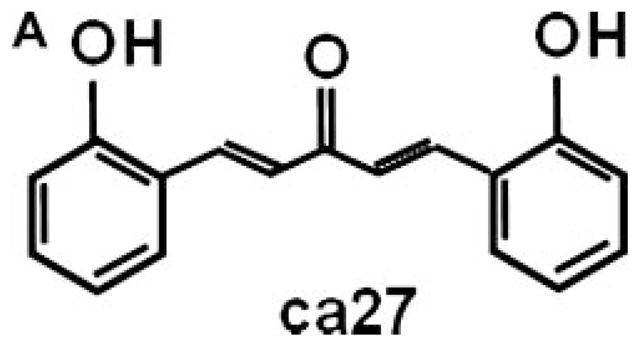
Structure of ca27 and down-regulation of AR protein expression in prostate cancer cell lines. ca27 (1,5-Bis(2-hydroxyphenyl)-1,4-pentadien-3-one) (A) consists of two phenolic rings with symmetrical hydroxyl groups on the ortho position of the aryl rings, which are linked by an unsaturated 5-carbon spacer with a single carbonyl. The synthesis of ca27 was previously described in Weber et al. 2006 [14]. Down-regulation of AR protein expression ca27 in LNCaP, C4-2 and LAPC-4 cells. LNCaP (B), C4-2 (C) and LAPC-4 (D) cells were treated with 1 and 5 μM ca27. AR protein was measured by western blotting and densitometric analysis (ratio AR: β-actin) after 12 hours. LNCaP (E) cells were treated with 20 μM ca27 or curcumin for 72 hours AR protein expression was measured and quantitated as described above. LNCaP (F) cells were pretreated with 10 μ MMG132f or 1 hour before the addition of 5 μM ca27 for 6 hours. One representative western blot is shown; bars in the graph represent the average of triplicate values + standard deviation. * denote P < 0.05comparedto control.
In the current study, we have demonstrated that ca27 mediates the down-regulation of AR protein expression and activity. We further provide a potential mechanism of action for ca27 on the AR by studying its effect on the redox status in prostate cancer cells. We show that ca27 induced the generation of intracellular reactive oxygen species (ROS) by the 2′,7′-dichlorofluorescein diacetate (DCF) assay. In support of this finding, ca27 increased the activation of the cellular redox sensor, NF-E2-related factor 2 (Nrf2), followed by expression of the Nrf2 regulated detoxification genes, NAD(P)H: quinone oxidoreductase 1 (NQO1) and aldoketoreductase 1C1 (AKR1C1). Finally, we show that the antioxidant N-acetyl-L-cysteine abrogates ca27 mediated AR down-regulation, which provides further support that ca27 induced AR protein loss is mediated by oxidative stress. Importantly, ca27 and similar curcumin analogs represent a novel class of agents for the elucidation of mechanisms of AR down-regulation in prostate cancer cells which could lead to effective new anti-androgenic strategies for the treatment of advanced prostate cancer.
MATERIALS AND METHODS
Chemical Reagents
The curcumin analog 27 (ca27) (1,5-Bis(2-hydroxyphenyl)-1,4-pentadien-3-one) was synthesized and characterized as previously described [14]. This diphenolic chemical was solubilized in 100% dimethyl sulfoxide (DMSO) stored protected from light at 4°C. The synthetic androgen methyltrienolone (R1881) was from Perkin Elmer/NEN Life Science Products (Boston, MA). MG132, N-acetyl-L-cysteine (NAC) and actinomycin D (Act D) were from Sigma Chemical Co. (St. Louis, MO).
Cell Culture and Treatment Protocols
The human prostate cancer cell lines LNCaP (American Type Culture Collection, Manassas, VA), C4-2 (gift from Dr. G.N. Thalmann, University of Bern, Switzerland) and a variant of the LAPC-4 (acquired from Dr. George Wilding, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) supplemented with 5% heat-inactivated fetal bovine serum (FBS) and streptomycin–penicillin antibiotics (DMEM/FBS). To evaluate androgenic responses cells were cultured in DMEM containing 4% charcoal-stripped FBS and 1% heat-inactivated FBS (DMEM/CSS). All cells were maintained at 37°C in a humidified 5% CO2 atmosphere. ca27 was added to the cells for the indicated lengths of time and final concentrations. Vehicle controls never amounted to a final concentration of >0.1% DMSO.
Cell Proliferation and Viability Assays
Cells were plated in quadruplicate in 12-well tissue culture plates (Invitrogen) in DMEM/FBS and treated with ca27 at the indicated final concentrations for 96 hr. After cell detachment in 2.5% Trypsin/EDTA (Invitrogen), cell proliferation was determined by total cell count in a hemacytometer by light microscopy. Viability was determined by trypan blue dye exclusion (0.4%; Sigma). Results are expressed as percent of vehicle control.
Promoter Activation Assays
Cells were cultured in quadruplicate in 24-well plates (Invitrogen) in DMEM/CSS. After 48 hr, cells were co-transfected with a reporter plasmid carrying a mouse mammary tumor virus (MMTV) promoter regulating luciferase cDNA expression [17] and a control plasmid carrying a thymidine kinase (TK) promoter regulating Renilla luciferase cDNA expression (Promega, Madison, WI) using Lipofectamine 2000 transfection agent (Invitrogen). Twenty-four hours post-transfection, cells were treated with ca27 at the indicated concentrations for 24 hr. After stimulation with 1 nM R1881 for 6 hr, whole cell extracts were generated using Cell Culture Lysis Reagent (Promega, Madison, WI). Luciferase activity was measured using the Luciferase Assay Substrate kit (Promega, Madison, WI) and relative luciferase units determined on a Perkin Elmer Victor3V 1420 counter and analyzed using Wallac 1420 software (Perkin Elmer, Turku, Finland). Cells were cultured as described above and co-transfected with a reporter plasmid carrying an antioxidant response element promoter regulating luciferase cDNA expression, pNQO1hARE [18] and the control TK promoter plasmid. Forty-eight hours post-transfection, cells were treated with the indicated concentrations of ca27 for 16 hr. Luciferase activity was determined as outlined above. Normalized luciferase expression is expressed as a percent of vehicle control.
AR activation was further measured using the multifunctional androgen receptor screening (MARS) assay [19]. Androgen independent PC-3 human prostate cancer cells were co-transfected with a wild-type AR expressing plasmid and a plasmid carrying an MMTV promoter containing an AR response element driving destabilized enhanced green fluorescent protein (dsEGFP). In this assay, AR activation is stimulated by R1881 at 1 nM. Images of fluorescent cells were captured using an Olympus IX70 inverted fluorescent microscope and fluorescence was quantified by ImageJ software [20]. The number of fluorescent cells was expressed as percent of control.
mRNA Expression Analysis by Quantitative (Real Time) Reverse Transcriptase Polymerase Chain Reaction (qRT-PCR)
Cells were cultured in quadruplicate in 24-well plates (Invitrogen) in DMEM/FBS and treated with ca27 for 3 or 12 hr at the indicated concentrations. Total RNA was extracted using TRIzol Reagent (Invitrogen) and cDNA was prepared using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). PCR was performed using an Applied Biosystems 7900HT Fast Real-Time PCR System (Carlsbad, CA). PCR cycling parameters were 95°C for 10 min followed by 40 cycles of 95°C for 15 sec, and 60°C for 1 min. Forward and reverse primers for the AR and the normalization control gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were available in the QuantiTect Primers Assays from Qiagen (Valencia, CA). Forward and reverse primers for PSA, NQO1, AKR1C1 and MafG were purchased from Integrated DNA Technologies (Coralville, IA). PSA forward primer sequence is 5′-CGCTGGACAGGGGGCAAAA-3′ and the reverse primer sequence is 5′-ACAAGTGGGCCCCCAGAA-TCA-3′. NQO1 forward primer sequence is 5′-TG-AGCTCGAGCCCCGGACTGCACCAGA-3′ and the reverse primer sequence is 5′-CTACCGCGGCAAGT-CAGGGAAGCCTGGAAAGAT-3′. AKR1C1 forward primer sequence is 5′-GATGGCCTAAACAGAAA-TGTGCGAT-3′ and the reverse primer sequence is 5′-GGATAATTAGGGGGGCCAGCAA-3′. MafG forward primer sequence is 5′-GCTGTGCCCCCGGG-TTATGA-3′ and the reverse primer sequence is 5′-CCGTCAGGCTGGTGCCATTCT-3′. AR, PSA, NQO1, AKR1C1, and MafG mRNA expression levels normalized to GAPDH were determined using the ΔΔCt method and are shown relative to control.
ROS Detection by DCF
Cells were cultured in 96-well plates (Corning Inc., Corning, NY) in DMEM/FBS for 48 hr and then treated with ca27 for 1 hr at the indicated concentrations. Cells were analyzed for the formation of ROS by use of the fluorescent probe, DCF (Invitrogen) as described by Basu et al. [21]. DCF fluorescent units per well were measured 1 hr after DCF addition. DNA content per well was measured by the Hoechst 33258 dye (Sigma) [22]. Fluorescence measurements for both the DCF assay and Hoechst dye were taken using a TECAN plate reader (TECAN Austria GmbH, Salzburg, Austria) and analyzed with Magellan software. Over 12 replicates were used per treatment group. Hoechst dye normalized DCF fluorescent units are shown relative to control.
Protein Expression by Western Blot
Cells were cultured in quadruplicate in 12-well plates (Invitrogen) in DMEM/FBS and treated with ca27 for 12 hr at the indicated concentrations. Cells were washed in cold phosphate buffered saline (PBS) and whole cell extracts were generated using 1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.1 mg/ml phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and 10 μg/ml aprotinin in PBS. Protein concentrations were determined using the BCA Proteins Assay kit (Pierce Biotechnology, Rockford, IL). Protein (30 μg) were size-separated by SDS polyacryalmide gel electrophoresis (SDS–PAGE) in triplicate in 12.5% gels (BioRad, Hercules, CA) and electro-transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA) using a GENIE wet transfer system (Idea Scientific, Minneapolis, MN). Membranes were blocked in Trizma base (Tris) buffered saline (TBS) containing 5% nonfat dry milk at 4°C and then incubated with mouse anti-AR monoclonal antibody (441; Santa Cruz Biotechnology, Santa Cruz, CA) or mouse anti-β-actin monoclonal antibody (A5441; Sigma) at the concentrations indicated by the manufacturers. After washing in TBS, the membranes were incubated with a horseradish peroxidase-conjugated goat anti-mouse IgG (Biomeda, Foster City, CA). Bound antibodies were detected using Western Lightening Chemiluminescence Reagent Plus (Boston, MA) on a Kodak Image Station 4000MM (Rochester, NY). Band intensities were determined by densitometric analysis (ratio AR: β-actin) using Kodak Molecular Imaging Software (Rochester, NY). AR expression is shown relative to DMSO control.
Statistical Analysis
Significant differences in values between groups were assessed using the unpaired t-test with Sigma-Stat 3.1 software (Systat Software, San Jose, CA). P-values of <0.05 were used to signify statistical significance.
RESULTS
Inhibition of AR Expression by ca27 in Human Prostate Cancer Cells
The effects of the synthetic curcumin analog ca27 (Fig. 1A) were first determined on the endogenous AR protein expression in different human prostate cancer cell lines, i.e., LNCaP, C4-2, and LAPC-4. The cells were treated with ca27 for 12 hr at concentrations in the low micromolar range of 1 to 5 μM. Western blot analysis and densitometric quantitation revealed a significant decrease in AR protein expression in LNCaP (Fig. 1B), C4-2 (Fig. 1C), and LAPC-4 (Fig. 1D) cells treated with 5 μM ca27. ca27 (5 μM) led to a significant reduction of AR protein expression to approximately 30% of control within 12 hr for all the cell lines tested. In addition, there was a significant decrease in AR protein expression in LAPC-4 (Fig. 1D) cells treated with 1 μM ca27. Curcumin did not down-regulate the AR in our experimental system, as shown in Figure 1E. C4-2 cells treated with 20 μM ca27 for 72 hr demonstrated a significant loss of AR protein expression, whereas treatment with up to 20 μM curcumin for 72 hr did not inhibit AR protein expression (Fig. 1E). Similar results were observed in LNCaP cells (data not shown). To determine whether proteasomal degradation is involved in AR down-regulation, we used the proteasomal inhibitor MG132. As shown in Figure 1F, loss of AR protein expression by ca27 is independent of MG132 administration. LNCaP cells pretreated with 10 μM MG132 for 1 hr and then with 5 μM ca27 for 6 hr showed no inhibition of protein down-regulation in the presence of the proteasomal inhibitor (Fig. 1F). Collectively, these data indicate that ca27 mediates the down-regulation of endogenous AR protein in LNCaP, C4-2, and LAPC-4 prostate cancer cells within 3 hr of treatment independent of proteasomal degradation.
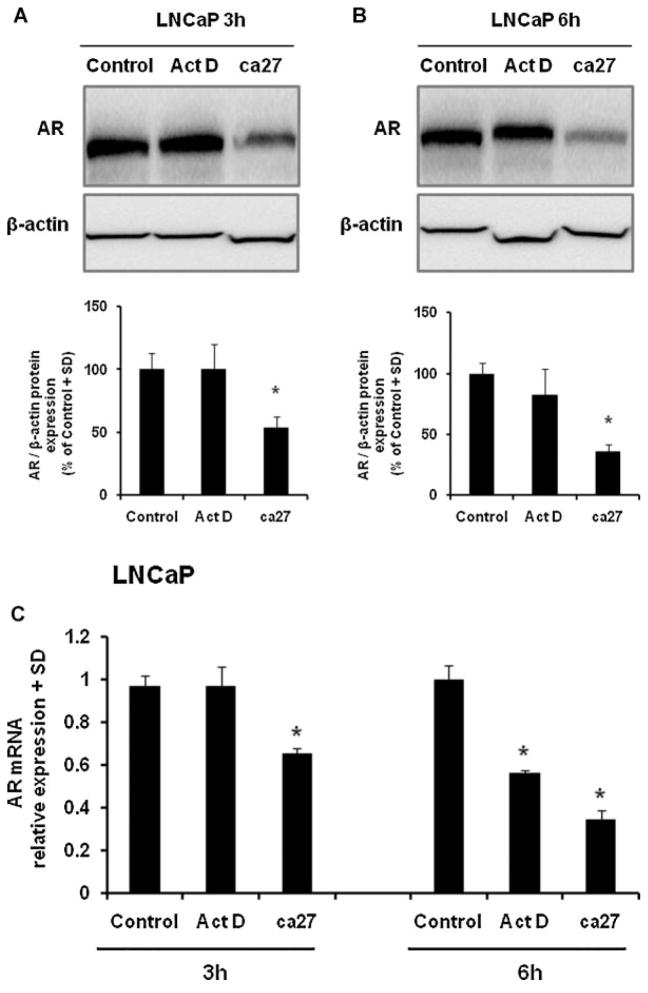
To test whether ca27 affects AR protein levels independent of mRNA transcription, we used the transcription inhibitor actinomycin D (Act D). LNCaP cells were treated with 10 μM Act D or 5 μM ca27 for 3 and 6 hr (Fig. 2). AR protein expression was significantly inhibited by 5 μM ca27 after 3 hr (Fig. 2A). At this time point, AR mRNA and protein expression were unaffected by Act D (Fig. 2A and C). However, Act D significantly inhibited AR mRNA expression after 6 hr (Fig. 2C) but did not inhibit AR protein expression at this time point (Fig. 2B). Together, these data indicate that ca27 at least in part down-regulates AR protein levels independent of its effect on AR mRNA transcription.
Fig. 2.
Down-regulation of endogenous AR protein expression by the synthetic curcumin analog ca27 in LNCaP cells after 3 hr. LNCaP (A) cells were treated with10 μM Act D or 5 μMca27 for 3 hr or 6 hr. AR protein (A and B) was measured by Western blotting and densitometric analysis (ratio AR: β-actin) after the indicated time. One representative Western blot is shown; bars in the graph represent the average of quadruplicate values + SD. AR and GAPDH mRNAs (C) were measured by qRT-PCR. Bars represent the average of quadruplicate values + SD. AR expression normalized to GAPDH is shown relative to control. * denotes P < 0.05comparedto control.
Inhibition of Cell Growth and Induction of Cell Death byca27 in Human Prostate Cancer Cells
The anti-proliferative effects of ca27 were tested on LNCaP and C4-2 prostate cancer cells. Due to the relatively long doubling time of LNCaP and C4-2 of approximately 48 hr, cell proliferation data was analyzed after 96 hr of treatment. The effect of ca27 on prostate cancer cell growth was determined by cell counts upon treatment with ca27 concentrations between 0.5 μM and 15 μM. As shown in Figure 3A, ca27 at ≥10 μM markedly inhibited growth of both LNCaP and C4-2 cells. Using trypan blue exclusion, we also determined the extent of cell death induced by ca27. As shown in Figure 3B, the rate of cell death increased extensively and variably at concentrations of >2.5 μM for C4-2 cells and >10 μM for LNCaP cells. These data indicate that the synthetic curcumin analog ca27 both inhibited prostate cancer cell growth and induced cell death. Of note, the loss of AR protein expression occurs within a shorter exposure time to ca27 and at lower concentrations (Fig. 1B and C), demonstrating that it precedes the effects on cell viability. Nevertheless, the loss of AR expression may contribute to cell growth inhibition and death, although a pleiotropic effect of ca27 acting through additional pathways cannot be excluded.
Fig. 3.
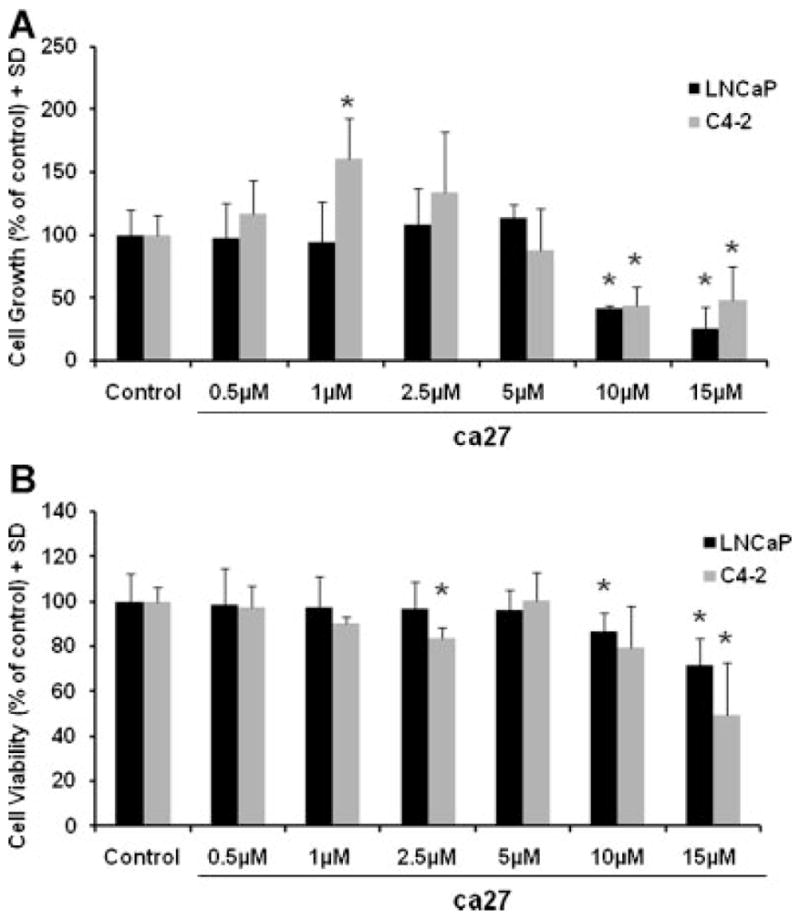
Growth inhibition (A) and induction of cell death (B) in LNCaP and C4-2 human prostate cancer cells by the synthetic curcumin analog ca27. Cell growth and death were determined by total cell counts and trypan blue positive cell counts, respectively. Cells were cultured in the presence of 0.5,1, 2.5, 5,10, or15 μM ca27 for 96 hr. Bars represent the average of quadruplicate values + SD. Cell growth and cell viability are expressed as percent of control. * denotes P < 0.05comparedto control.
Inhibition of AR Activation by ca27 in Human Prostate Cancer Cells
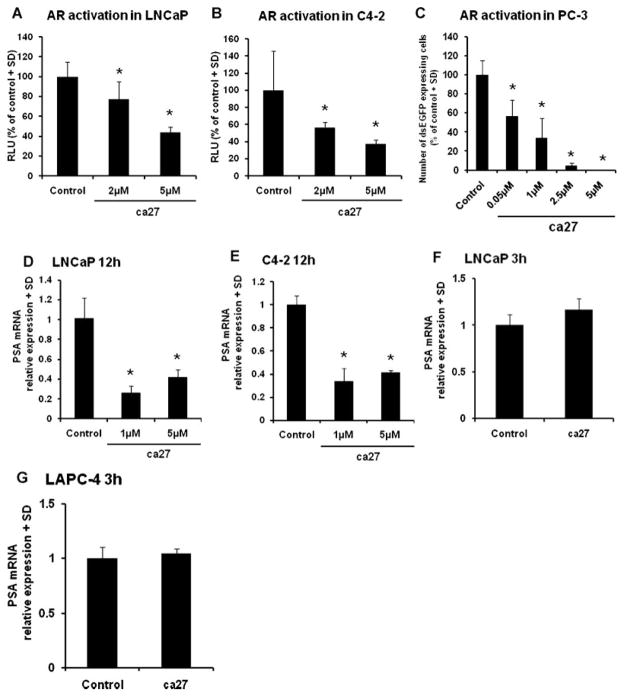
Other reports demonstrating that curcumin analogs have inhibitory action against the AR [23–25] prompted us to test the effect of ca27 on AR function. LNCaP and C4-2 cells (Fig. 4A and B) were transiently transfected with a reporter plasmid expressing luciferase regulated by the MMTV promoter containing androgen responsive elements [17], cultured in medium containing charcoal stripped serum, and treated for 24 hr with increasing concentrations of ca27. AR activation measured by luciferase activity was determined 6 hr after addition of 1 nM R1881 synthetic androgen. As shown in Figure 4A, ca27 significantly inhibited AR activation in LNCaP cells at 5 μM. ca27 affected AR activation similarly in C4-2 cells, with more variation and potentially at lower concentrations of 2 μM (Fig. 4B).
Fig. 4.
Inhibition of AR activation and endogenous PSA expression by the synthetic curcumin analog ca27 in LNCaP, C4-2, and PC-3 cells. A and B: LNCaP (A) and C4-2 (B) cells were co-transfected with AR reporter plasmid driving fire fly luciferase and aTK reporter plasmid driving Renilla luciferase. Cells were treated with ca27 at 2 and 5 μM for 24 hr. Normalized luciferase activity (relative luciferase units, RLU) was determined 6 hr after addition of1 nM R1881synthetic androgen. C: MARS assay (21): AR- and dsEGFP-transfected PC-3 cells were treated with increasing concentrations of ca27 for 24 hr and stimulated with 1 nM R1881. Bars in A^C represent the average of quadruplicate values + SD. AR activation is expressed as percent of control. D and E: LNCaP (D) andC4-2 (E) cells were treated with 1 and 5 μMca27. PSA and GAPDH mRNAs were measured by qRT-PCR after12 hr. Bars represent the average of quadruplicate values + SD. PSA expression normalized to GAPDH is shown relative to control. LNCaP (F) and LAPC-4 (G) cells were treatedwith5 μMca27 for 3 hr. Bars represent the average of triplicate values + SD. PSA expression normalized to GAPDH is shown relative to vehicle control. * denotes P < 0.05, respectively compared to control.
The ability of ca27 to inhibit AR activation was confirmed using the MARS assay developed to screen for compounds with antagonistic and agonistic effects on androgenic activity [19]. The MARS assay features androgen independent PC-3 human prostate cancer cells transiently co-transfected with an expression vector for the wild-type human AR and a plasmid carrying an androgen-sensitive promoter regulating the expression of destabilized enhanced GFP [19]. In this sensitive assay, ca27 inhibited AR activation at low micromolar concentrations. In particular, ca27 above 0.05 μM proved to be a potent inhibitor of AR activation (Fig. 4C). Collectively, these data indicate that ca27 is a potent inhibitor of AR activation.
Inhibition of Prostate Specific Antigen Expression by ca27 in Human Prostate Cancer Cells
To corroborate ca27 mediated AR down-regulation, we analyzed the effect of ca27 on the well-established transcriptional target of the AR, prostate specific antigen (PSA). LNCaP and C4-2 cells were treated with 1 and 5 μM ca27 for 12 hr, followed by assessment of endogenous PSA mRNA expression by qRT-PCR. In agreement with the observations on AR, PSA expression was significantly inhibited by 1 μM ca27 at 12 hr (Fig. 4D and E). Further, the effect of ca27 on PSA mRNA expression was tested after 3 hr when AR protein expression was significantly reduced as previously shown in Figures 1 and 2. At this time point ca27 did not reduce PSA mRNA expression in LNCaP or LAPC-4 cells (Fig. 4F and G). Together, these data indicate that ca27 is able to rapidly affect a biologically important downstream target of androgenic activity in prostate cancer cells, i.e., PSA. Further, the lack of PSA mRNA reduction after the short exposure time of 3 hr supports that ca27’s effect on PSA mRNA is a result of reduced AR activity due to AR down-regulation.
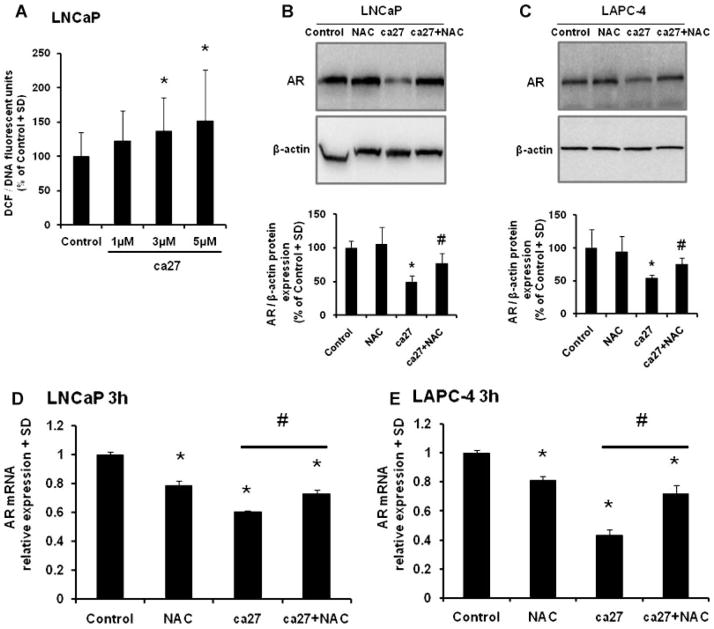
Increased Cellular Oxidative Stressbyca27 Leads to AR Down-Regulation in LNCaP Cells
Given the rapid action of ca27, we evaluated the status of oxidative stress upon ca27 treatment in human prostate cancer cells. LNCaP cells were treated for 1 hr with 1–5 μM ca27 and assayed for the production of ROS as measured by DCF fluorescence. Treatment of LNCaP cells with 3 μM ca27 led to a significant production of ROS (Fig. 5A). In order to determine if this significant increase in oxidative stress by ca27 induces the down-regulation of AR protein expression, LNCaP (Fig. 5B) and LAPC-4 (Fig. 5C) cells were simultaneously treated with ca27 and the antioxidant NAC for 3 hr. ca27 (5 μM) significantly inhibited AR protein expression after this short incubation time in both cell lines. Further, NAC prevented ca27 mediated AR protein loss in both LNCaP and LAPC-4 cells. To determine if the down-regulation of AR protein expression could be due to the inhibition of AR mRNA by ca27, LNCaP, and LAPC-4 cells were treated with 5 μM ca27 for 3 hr and AR mRNA was measured by qRT-PCR. In agreement with our previous result (Fig. 2C), within this short time period ca27 significantly inhibits AR mRNA expression in both cell lines (Fig. 5D and E). Further, AR mRNA expression is recovered when cells are simultaneously treated with ca27 and NAC demonstrating that the alleviation of oxidative stress induced by ca27 prevents the inhibition of AR expression. This result supports the hypothesis that induction of cellular oxidative stress by ca27 contributes to the down-regulation of AR expression in human prostate cancer cells.
Fig. 5.
Increased ROS generation induced by the synthetic curcumin analog ca27 and prevention of AR down-regulation by antioxidant NAC in LNCaP cells. LNCaP cells were treated with increasing concentrations (1, 3, and 5 μM) ofca27 for1 hr. Increased ROS production was measured by DCF fluorescence and normalized to DNA content (A). LNCaP (B) and LAPC-4 (C) cells were treated for 3 hr with or without 5 mMNAC in the presence or absence of 5 μ Mca27 and assayed for AR protein expression by Western blot; one representative Western blot is shown. Protein expression was quantitated by densitometry. Bars represent the average of triplicate values + SD. AR expression normalized to β-actin is shown relative to vehicle control. LNCaP (D) and LAPC-4 (E) cells were treated for 3 hr with or without 5 mMNAC in the presence or absence of 5 μM ca27 and assayed for AR mRNA expression and normalized to GAPDH bar graph shown is relative to control. * denotes P < 0.05, respectively compared to control. #denotes P < 0.05, respectively compared to ca27 treatment.
Activation of Nrf2 and Up-Regulation of Nrf2 Regulated Genesbyca27
A typical downstream effect of cellular oxidative stress is the activation of the critical cellular redox sensor Nrf2. The increased ROS generation by ca27 treatment led us to investigate the activation status of Nrf2. A 5 μM ca27 treatment in LNCaP cells significantly increased Nrf2 activation, as measured by an antioxidant response element promoter driving a luciferase reporter (Fig. 6A). In addition, in LAPC-4 cells there was a significant activation of Nrf2 by 1 μM ca27 (Fig. 6B). This result demonstrates that ca27 leads to increased transcriptional activation by Nrf2. In addition, these concentrations are in agreement with the induction of AR protein down-regulation in the LNCaP and LAPC-4 cells as shown in Figure 1B and D. To further illustrate activation of Nrf2, we evaluated Nrf2 regulated genes such as NQO1, AKR1C1, and MafG. LNCaP cells were treated with 5 μM ca27 for 3 hr and NQO1, AKR1C1, and MafG mRNA expression was measured by qRT-PCR. NQO1, AKR1C1 and MafG mRNA expression were increased ≥2-fold by ca27 treatment in comparison to the vehicle control (Fig. 6C). Collectively, these results corroborate the induction of oxidative stress by ca27 by demonstrating the activation of Nrf2 and the increased expression of Nrf2 regulated genes.
Fig. 6.
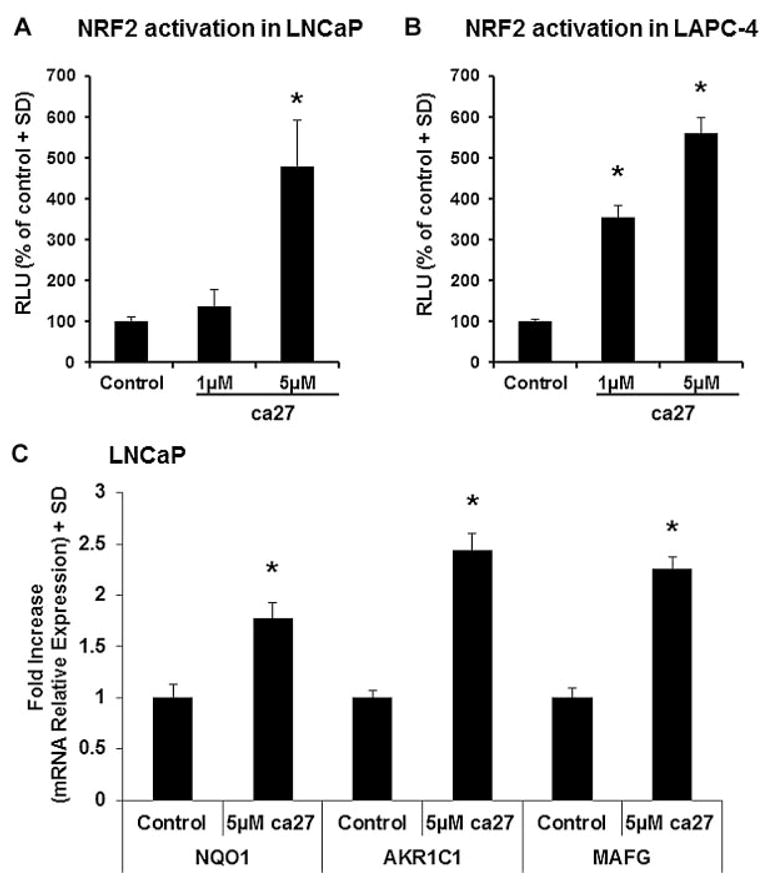
Nrf2activation and up-regulation ofNrf2 regulated genes in LNCaP and LAPC-4cellsby the synthetic curcumin analog ca27. LNCaP (A) and LAPC-4 (B) cells were co-transfected with Nrf2 reporter plasmid driving luciferase and TK reporter plasmid driving Renilla luciferase. Normalized luciferase activity was determined 16 hr post-treatment with 1 and 5 μM ca27. Bars represent the average of quadruplicate values + SD. Nrf2 activation is expressed as % of control. LNCaP cells (C) were treated with vehicle control or 5 μM ca27 for 3 hr. NQO1, AKR1C1, and MafG mRNA expression was measured by qRT-PCR. Bars represent the average of triplicate values + SD. NQO1, AKR1C1and MafG expression normalized to GAPDH is shown relative to control. * denotes P < 0.05, respectively compared to control.
DISCUSSION
The development of prostate cancer relies initially on androgenic activation of the AR by testosterone and its more active metabolite dihydrotestosterone (DHT) [1–2]. While AR activation in normal prostatic tissue represents part of normal physiology and maintains normal differentiation of epithelial cells, in the malignant setting it leads to the expression of target genes that promote tumorigenesis and cancer progression [11,26]. Clinically, the persistence of AR expression and function in androgen ablation resistant prostatic tissue is manifested by the successful yet transient application of second line androgen ablation strategies after primary failure, and by symptoms associated with androgen withdrawal [27–29]. Furthermore, this stage of disease is characterized by a number of molecular mechanisms supporting the function of the AR in very low or even absent levels of DHT [10,30,31]. Importantly, AR function under these conditions is still essential for prostate epithelial cell survival, as targeted AR down-regulation in androgen ablation resistant prostate cancer cell and animal models leads to cell growth inhibition [8–9]. Therefore, given the persisting importance of the AR and its signaling axis in advanced prostate cancer, it remains a prominent and promising target for this stage of disease.
The natural product curcumin (diferuloylmethane) has been shown to inhibit many targets in prostate epithelial cells with an importance in cancer formation and progression. Among these targets are transcription factors, receptors, intracellular kinases, cytokines, and growth factors [32]. Curcumin’s effect on the AR and on its target PSA has been demonstrated by several independent investigators using both endogenously expressed AR in LNCaP cells and ectopically expressed AR in PC-3 cells [33,34]. However, in these reports curcumin was used at relatively high concentrations, typically at ≥20 μM. It has previously been reported that curcumin has poor bioavailability, which has been determined in both animal models and humans [35]. This limitation has led researchers to generate a variety of synthetic analogs of curcumin and to investigate their capability to affect a number of molecular pathways implicated in tumorigenesis and cancer progression [16,36–39]. Typical structure modifications include the introduction of substituents on the biphenyl moieties and modifications of the length of the linker between the biphenyl rings. A specific group of such analogs has been exploited towards their ability to inhibit AR function [23–25], and some of these agents have been shown to down-regulate the expression of AR [24].
Along this line, we report here on the anti-androgenic action of curcumin analog ca27, which originates from our previously reported chemical libraries [12–14]. In particular, we have shown that ca27 at concentrations below those typically used for curcumin inhibits the growth of LNCaP and C4-2 human prostate cancer cells. Our data indicate that the observed growth inhibition and cell death of prostate cancer cells by ca27 could be in part mediated by the suppression of AR function. In fact, AR protein expression is significantly down-regulated by ca27 within 3 hr of treatment in various human prostate cancer cell lines. This rapid loss of AR protein expression could be due in part to the initial concomitant loss of AR mRNA expression. However, our investigations using the transcriptional inhibitor actinomycin D at multiple time points indicate an additional post-transcriptional inhibitory effect of ca27 on AR protein. Further, ca27 demonstrates selectivity of mRNA modulation as ca27 significantly reduced AR but not PSA mRNA expression in LNCaP and LAPC-4 cells, indicating that PSA mRNA reduction is a result of reduced AR activity due to AR down-regulation.
Evaluation of ca27 induced AR protein down-regulation indicated a distinct mechanism. Accordingly, we examined the actions of a well-established AR degradation mechanism, the ubiquitin-proteasomal pathway [40,41], and found that ca27 mediated loss of AR expression was not prevented by the proteasomal inhibitor MG132. This indicates an alternative down-regulation pathway for the AR activated by ca27. Accordingly, we show here that a potential mechanism for ca27 mediated AR down-regulation is through the induction of cellular oxidative stress. We demonstrate the pro-oxidant activity of ca27 by the increased ROS generation in human prostate cancer cells. The induction of ROS by ca27 was further demonstrated by the transcriptional activation of a known cellular redox sensor, the transcription factor Nrf2 [42]. Further, the expression of Nrf2 regulated detoxification genes, NQO1 and AKR1C1 [42,43], were significantly increased by ca27. This is in agreement with a previous study by Dinkova-Kostova et al. [44] who reported that the identical structure induces NQO1 activity in murine hepatoma and papilloma cells. Further, ca27 induced the mRNA expression of the small Maf protein, MafG. MafG is a known heterodimerization partner of Nrf2 and leads to Nrf2 transcriptional activity, and MafG expression has been shown to be regulated by Nrf2 transcriptional activity under oxidative stress conditions [45]. Evidence that AR down-regulation is mediated by ca27 induced ROS generation is provided by our data showing that AR loss is attenuated by the addition of the antioxidant NAC. Finally, the generation of cellular oxidative stress by ca27 could partially explain the proteasomal-independent down-regulation of the AR observed in this study, as previous studies have demonstrated that increased cellular oxidative stress can lead to protein aggregates, which inhibit the functions of the proteasome [46,47]. While the exact mechanism(s) of ca27 mediated AR protein down-regulation is at present unknown, it seems to entail oxidative stress mediated pathways. Our results are in agreement with two recent studies showing that AR mRNA transcription was inhibited in LNCaP and rat hepatoma cells by the pro-oxidant tert-butyl hydro-peroxide [48], and that the black seed oil ingredient thymoquinone induces oxidative stress and affects AR expression [49].
CONCLUSIONS
We conclude that the curcumin analog ca27 represents a lead structure with anti-androgenic activity in human prostate cancer cells, possibly through the induction of oxidative stress. Therefore, ca27 and similar compounds can be exploited as molecular tools to study pathways relevant to AR protein down-regulation. By extension, given the prominent role of the AR in prostate cancer [2,4,10,11] and because AR degradation has been recognized as an effective therapeutic strategy [9–10], we propose that ca27 is a potential lead in the development of novel therapeutics for prostate cancer. This is in agreement with recent reports on other compounds derived from natural products with similar anti-androgenic activities mediated by oxidative stress [50], and may represent an emerging theme for novel prostate cancer therapeutics.
Acknowledgments
Grant sponsor: Pfizer Safety Scholars Fellowship; Grant sponsor: National Institutes of Health (NIH) Grant; Grant number: RR0164880; Grant sponsor: Department of Defense (DOD) Prostate Cancer Program Grant; Grant number: PC060864/W81XWH-07-1-0081; Grant sponsor: NIH Grant; Grant number: 1 R03 CA133941; Grant sponsor: University of New Mexico Cancer Center Support Grant; Grant number: NIH/NCI P30CA118110; Grant sponsor: DOD Breast Cancer Program Grant; Grant number: BC043125.
The Departmental offices of the UNM Biochemistry and Molecular Biology Department and the College of Pharmacy Pharmaceutical Sciences Department are acknowledged for administrative support. This work is supported by a Pfizer Safety Scholars Fellowship (to A.M. Fajardo), National Institutes of Health (NIH) Grant RR0164880 (to M. Bisoffi), Department of Defense (DOD) Prostate Cancer Program Grant PC060864/W81XWH-07-1-0081 (to M. Bisoffi), NIH Grant 1 R03 CA133941 (to T.A. Thompson), and University of New Mexico Cancer Center Support Grant NIH/NCI P30CA118110. Research on the development of curcumin analogs is supported in part by DOD Breast Cancer Program Grant BC043125 (to D.L. Vander Jagt).
Abbreviations
- AR
androgen receptor
- ca27
curcumin analog 27
- ROS
reactive oxygen species
- NAC
N-acetyl-L-cysteine
- PSA
prostate specific antigen
References
- 1.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105(9):3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 3.Klotz L. Hormone therapy for patients with prostate carcinoma. Cancer. 2000;88(12 Suppl):3009–3014. doi: 10.1002/1097-0142(20000615)88:12+<3009::aid-cncr17>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Simmons MN, Klein EA. Combined androgen blockade revisited: Emerging options for the treatment of castration-resistant prostate cancer. Urology. 2009;73(4):697–705. doi: 10.1016/j.urology.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick JM. Is hormone ablation still the right choice for advanced prostate cancer? BJU Int. 2007;100(Suppl 2):36–39. doi: 10.1111/j.1464-410X.2007.06952.x. [DOI] [PubMed] [Google Scholar]
- 6.Chodak GW, Kranc DM, Puy LA, Takeda H, Johnson K, Chang C. Nuclear localization of androgen receptor in heterogeneous samples of normal, hyperplastic and neoplastic human prostate. J Urol. 1992;147(3 Pt 2):798–803. doi: 10.1016/s0022-5347(17)37389-5. [DOI] [PubMed] [Google Scholar]
- 7.Sadi MV, Walsh PC, Barrack ER. Immunohistochemical study of androgen receptors in metastatic prostate cancer. Comparison of receptor content and response to hormonal therapy. Cancer. 1991;67(12):3057–3064. doi: 10.1002/1097-0142(19910615)67:12<3057::aid-cncr2820671221>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Eder IE, Culig Z, Ramoner R, Thurnher M, Putz T, Nessler-Menardi C, Tiefenthaler M, Bartsch G, Klocker H. Inhibition of LncaP prostate cancer cells by means of androgen receptor antisense oligonucleotides. Cancer Gene Ther. 2000;7(7):997–1007. doi: 10.1038/sj.cgt.7700202. [DOI] [PubMed] [Google Scholar]
- 9.Snoek R, Cheng H, Margiotti K, Wafa LA, Wong CA, Wong EC, Fazli L, Nelson CC, Gleave ME, Rennie PS. In vivo knockdown of the androgen receptor results in growth inhibition and regression of well-established, castration-resistant prostate tumors. Clin Cancer Res. 2009;15(1):39–47. doi: 10.1158/1078-0432.CCR-08-1726. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8(4):440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen KE, Scher HI. Starving the addiction: New opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15(15):4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber WM, Hunsaker LA, Abcouwer SF, Deck LM, Vander Jagt DL. Anti-oxidant activities of curcumin and related enones. Bioorg Med Chem. 2005;13(11):3811–3820. doi: 10.1016/j.bmc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Weber WM, Hunsaker LA, Gonzales AM, Heynekamp JJ, Orlando RA, Deck LM, Vander Jagt DL. TPA-induced up-regulation of activator protein-1 can be inhibited or enhanced by analogs of the natural product curcumin. Biochem Pharmacol. 2006;72(8):928–940. doi: 10.1016/j.bcp.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Weber WM, Hunsaker LA, Roybal CN, Bobrovnikova-Marjon EV, Abcouwer SF, Royer RE, Deck LM, Vander Jagt DL. Activation of NFkappaB is inhibited by curcumin and related enones. Bioorg Med Chem. 2006;14(7):2450–2461. doi: 10.1016/j.bmc.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Mosley CA, Liotta DC, Snyder JP. Highly active anticancer curcumin analogues. Adv Exp Med Biol. 2007;595:77–103. doi: 10.1007/978-0-387-46401-5_2. [DOI] [PubMed] [Google Scholar]
- 16.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76(11):1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Thompson TA, Gould MN, Burkholder JK, Yang NS. Transient promoter activity in primary rat mammary epithelial cells evaluated using particle bombardment gene transfer. In Vitro Cell Dev Biol. 1993;29A(2):165–170. doi: 10.1007/BF02630949. [DOI] [PubMed] [Google Scholar]
- 18.Moinova HR, Mulcahy RT. An electrophile responsive element (EpRE) regulates beta-naphthoflavone induction of the human gamma-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. J Biol Chem. 1998;273(24):14683–14689. doi: 10.1074/jbc.273.24.14683. [DOI] [PubMed] [Google Scholar]
- 19.Dennis MK, Bowles HJ, MacKenzie DA, Burchiel SW, Edwards BS, Sklar LA, Prossnitz ER, Thompson TA. A multifunctional androgen receptor screening assay using the high-throughput Hypercyt flow cytometry system. Cytometry A. 2008;73(5):390–399. doi: 10.1002/cyto.a.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with imageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 21.Basu HS, Thompson TA, Church DR, Clower CC, Mehraein-Ghomi F, Amlong CA, Martin CT, Woster PM, Lindstrom MJ, Wilding G. A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2009;69(19):7689–7695. doi: 10.1158/0008-5472.CAN-08-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ripple MO, Henry WF, Rago RP, Wilding G. Prooxidant-anti-oxidant shift induced by androgen treatment of human prostate carcinoma cells. J Natl Canc Inst. 1997;89(1):40–48. doi: 10.1093/jnci/89.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsu H, Xiao Z, Ishida J, Nagai M, Wang HK, Itokawa H, Su CY, Shih C, Chiang T, Chang E, Lee Y, Tsai MY, Chang C, Lee KH. Antitumor agents. 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J Med Chem. 2002;45(23):5037–5042. doi: 10.1021/jm020200g. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Shih CC, Lee KH. Novel anti-prostate cancer curcumin analogues that enhance androgen receptor degradation activity. Anticancer Agents Med Chem. 2009;9(8):904–912. doi: 10.2174/187152009789124655. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Geng G, Shi Q, Sauriol F, Wu JH. Design and synthesis of androgen receptor antagonists with bulky side chains for overcoming antiandrogen resistance. J Med Chem. 2009;52(17):5546–5550. doi: 10.1021/jm801218k. [DOI] [PubMed] [Google Scholar]
- 26.Kung HJ, Evans CP. Oncogenic activation of androgen receptor. Urol Oncol. 2009;27(1):48–52. doi: 10.1016/j.urolonc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006;175(1):27–34. doi: 10.1016/S0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- 28.Muthuramalingam SR, Patel K, Protheroe A. Management of patients with hormone refractory prostate cancer. Clin Oncol (R Coll Radiol) 2004;16(8):505–516. doi: 10.1016/j.clon.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Van Allen EM, Ryan CJ. Novel secondary hormonal therapy in advanced prostate cancer: An update. Curr Opin Urol. 2009;19(3):315–321. doi: 10.1097/MOU.0b013e328329b73a. [DOI] [PubMed] [Google Scholar]
- 30.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: Androgen-receptor cofactors and bypass pathways. BJU Int. 2005;95(9):1327–1335. doi: 10.1111/j.1464-410X.2005.05527.x. [DOI] [PubMed] [Google Scholar]
- 31.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23(32):8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal BB. Prostate cancer and curcumin: Add spice to your life. Cancer Biol Ther. 2008;7(9):1436–1440. doi: 10.4161/cbt.7.9.6659. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, Rhim JS. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002;21(4):825–830. [PubMed] [Google Scholar]
- 34.Tsui KH, Feng TH, Lin CM, Chang PL, Juang HH. Curcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. J Androl. 2008;29(6):661–668. doi: 10.2164/jandrol.108.004911. [DOI] [PubMed] [Google Scholar]
- 35.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bio-availability of curcumin: Problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 36.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12(14):3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Basile V, Ferrari E, Lazzari S, Belluti S, Pignedoli F, Imbriano C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem Pharmacol. 2009;78(10):1305–1315. doi: 10.1016/j.bcp.2009.06.105. [DOI] [PubMed] [Google Scholar]
- 38.Ishida J, Ohtsu H, Tachibana Y, Nakanishi Y, Bastow KF, Nagai M, Wang HK, Itokawa H, Lee KH. Antitumor agents. Part 214: Synthesis and evaluation of curcumin analogues as cytotoxic agents. Bioorg Med Chem. 2002;10(11):3481–3487. doi: 10.1016/s0968-0896(02)00249-3. [DOI] [PubMed] [Google Scholar]
- 39.Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, Kakudo Y, Takahashi A, Takahashi S, Kato S, Suzuki T, Ishioka C, Iwabuchi Y, Shibata H. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5(10):2563–2571. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- 40.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21(15):4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheflin L, Keegan B, Zhang W, Spaulding SW. Inhibiting proteasomes in human HepG2 and LNCaP cells increases endogenous androgen receptor levels. Biochem Biophys Res Commun. 2000;276(1):144–150. doi: 10.1006/bbrc.2000.3424. [DOI] [PubMed] [Google Scholar]
- 42.Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36(10):1199–1207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- 43.Burchiel SW, Thompson TA, Lauer FT, Oprea TI. Activation of dioxin response element (DRE)-associated genes by benzo(a)-pyrene 3,6-quinone and benzo(a)pyrene 1,6-quinone in MCF-10A human mammary epithelial cells. Toxicol Appl Pharmacol. 2007;221(2):203–214. doi: 10.1016/j.taap.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci USA. 2001;98(6):3404–3409. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katsuoka F, Motohashi H, Engel JD, Yamamoto M. Nrf2 transcriptionally activates the mafG gene through an antioxidant response element. J Biol Chem. 2005;280(6):4483–4490. doi: 10.1074/jbc.M411451200. [DOI] [PubMed] [Google Scholar]
- 46.Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol Chem. 2008;389(3):203–209. doi: 10.1515/BC.2008.029. [DOI] [PubMed] [Google Scholar]
- 47.Szweda PA, Friguet B, Szweda LI. Proteolysis, free radicals, and aging. Free Radic Biol Med. 2002;33(1):29–36. doi: 10.1016/s0891-5849(02)00837-7. [DOI] [PubMed] [Google Scholar]
- 48.Shi L, Ko S, Kim S, Echchgadda I, Oh TS, Song CS, Chatterjee B. Loss of androgen receptor in aging and oxidative stress through Myb protooncoprotein-regulated reciprocal chromatin dynamics of p53 and poly(ADP-ribose) polymerase PARP-1. J Biol Chem. 2008;283(52):36474–36485. doi: 10.1074/jbc.M805980200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koka PS, Mondal D, Schultz M, Abdel-Mageed AB, Agrawal KC. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: Role of reactive oxygen species. Exp Biol Med (Maywood) 2010;235(6):751–760. doi: 10.1258/ebm.2010.009369. [DOI] [PubMed] [Google Scholar]
- 50.Ketola K, Vainio P, Fey V, Kallioniemi O, Iljin K. Monensin is a potent inducer of oxidative stress and inhibitor of androgen signaling leading to apoptosis in prostate cancer cells. Mol Cancer Ther. 2010;9(12):3175–3185. doi: 10.1158/1535-7163.MCT-10-0368. [DOI] [PubMed] [Google Scholar]