Abstract
5-Methylaminomethyl-2-selenouridine (mnm5Se2U) is found in the first position of the anticodon in certain tRNAs from bacteria, archaea and eukaryotes. This selenonucleoside is formed in Escherichia coli from the corresponding thionucleoside mnm5S2U by the monomeric enzyme YbbB. This nucleoside is present in the tRNA of Methanococcales, yet the corresponding 2-selenouridine synthase is unknown in archaea and eukaryotes. Here we report that a bipartite ybbB ortholog is present in all members of the Methanococcales. Gene deletions in Methanococcus maripaludis and in vitro activity assays confirm that the two proteins act in trans to form in tRNA a selenonucleoside, presumably mnm5Se2U. Phylogenetic data suggest a primal origin of seleno-modified tRNAs.
Keywords: selenouridine, tRNA 2-selenouridine synthase, YbbB, archaea, Methanococcus maripaludis
1. Introduction
Selenium is an essential trace mineral for a subset of organisms from all three domains of life. It is present in the active site of selenium-dependent enzymes as the rare amino acid selenocysteine [1,2] and is also found in the form of nucleoside modifications in certain tRNAs [3-7]. The most abundant selenonucleoside is 5-methylaminomethyl-2-selenouridine (mnm5Se2U) which has been identified in tRNA species from bacteria [8,9], archaea [7,10], mammals [11], and plants [12]. This nucleoside modification has been shown to commonly occur at the wobble position of the anticodon in a fraction of tRNAGlu, tRNAGln, and tRNALys acceptors where it is thought to play a role in the fine tuning of codon-anticodon interactions [13]. The mechanism by which mnm5Se2U is formed has only been partially characterized in bacteria and archaea. In Escherichia coli, the uridine at the wobble position 34 of the tRNA anticodon is first thiolated by MnmA and IscS to form 2-thiouridine [14,15], and is then further modified by MnmE to 5-methylaminomethyl-2-thiouridine (mnm5S2U) [16]. Finally, the sulfur atom in 2-thiouridine is replaced with a selenium atom using selenophosphate (provided by selenophosphate synthetase; SelD), as selenium-donor [14,17,18]. A tRNA 2-selenouridine synthase catalyzing this reaction has first been discovered in Salmonella typhimurium cell extract [17] and has recently been identified and partially characterized as the product of the ybbB gene in E. coli [19].
YbbB is composed of an N-terminal catalytic rhodanese domain and a C-terminal domain containing a P-loop (Walker A) motif [19]. Rhodanese-like proteins are generally sulfurtransferases which catalyze the transfer of sulfane sulfur from a donor molecule to a thiophilic acceptor [20]. They represent a large and heterogeneous superfamily of proteins which can be grouped into four distinct classes: (I) Proteins composed of a single catalytic rhodanese domain (e.g., GlpE, PspE), (II) fusion proteins composed of two (e.g., Rhobov, RhdA), three (e.g., YnjE) or up to six rhodanese domains [21], (III) multidomain proteins where the rhodanese domain is paired with domains of different functions (e.g., YbbB, ThiI), and (IV) rhodanese-like proteins with an elongated active-site loop (e.g., CDC25A) (reviewed in [20,22]). While the function of the P-loop domain in YbbB is still unknown, it has been shown that its rhodanese domain is responsible for catalyzing the selenium transfer reaction, and a conserved cysteine residue in the active site of this domain has been predicted to be involved in the formation of a perselenide [19]. No obvious homolog of E. coli YbbB has been identified in the genomes of most archaea or eukaryotes and the mechanism by which 2-selenouridine is synthesized in these organisms is yet unknown. The archaeon Methanocaldococcus jannaschii, however, is predicted to possess an YbbB-like enzyme consisting of a separately encoded rhodanese and P-loop domain [19]. Yet the presence of the presumed modified nucleoside precursor mnm5S2U has been established by LC-MS in the tRNA of five members of the Methanococcales [23], but orthologs of the bacterial enzymes involved in its synthesis are missing in archaea.
In the present study, we identified the putative bipartite ortholog of the bacterial YbbB gene in twelve additional archaeal genomes, and confirmed its function as a tRNA 2-selenouridine synthase through biochemical and genetic experiments. A phylogenetic analysis of bacterial and archaeal versions of this enzyme provides insights into the early evolution of tRNA 2-selenouridine synthase.
2. Material and methods
2.1. Computation and phylogenetic analysis
The identification of ybbB genes was carried out via the “IMG Genome Blast tool” on the DOE JGI website (http://img.jgi.doe.gov) using the E. coli YbbB sequence as a query against “All finished, permanent draft, and draft” genomes, with the “Compute bidirectional best hits also” option selected. For all archaeal sequences the output result was also verified by testing for the co-occurrence of selD genes. Single-domain rhodanese-like proteins closely related to YbbB were identified using BLAST and downloaded from NCBI.
For molecular evolutionary analyses, the structures of the E. coli rhodanese proteins GlpE (PDB code 1gn0) and PspE (PDB code 2jtq), and of the 2-selenouridine synthase rhodanese domain from Clostridium difficile (PDB code 3g5j) were aligned using Multiseq in VMD 1.9 [24]. YbbB and rhodanese-like protein sequences were aligned to the resulting structural alignment using CLUSTAL W [25]. Phylogenies were determined by the Neigbor-Joining method [26] applying the JTT matrix-based model [27]. All ambiguous positions were removed for each sequence pair. Five hundred bootstrap resamplings were performed to assess the support for individual branches. MEGA5 [28] was used to conduct all evolutionary analyses.
2.2. Microbial strains and growth conditions
M. maripaludis strains were grown at 37°C on solid or liquid McCas medium under strictly anaerobic conditions with 276 kPa H2/CO2 gas (80:20 [vol/vol]) as described before [29]. Puromycin (2.5 μg/ml), neomycin (1.0 mg/ml), or 8-azahypoxanthine (0.25 mg/ml) was added to the medium as needed. E. coli Dh5α, grown at 37°C on Luria-Bertani medium supplemented with ampicillin (100 μg/ml) was used for plasmid cloning.
2.3. Cloning, expression and purification of recombinant proteins
Standard methods were used for isolation of genomic DNA from M. maripaludis, and for plasmid and genomic DNA extraction from E. coli. For recombinant protein preparation, MMP0899 and MMP0900 were cloned into pET15b plasmid (Novagen, Madison, WI) individually. E. coli YbbB and SelD were expressed from strains derived from the ASKA library [30]. Proteins were expressed in autoinduction medium [31] at 20°C for 24 h and His6-tagged proteins were purified on Talon resin (Clontech, Palo Alto, CA) according to the manufacturer’s manual. More details can be found in the
2.4. Gene replacement and complementation experiments in M. maripaludis
The putative operon encoding MMP0899 and MMP0900 was replaced by a puromycin resistance marker (pac cassette) in the M. maripaludis S2 derivative strain Mm900 as described previously [32], yielding M. maripaludis ΔselU (details shown in Supplementary data). For genetic complementation experiments the single genes encoding MMP0899 and MMP0900, or the operon comprising the MMP0899 and MMP0900 genes was cloned into the M. maripaludis expression plasmid pMEV2 [33] under the control of the constitutive Methanococcus hmvA promoter (details shown in Supplementary data). M. maripaludis ΔselU was transformed with the resulting plasmids (pMEV2-MMP0899, pMEV2-MMP0900, and pMEV2-MMP0899-900, respectively) as described [32].
2.5. Metabolic labeling with [75Se]selenite
For radio-labeling, M. maripaludis cultures grown to an A600 of 0.8 were diluted 1:50 in 5 ml McCas medium supplemented with 7.5 μCi [75Se]selenite, and incubated at 37°C for 48 h, as described [32]. Cells were harvested by centrifugation, washed in McCas medium, and lysed by resuspending in dH2O. Cell lysates were subjected to SDS/PAGE, followed by autoradiography.
2.6. tRNA isolation
Total M. maripaludis RNA was isolated from a 4-liter culture of the M. maripaludis ΔselU strain by standard acid phenol/chloroform extraction. Total tRNA was then further purified by anion exchange chromatography (details shown in Supplementary data).
2.7. In vitro tRNA selenation
[75Se]Selenide was generated by incubating 1 mM [75Se]selenite (10 μCi) in 500 mM Tris-HCl, pH 8.5, and 250 mM DTT in an anaerobic chamber at 37°C for 2 hours. Selenation assays were carried out according to [19]. Briefly, a reaction mixture containing 50 mM Tris-HCl, pH 7.2, 20 mM KCl, 4 mM MgCl2, 2 mM ATP, 100 μM HSe (1 μCi of [75Se]selenide), 50 μM bulk tRNA, 10 μM of E. coli SelD, and 10 μM of either MMP0899, MMP0900, or MMP0899+MMP0900 was incubated at 37°C in an anaerobic chamber for 30 min. The reaction was stopped by addition of phenol to the mixture. Total tRNA was then isolated by phenol/chloroform extraction and loaded onto a 12% Urea-polyacrylaminde gel. After electrophoresis [75Se]selenium incorporation into tRNA was visualized by phosphoimaging.
3. Results and discussions
3.1. Computational identification of tRNA 2-selenouridine synthase homologs
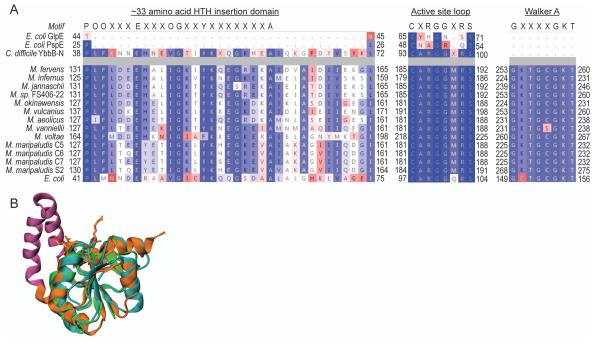
A comprehensive search of the IMG genome database with the E. coli YbbB ORF as query identified homologs in 579 of the 2508 bacterial genomes. Surprisingly, we also discovered YbbB homologs in the genomes of several unicellular eukaryotes, including green algae, chrysophytes and amoeba. Within the archaeal domain YbbB homologs are restricted only to members of the Methanococcales, and are present in all of the 13 available genomes from this order. While all bacterial and eukaryotic YbbB proteins are encoded by a single gene, in all of the archaeal YbbB homologs the rhodanese and P-loop domains are found as two separate polypeptides encoded in a putative operon. The gene order in this operon is strictly conserved in all methanococcal genomes with the gene encoding the P-loop domain always immediately upstream of the gene encoding the rhodanese domain. This preserved gene organization suggests a common physiological function shared by these two proteins. In addition, all 13 archaea also encode selenophosphate synthetase (SelD) (Supplementary Table 1), which produces selenophosphate, the selenium donor for both, selenocysteine and YbbB-catalyzed selenouridine formation [34]. This in agreement with an earlier report stating that in bacteria ybbB usually coexists with selD [35]. Unique signature motifs have been described for bacterial YbbB, in both the rhodanese and P-loop domain [19]. To identify these motifs in the archaeal YbbB orthologs, we aligned the concatenated amino acid sequences of each of the archaeal rhodanse and corresponding P-loop domains against the E. coli YbbB sequence. In addition, we compared the archaeal rhodanese-like proteins to the structure of the YbbB rhodanese domain from Clostridium difficile. The concatenated archaeal YbbB sequences are ~20% larger than that of E. coli YbbB and share only ~15% amino acid identity with it. Sequence alignments show, however, that the predicted active site loop containing the YbbB-characteristic CXRGGXRS motif near the C-terminus of the rhodanese domain and the Walker A motif (GX4GKT) at the N-terminus of the P-loop domain are highly conserved among the archaeal and bacterial YbbB sequences (Fig. 1A). A ~33 amino acid insertion immediately upstream of the rhodanese active site loop has been reported to be characteristic for bacterial YbbB homologs [19]. The C. difficile structure reveals that this insertion corresponds to a helix-turn-helix motif (Fig 1B) which is absent from other single-domain rhodanese proteins but also occurs in the archaeal rhodanese-like proteins (Fig 1A). In summary these findings strongly indicate that the identified archaeal YbbB orthologs represent a bipartite version of bacterial tRNA 2-selenouridine synthase.
Figure 1. Sequence alignment of bacterial and archaeal tRNA 2-selenouridine synthases.
(A) Structure based alignment of the E. coli single-domain rhodaneses GlpE and PspE and the YbbB rhodanese domain from C. difficile aligned with concatenated archaeal tRNA 2-selenouridine synthase sequences. The E. coli YbbB sequence is shown as a reference. Amino acids are colored according to sequence similarity (BLOSUM 50). The Rhodanese active site motif (CXRGGXRS), the P-loop Walker A motif, (GX4GKT), and the conserved ~33 amino acid HTH insertion motif (POOX3EX3OGX2YX7A, where O represents a hydrophobic amino acid) are shown. Residue numbering is shown adjacent to each sequence. (B) Structural comparison of GlpE (blue), PspE (green), and the YbbB rhodanese domain (orange). The ~33 amino acid HTH insertion is highlighted in purple and active site residues in the YbbB rhodanese domain are shown.
3.2. In vivo analysis of tRNA 2-selenouridine synthase activity
In M. maripaludis S2 the YbbB-like rhodanese and P-loop domains are encoded by MMP0900 and MMP0899, respectively. To test if the gene products of these two ORFs can function as tRNA 2-selenouridine synthase in vivo we generated an M. maripaludis deletion mutant in which we replaced the putative operon encoding MMP0899 and MMP0900 with a pac-cassette. As in E. coli [19], the resulting M. maripaludis ΔselU strain had no obvious growth defect under standard cultivation conditions (Supplementary Fig. 1). To directly follow selenium incorporation into tRNAs, wild-type and deletion mutant were grown in the presence of [75Se]selenite, followed by separation of cell extracts on a polyacrylamide gel and visualization by autoradiography (Fig. 2). In wild-type cell extract, selenium incorporation occurred into both proteins and tRNAs (Fig. 2, lanes 1 and 2), whereas in extracts from the deletion strain only selenoproteins but no selenium-modified tRNAs were detectable (Fig. 2, lane 3). This demonstrates that only one type of selenium-modification (presumably 2-selenouridine) is present in archaeal tRNAs. To confirm that both, the rhodanese and P-loop domain containing subunits are required for this tRNA modification, the deletion mutant was transformed with a plasmid encoding either MMP0899, or MMP0900, or both genes together under the control of a constitutive Methanococcus promoter. Only transformants complemented with both, MMP0899 and MMP0900, were able to incorporate [75Se]selenium into tRNAs (Fig. 2, lane 6), demonstrating that MMP0899 and MMP0900 have to act in trans to reconstitute tRNA 2-selenouridine synthase activity.
Figure 2. Metabolic labeling of protein and tRNA.
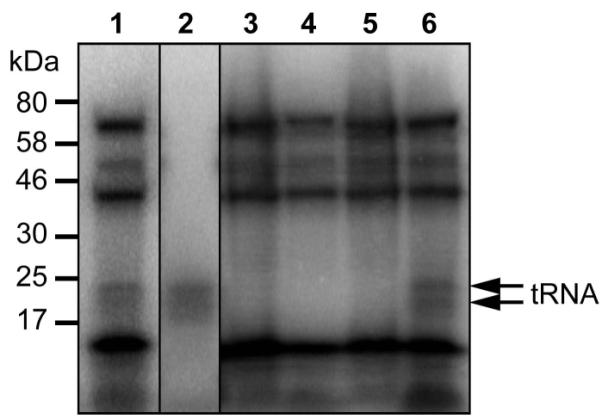
Cell extracts of wild-type M. maripaludis Mm900 (lane 1), Mm900, digested with proteinase K (lane 2), Mm900 ΔselU (lane 3), Mm900 ΔselU, containing the MMP0899 expression vector (lane 4), Mm900 ΔselU, containing the MMP0900 expression vector (lane 5), and Mm900 ΔselU, containing the MMP0899-MMP0900 expression vector (lane 6) were prepared following growth on McCas medium in the presence of [75Se]selenite. Extracts were separated on a SDS-PAGE gel (4–20%), and the insertion of 75Se into macromolecules was followed by autoradiography. Migration position of 75Se-labelled RNA is indicated on the right.
3.3. In vitro analysis of tRNA 2-selenouridine synthase activity
To further characterize the enzyme in vitro, we cloned both MMP0899 and MMP0900 into plasmids for over-expression in E. coli, and purified the recombinant proteins separately. A coupled assay system was then used to test tRNA 2-selenouridine synthase activity in vitro. [75Se]selenophosphate was produced from [75Se]selenide and ATP by recombinant E. coli selD and subsequent YbbB-mediated formation of 75Se-labeled tRNA was shown by urea PAGE. As a tRNA substrate for this reaction we used total tRNA purified from the M. maripaludis ΔselU strain which lacks selenium modified tRNAs.
We found that recombinant E. coli YbbB is active in selenation of M. maripaludis tRNA (Fig. 3, lane 1), while neither MMP0899 nor MMP0900 could catalyze tRNA selenation by themselves (Fig. 3, lane 2 and 3). Only when both MMP0899 and MMP0900 are present in the reaction tRNA selenation occurs (Fig. 3, lane 4). This result is consistent with the in vivo 75Se labeling data, demonstrating that both proteins are required for the tRNA 2-selenouridine synthase activity. The data show that under our conditions the activity of the in vitro reaction was low. This may indicate that additional factors (proteins and/or substrates) might be missing.
Figure 3. In vitro tRNA selenation by tRNA 2-selenouridine synthase.
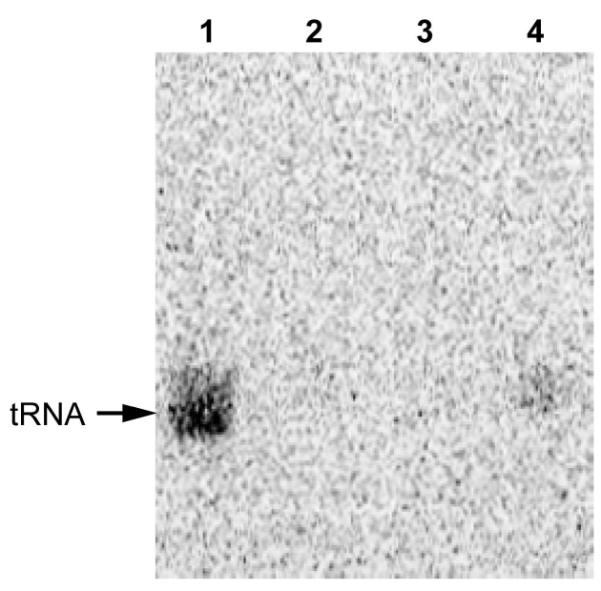
E. coli YbbB (lane 1), MMP0899 (lane 2), MMP0900 (lane 3) or MMP0899 and MMP0900 (lane 4) were incubated with total tRNAs from the M. maripaludis Mm900 ΔselU strain and [75Se]selenophosphate to generate selenated tRNAs. tRNAs were extracted from the reaction and resolved by 12% Urea-PAGE, followed by autoradiography. Migration position of 75Se-labeled tRNAs is indicated on the left.
E. coli YbbB was shown to bind its tRNA substrate very tightly [19]. We confirmed this finding during E. coli YbbB purification (data not shown). However, no tRNA was bound to either MMP0899 or MMP0900 during purification (data not shown). This might indicate that both domains of YbbB are involved in tRNA interaction and that a single domain alone might not be able to bind to tRNA efficiently.
3.4. Phylogeny
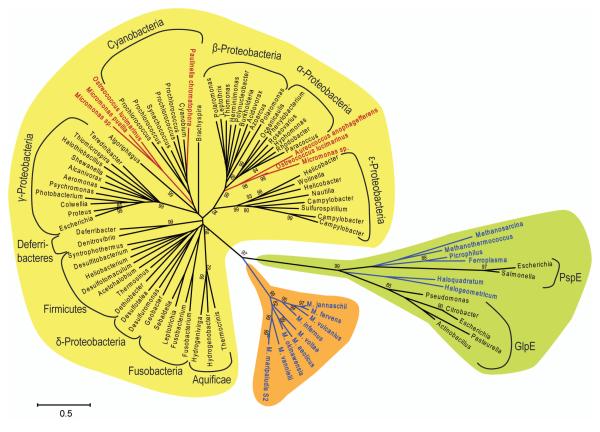
In order to reconstruct the phylogenetic relationship between YbbB homologs identified in bacterial, archaeal, and eukaryotic genomes we assembled amino acid alignments of full-length YbbB proteins (i.e. the rhodanese and P-loop domains of the archaeal YbbB homologs were concatenated). The use of an outgroup, consisting of 7 bacterial and 6 archaeal single-domain rhodanese-like proteins with close homology to the YbbB rhodanese domain, allowed us to place an evolutionary root between YbbB proteins. YbbB phylogeny (Fig 4) is mostly in accordance with the standard taxonomy, demonstrating that the evolution of this enzyme is largely characterized by vertical gene flow and gene loss. All YbbB sequences identified in eukaryotic genomes, however, branch off within the bacterial lineage with either cyanobacterial or alpha-proteobacterial sequences as their closest evolutionary neighbors. This provides evidence that YbbB has been horizontally transferred to these organisms, and most likely originated from their bacterial photosynthetic endosymbionts. On the other hand, all archaeal YbbB homologs cluster together in a separate clade. The phylogenetic root is placed with high statistical support between the bacterial and archaeal lineages, providing evidence that tRNA 2-selenouridine synthase must have been already present in the last common ancestor of bacteria and archaea from which it has been vertically inherited to both these domains. This finding suggests that the 2-selenouridine modification in certain tRNAs was an early invention of nature.
Figure 4. tRNA 2-selenouridine synthase phylogeny.
Neighbor-joining tree of bacterial and archaeal YbbB homologs (shaded in yellow and orange, respectively), and representative groups of closely related single-domain rhodanese-like proteins (shaded in green), which include bacterial rhodanese (GlpE) and phage shock protein (PspE). Organism names are color-coded: Archaea (blue), Eukarya (red), and Bacteria (black). Only bootstrap values ≥80% are shown. Scale bar represents 0.5 changes per site.
It seems likely that the fusion of two genes separately encoding the rhodanese-like and P-loop domains gave rise to bacterial YbbB. Assuming that this fusion event has taken place during the early evolution of the bacterial lineage, the archaeal version of tRNA 2-selenouridine synthase may more closely resemble the ancestral form of this enzyme. It cannot be fully excluded, however, that a fused version of YbbB was already present in the last common ancestor of archaea and bacteria. In that case a secondary split of YbbB would have occurred in the last common ancestor that gave rise to the Methanococcales. It is yet unknown how tRNA 2-selenouridine synthase specifically recognizes its substrate tRNAs, by which molecular mechanism the sulfur atom in 2-thiouridine is replaced with Se, and what role the P-loop domain of this enzyme plays in this reaction. A tRNA 2-selenouridine synthase : tRNA co-crystal structure will provide answers to these questions and the availability of a functional bipartite tRNA 2-selenouridine synthase with separately encoded rhodanese and P-loop domains may turn out handy for future structural and biochemical studies.
Supplementary Material
A bipartite archaeal tRNA 2-selenouridine synthase (SelU) was identified in silico.
Both proteins are required to catalyze tRNA selenation in vivo and in vitro.
SelU presumably was present in the last common ancestor of bacteria and archaea.
Acknowledgements
We thank Jiqiang Ling and Patrick O’Donoghue for helpful discussions and critical reading of the manuscript. M.J.H. held a Feodor Lynen Postdoctoral Fellowship of the Alexander von Humboldt Foundation (Bonn, Germany). This work was supported by grants from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy (grant DE-FG02-98ER20311), the National Institute of General Medical Sciences (grant GM22854), and by COMBREX, via the National Institute of General Medical Sciences (1RC2GM092602-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Böck A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B, Zinoni F. Selenocysteine: the 21st amino acid. Mol Microbiol. 1991;5:515–20. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- [2].Zinoni F, Birkmann A, Stadtman TC, Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc. Natl. Acad. Sci. USA. 1986;83:4650–4. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen CS, Stadtman TC. Selenium-containing tRNAs from Clostridium sticklandii: cochromatography of one species with L-prolyl-tRNA. Proc. Natl. Acad. Sci. USA. 1980;77:1403–7. doi: 10.1073/pnas.77.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen CS, Wen TN, Chang JH. Selenium-containing tRNA of a higher plant. Curr. Top. Cell. Regul. 1985;27:509–16. doi: 10.1016/b978-0-12-152827-0.50051-7. [DOI] [PubMed] [Google Scholar]
- [5].Ching WM. Occurrence of selenium-containing tRNAs in mouse leukemia cells. Proc. Natl. Acad. Sci. USA. 1984;81:3010–3. doi: 10.1073/pnas.81.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hoffman JL, McConnell KP. The presence of 4-selenouridine in Escherichia coli tRNA. Biochim. Biophys. Acta. 1974;366:109–13. doi: 10.1016/0005-2787(74)90323-2. [DOI] [PubMed] [Google Scholar]
- [7].Ching WM, Wittwer AJ, Tsai L, Stadtman TC. Distribution of two selenonucleosides among the selenium-containing tRNAs from Methanococcus vannielii. Proc. Natl. Acad. Sci. USA. 1984;81:57–60. doi: 10.1073/pnas.81.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wittwer AJ, Stadtman TC. Biosynthesis of 5-methylaminomethyl-2-selenouridine, a naturally occurring nucleoside in Escherichia coli tRNA. Arch. Biochem. Biophys. 1986;248:540–50. doi: 10.1016/0003-9861(86)90507-2. [DOI] [PubMed] [Google Scholar]
- [9].Wittwer AJ, Tsai L, Ching WM, Stadtman TC. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino)methyl]-2-selenouridine. Biochemistry. 1984;23:4650–5. doi: 10.1021/bi00315a021. [DOI] [PubMed] [Google Scholar]
- [10].Politino M, Tsai L, Veres Z, Stadtman TC. Biosynthesis of selenium-modified tRNAs in Methanococcus vannielii. Proc. Natl. Acad. Sci. USA. 1990;87:6345–8. doi: 10.1073/pnas.87.16.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mizutani T, Watanabe T, Kanaya K, Nakagawa Y, Fujiwara T. Trace 5-methylaminomethyl-2-selenouridine in bovine tRNA and the selenouridine synthase activity in bovine liver. Mol. Biol. Rep. 1999;26:167–72. doi: 10.1023/a:1006907920395. [DOI] [PubMed] [Google Scholar]
- [12].Huang KX, An YX, Chen ZX, Xu HB. Isolation and partial characterization of selenium-containing tRNA from germinating barley. Biological Trace Element Research. 2001;82:247–57. doi: 10.1385/BTER:82:1-3:247. [DOI] [PubMed] [Google Scholar]
- [13].Ching WM. Characterization of selenium-containing tRNAGlu from Clostridium sticklandii. Arch. Biochem. Biophys. 1986;244:137–46. [Google Scholar]
- [14].Mihara H, et al. The iscS gene is essential for the biosynthesis of 2-selenouridine in tRNA and the selenocysteine-containing formate dehydrogenase H. Proc. Natl. Acad. Sci. USA. 2002;99:6679–83. doi: 10.1073/pnas.102176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kambampati R, Lauhon CT. MnmA and IscS are required for in vitro 2-thiouridine biosynthesis in Escherichia coli. Biochemistry. 2003;42:1109–17. doi: 10.1021/bi026536+. [DOI] [PubMed] [Google Scholar]
- [16].Elseviers D, Petrullo LA, Gallagher PJ. Novel E. coli mutants deficient in biosynthesis of 5-methylaminomethyl-2-thiouridine. Nucleic Acids Res. 1984;12:3521–34. doi: 10.1093/nar/12.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Veres Z, Stadtman TC. A purified selenophosphate-dependent enzyme from Salmonella typhimurium catalyzes the replacement of sulfur in 2-thiouridine residues in tRNAs with selenium. Proc. Natl. Acad. Sci. USA. 1994;91:8092–6. doi: 10.1073/pnas.91.17.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Veres Z, Tsai L, Scholz TD, Politino M, Balaban RS, Stadtman TC. Synthesis of 5-methylaminomethyl-2-selenouridine in tRNAs: 31P NMR studies show the labile selenium donor synthesized by the selD gene product contains selenium bonded to phosphorus. Proc. Natl. Acad. Sci. USA. 1992;89:2975–9. doi: 10.1073/pnas.89.7.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wolfe MD, Ahmed F, Lacourciere GM, Lauhon CT, Stadtman TC, Larson TJ. Functional diversity of the rhodanese homology domain: the Escherichia coli ybbB gene encodes a selenophosphate-dependent tRNA 2-selenouridine synthase. J. Biol. Chem. 2004;279:1801–9. doi: 10.1074/jbc.M310442200. [DOI] [PubMed] [Google Scholar]
- [20].Bordo D, Bork P. The rhodanese/Cdc25 phosphatase superfamily. Sequence-structure-function relations. EMBO Rep. 2002;3:741–6. doi: 10.1093/embo-reports/kvf150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hänzelmann P, Dahl JU, Kuper J, Urban A, Müller-Theissen U, Leimkühler S, Schindelin H. Crystal structure of YnjE from Escherichia coli, a sulfurtransferase with three rhodanese domains. Protein science : a publication of the Protein Society. 2009;18:2480–91. doi: 10.1002/pro.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cipollone R, Ascenzi P, Visca P. Common themes and variations in the rhodanese superfamily. IUBMB life. 2007;59:51–9. doi: 10.1080/15216540701206859. [DOI] [PubMed] [Google Scholar]
- [23].McCloskey JA, Graham DE, Zhou S, Crain PF, Ibba M, Konisky J, Söll D, Olsen GJ. Post-transcriptional modification in archaeal tRNAs: identities and phylogenetic relations of nucleotides from mesophilic and hyperthermophilic Methanococcales. Nucleic Acids Res. 2001;29:4699–706. doi: 10.1093/nar/29.22.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roberts E, Eargle J, Wright D, Luthey-Schulten Z. MultiSeq: unifying sequence and structure data for evolutionary analysis. BMC Bioinformatics. 2006;7:382. doi: 10.1186/1471-2105-7-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–25. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- [27].Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Computer applications in the biosciences : CABIOS. 1992;8:275–82. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- [28].Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moore BC, Leigh JA. Markerless mutagenesis in Methanococcus maripaludis demonstrates roles for alanine dehydrogenase, alanine racemase, and alanine permease. J. Bacteriol. 2005;187:972–9. doi: 10.1128/JB.187.3.972-979.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–9. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- [31].Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 2005;41:207–34. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- [32].Hohn MJ, Palioura S, Su D, Yuan J, Söll D. Genetic analysis of selenocysteine biosynthesis in the archaeon Methanococcus maripaludis. Mol. Microbiol. 2011;81:249–58. doi: 10.1111/j.1365-2958.2011.07690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lin W, Whitman WB. The importance of porE and porF in the anabolic pyruvate oxidoreductase of Methanococcus maripaludis. Arch. Microbiol. 2004;181:68–73. doi: 10.1007/s00203-003-0629-1. [DOI] [PubMed] [Google Scholar]
- [34].Leinfelder W, Forchhammer K, Veprek B, Zehelein E, Böck A. In vitro synthesis of selenocysteinyl-tRNAUCA from seryl-tRNAUCA: involvement and characterization of the selD gene product. Proc. Natl. Acad. Sci. USA. 1990;87:543–7. doi: 10.1073/pnas.87.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Romero H, Zhang Y, Gladyshev VN, Salinas G. Evolution of selenium utilization traits. Genome Biol. 2005;6:R66. doi: 10.1186/gb-2005-6-8-r66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.