Abstract
Purpose
Blood-brain barrier (BBB) disruption is one of major consequences of radiation-induced normal tissue injury in the central nervous system. In the present study, we examined the effects of whole brain irradiation on matrix metalloproteinases (MMPs)/tissue inhibitors of metalloproteinases (TIMPs) and extracellular matrix (ECM) degradation in the brain.
Methods and Materials
Animals received either whole brain irradiation (a single dose of 10 Gy γ-rays or a fractionated dose of 40 Gy γ-rays total) or sham-irradiation, and were maintained for 4, 8, and 24 h following irradiation. The mRNA expression levels of MMPs and TIMPs in the brain were analyzed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR). The functional activity of MMPs was measured by in situ zymography and degradation of ECM was visualized by collagen type IV immunofluorescence staining.
Results
A significant increase in mRNA expression levels of MMP-2, MMP-9, and TIMP-1 was observed in irradiated brains compared to sham-irradiated controls. In situ zymography revealed a strong gelatinolytic activity in the brain 24 h post-irradiation and the enhanced gelatinolytic activity mediated by irradiation was significantly attenuated in the presence of anti-MMP-2 antibody. A significant reduction in collagen type IV immunoreactivity was also detected in the brain at 24 h after irradiation. In contrast, the levels of collagen type IV were not significantly changed at 4 and 8 h after irradiation compared with the sham-irradiated controls.
Conclusions
The present study demonstrates for the first time that radiation induces an imbalance between MMP-2 and TIMP-2 levels and suggests that degradation of collagen type IV, a major ECM component of BBB basement membrane, may have a role in the pathogenesis of brain injury.
Keywords: Whole brain irradiation, Matrix metalloproteinases, Tissue inhibitor of metalloproteinases, Extracellular matrix, Collagen type IV
INTRODUCTION
Radiation therapy continues to be a main treatment modality in the therapeutic management of brain tumors (1). About 200,000 individuals are treated with either partial large field or whole brain irradiation every year in the United States (2). The use of radiotherapy for treatment of brain tumors, however, is limited by the risk of radiation-induced injury to normal brain tissue that can subsequently lead to devastating functional deficits several months to years after treatment (3). Recent randomized, prospective trials also provide evidence that the addition of whole brain radiation therapy to stereotactic radiosurgery may cause a significant reduction in learning and memory in patients with brain metastasis (4). At present, there is sparse information on the etiology of radiation-induced damage to normal tissue in brain.
The extracellular matrix (ECM) is a complex of various proteins and proteoglycans, including collagens, laminin, fibronectin, and tenascin, which constitute the basal lamina of the blood-brain barrier (BBB) (5). Besides acting as a physical barrier to the passage of macromolecules and cells, ECM separates adjacent tissues, provides mechanical support for cell attachment, and serves as a substratum for cell migration and a medium of communication between cells (5). A number of studies have demonstrated that degradation and consequent rearrangement of ECM are critically involved in the breakdown of the BBB (6, 7). Despite a crucial role for ECM degradation in the BBB breakdown, the involvement of ECM remodeling in the pathophysiology of radiation-induced brain injury has not yet been investigated.
Matrix metalloproteinases (MMPs), a large family of ECM-degrading enzymes, have been implicated in the pathophysiological processes of neurodegenerative diseases by causing BBB disruption (8). The potential role of MMPs in brain injury in response to irradiation, however, remains largely unknown while evidence demonstrates that MMPs are associated with radiation-induced damage to various other tissues (8–11).
In the present study, we examined the critical role of MMPs in radiation-induced ECM degradation in brain to define the molecular mechanisms of BBB disruption and subsequent brain injury by whole brain irradiation. Our results provide the first novel evidence to demonstrate that MMP-2 plays a pivotal role in radiation-induced ECM degradation in brain.
METHODS AND MATERIALS
Animals
Fisher 344-Brown Norway (F344×BN) male rats and C57BL/6 male mice were purchased from Harlan Laboratories (Indianapolis, IN) and Jackson Laboratory (Bar Harbor, ME), respectively. Animals were housed on a 12/12 light-dark cycle with food and water provided ad libitum. Animal care was conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and this study was approved by the Institutional Animal Care and Use Committee.
Whole brain irradiation and tissue sample preparation
A single dose of whole brain irradiation procedure was carried out as described previously (12). Briefly, rats were anesthetized by ketamine/xylazine (i.p., 80/12 mg/kg) and received a single dose of 10 Gy (dose rate of 4.23 Gy/min) using a 137Cs γ-irradiator. Control rats were anesthetized but not irradiated. The animals were maintained for 4, 8, and 24 h post-irradiation. For real-time reverse transcriptase-polymerase chain reaction (RT-PCR), the rat brains were rapidly removed and two different brain regions (hippocampus and cortex) were dissected and immediately frozen in liquid nitrogen. For immunofluorescence staining and in situ zymography, the whole brains were rapidly removed after perfusion and immediately frozen in liquid nitrogen.
Fractionated whole brain irradiation was performed in mice as described previously (13). Briefly, mice were anesthetized by ketamine/xylazine (i.p., 100/15 mg/kg) and received a clinical fractionated dose of whole brain irradiation (total cumulative dose of 40 Gy in eight fractions of 5 Gy, twice a week for four weeks) using a 137Cs γ-irradiator. Mice in the control group were only anesthetized. The mice were maintained for 4, 8, and 24 h after the last fractionated dose of whole brain irradiation. The mouse brains were rapidly removed after perfusion and hemisected at the midline. Brains were then immediately frozen in liquid nitrogen.
Real-time RT-PCR
Quantitative real-time RT-PCR using TaqMan® probes and primers (Applied Biosystems, Foster City, CA) were employed for gene expression analyses as described previously (12). Amplification of individual genes was performed on the Applied Biosystems 7300 Real-Time PCR System using TaqMan® Universal PCR Master Mix and a standard thermal cycler protocol. TaqMan® Gene Expression Assay Reagents for rat MMP-2, rat MMP-3, rat MMP-7, rat MMP-9, rat MMP-10, rat MMP-12, rat TIMP-1, rat TIMP-2, rat glyceraldehydes-3-phosphate dehydrogenase (GAPDH), mouse MMP-2, mouse TIMP-2, and mouse GAPDH were used for specific probes and primers of PCR amplifications. The threshold cycle (CT) was determined and relative quantification was calculated by the comparative CT method as described previously (12).
In situ zymography
To detect and localize net gelatinolytic activity of MMPs in brain sections, in situ zymography was carried out as described previously (14). Briefly, 100 µg/ml fluorescein-conjugated DQ gelatin (Molecular Probes, Eugene, OR) was mixed with 0.2% agarose melted in reaction buffer at pH 7.6 (50 mM Tris-HCl, pH 7.5, 0.15M NaCl, 5 mM CaCl2 and 0.2 mM sodium azide). The brain sections (20-µm) were incubated with the reaction mixture prepared above for 24 h at 37°C in a moist dark chamber. The sections were then briefly washed with ice cold PBS and distilled water. Slides were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and examined using a Zeiss AXIO Imager A1m fluorescence microscope (Carl Zeiss MicroImaging, Thornwood, NY). Negative controls were prepared by incubation of tissue sections with non-immune rabbit serum (normal rabbit-IgG, Santa Cruz Biotechnology, Santa Cruz, CA) instead of the primary antibody, and rabbit anti-MMP-2 and rabbit anti-MMP-9 polyclonal antibodies (Santa Cruz Biotechnology) were added to the reaction to inhibit metalloproteinase activities. Images were acquired with 200× objective by AxioCam MRc5 Digital Imaging System. The MMP activity of acquired digital images was quantified with ImageJ software (NIH, Bethesda, MD).
Immunofluorescence staining
The brain sections (20-µm) were fixed in 4% paraformaldehyde for 15 min at room temperature, rinsed with PBS, and incubated in 0.5% Triton X-100 for 15 min. After washing with PBS, the non-specific binding sites were blocked with 3% bovine serum albumin (BSA) in PBS for 1 h at room temperature, followed by incubation with the primary antibody, goat anti-collagen type IV antibody (Southern Biotechnology, Birmingham, AL) diluted 1/500 in 1.5% BSA, overnight at 4°C. Sections were washed with PBS and incubated with secondary antibody, bovine anti-goat IgG conjugated with Texas Red (Santa Cruz Biotechnology), diluted 1/100 in PBS in the dark for 1 h. After washing with PBS, the sections were mounted in Vectashield mounting medium and examined using a Zeiss AXIO Imager A1m fluorescence microscope. Images were acquired with 100× objective by AxioCam MRc5 Digital Imaging System. The fluorescence intensity of acquired digital images was quantified by ImageJ software.
Statistical analysis
The statistical analysis of data was completed using SigmaStat 3.5 (SPSS, Chicago, IL). One-way analysis of variance (ANOVA) was used to compare mean responses among the treatments. For each endpoint, the treatment means were compared using the Bonferroni least significant difference procedure. Statistical probability of p<0.05 was considered significant.
RESULTS
A single dose of whole brain irradiation up-regulates mRNA expression of MMP-2, MMP-9, and TIMP-1 in rat brain
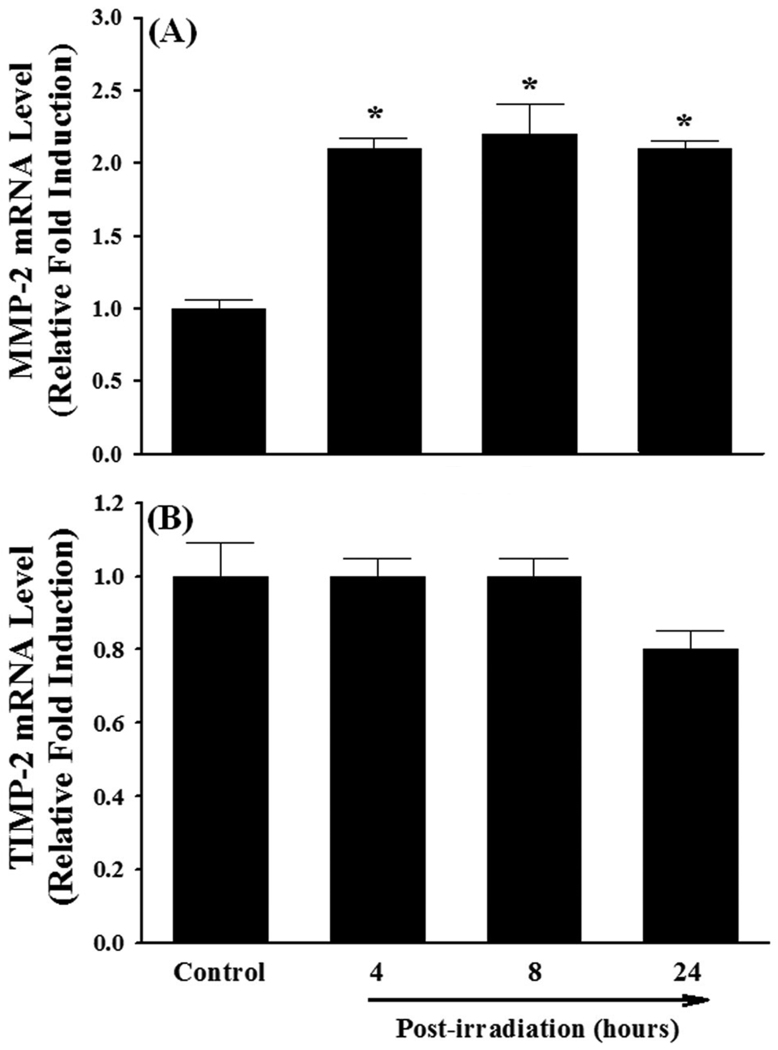
To examine whether a single dose of whole brain irradiation affects the MMPs/TIMPs system in brain, the mRNA expression levels of MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-12, TIMP-1, and TIMP-2 were determined by quantitative real-time RT-PCR. As indicated in Fig.1A, a significant up-regulation of MMP-2 mRNA expression was observed at 24 h post-irradiation in hippocampus (3.1-fold induction) and 8 and 24 h post-irradiation in cortex (3.0-fold and 6.4-fold) collected from irradiated rats compared to the sham-irradiated controls. In addition, a single dose of whole brain irradiation significantly increased mRNA expression levels of MMP-9 in hippocampus (1.6-fold at 4 h, 2.8-fold at 8 h, and 1.7-fold at 24 h post-irradiation) and cortex (1.9-fold at 8 h post-irradiation) (Fig.1B). In contrast, irradiation did not alter mRNA expression levels of MMPs-3, -7, -10, and -12 (data not shown). Furthermore, irradiation significantly up-regulated mRNA expression of TIMP-1 (Fig. 1C), a specific endogenous inhibitor of MMP-9, in hippocampus and cortex at 4 h (9.2-fold and 5.5-fold), 8 h (16-fold and 7.9-fold), and 24 h (15-fold and 6.1-fold) post-irradiation, while the mRNA expression of TIMP-2, a specific endogenous inhibitor of MMP-2, was not affected by irradiation (Fig. 1D). These results suggest that irradiation differentially regulates MMPs/TIMPs system in brain.
Fig. 1.
Effect of a single dose of whole brain irradiation on mRNA expression of MMPs and TIMPs in rat brain. Compared with sham-irradiated controls, a single dose of whole brain irradiation up-regulated mRNA expression of MMP-2 (A), MMP-9 (B), and TIMP-1 (C) in hippocampus and cortex, while the mRNA expression of TIMP-2 (D) was not affected by irradiation. Data shown are mean ± SEM for each group (n=4). *Statistically significant from control (p<0.05).
A single dose of whole brain irradiation enhances the gelatinase activity of MMPs in rat brain
To determine whether irradiation-mediated increases in MMP-2 and MMP-9 mRNA levels translate to elevated protein expression and functional activity, the histological distribution of the gelatinase activity was determined by in situ zymography using FITC-labeled DQ-gelatin. As illustrated in Fig. 2, a strong gelatinolytic activity was observed in rat brains 24 h after irradiation, whereas very little activity was detected in sham-irradiated control rat brains as well as in brains 4 and 8 h post-irradiation (Fig. 2A–2D). Quantitative analysis also demonstrated a significant increase in gelatinase activity in brains 24 h after irradiation compared to the sham-irradiated control rat brains (Fig. 2E). In addition, anti-rat MMP-2 and MMP-9 neutralizing antibodies were employed to further elucidate the critical role of either MMP-2 or MMP-9 in radiation-induced gelatinase activity. These antibodies were selected for their abilities to neutralize the biological activity of MMP-2 and MMP-9, respectively. As shown in Fig. 3, enhanced gelatinase activity in irradiated brain was significantly attenuated in the presence of MMP-2 neutralizing antibody (Fig. 3B and 3E). Interestingly, incubation with MMP-9 neutralizing antibody (Fig. 3C and 3E) and normal non-immune IgG (negative control, Fig. 3D) did not exert any significant effects on radiation-induced gelatinolytic activity. These data demonstrate that irradiation-induced MMP-2 expression is critically involved in enhanced gelatinase activity in brain.
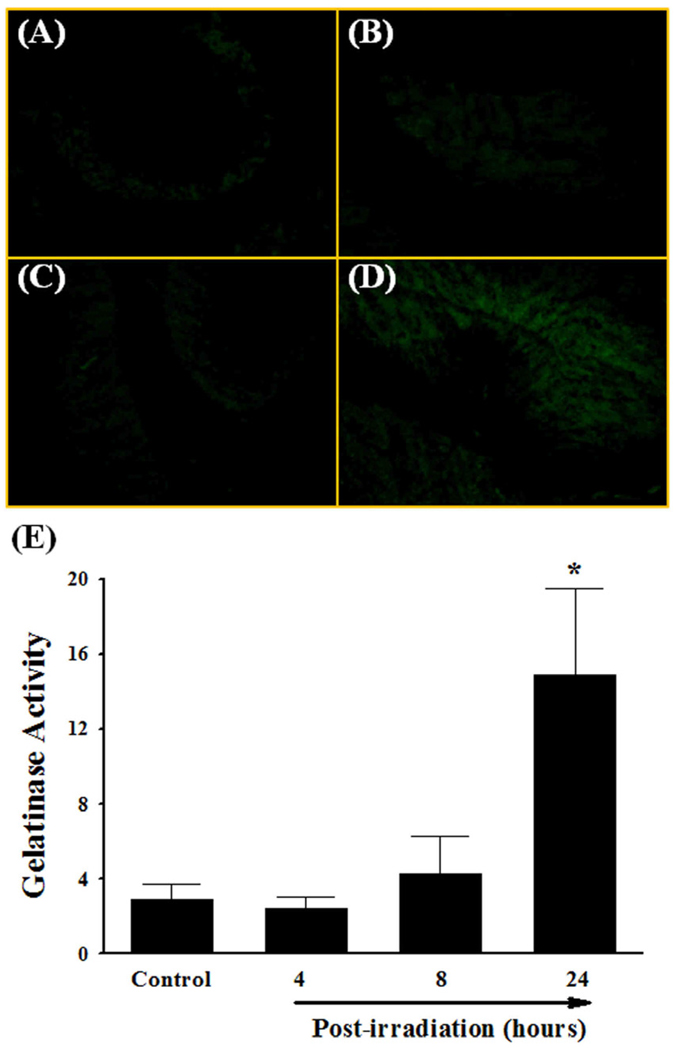
Fig. 2.
Effect of a single dose of whole brain irradiation on gelatinase activity in rat brain. The localization of green fluorescence indicates gelatinolytic activity of MMPs in rat brain (A–D). Quantitative analysis indicated a significant increase in gelatinase activity in brain 24 h after whole brain irradiation compared to sham-irradiated controls (E). Images (A–D) are as viewed under microscope at 200×. Data shown are mean ± SEM for each group (n=4). *Statistically significant from control (p<0.05). A: sham-irradiation (Control); B: 4 h post-irradiation; C: 8 h post-irradiation; D: 24 h post- irradiation; E: quantitative analysis of fluorescence intensity.
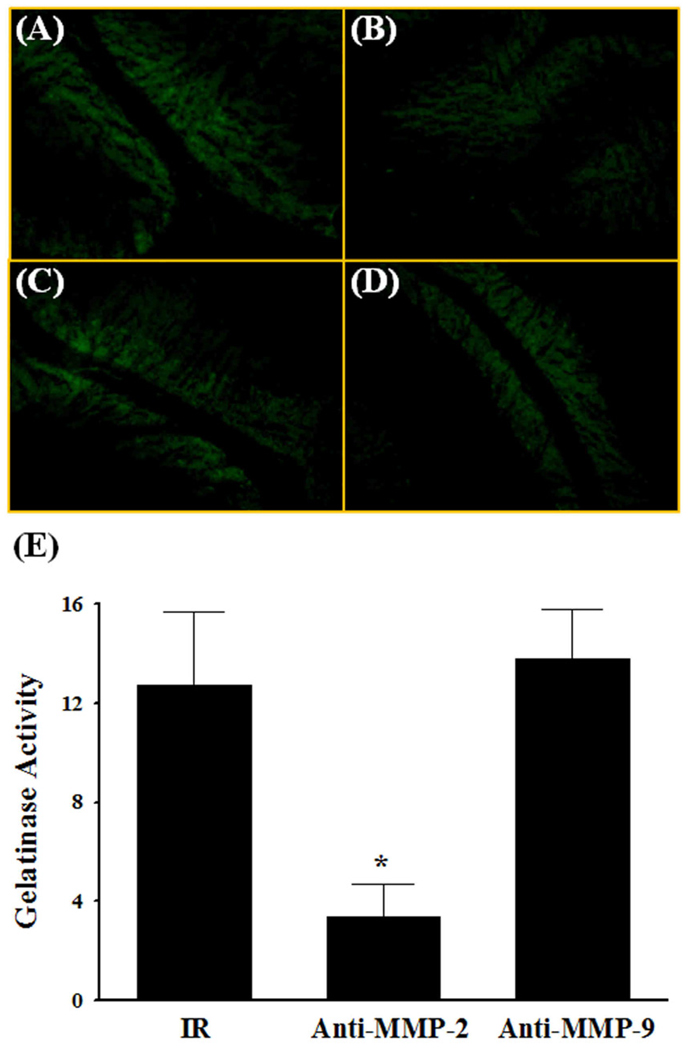
Fig. 3.
Effect of MMP-2 and MMP-9 neutralizing antibodies on radiation-induced gelatinase activity in rat brain. The localization of green fluorescence indicates gelatinolytic activity of MMPs in rat brain (A–D). Enhanced gelatinase activity in brains 24 h after a single dose of whole brain irradiation (IR) was significantly attenuated in the presence of MMP-2 neutralizing antibody (Anti-MMP-2) (E). Images (A–D) are as viewed under microscope at 200×. Data shown are mean ± SEM for each group (n=4). *Statistically significant from irradiated brain (p<0.05). A: 24 h post-irradiation; B: 24 h post-irradiation with anti-MMP-2 antibody; C: 24 h post-irradiation with anti-MMP-9 antibody; D: 24 h post-irradiation with normal non-immune IgG; E: quantitative analysis of fluorescence intensity.
A single dose of whole brain irradiation degrades ECM in rat brain
To investigate whether a single dose of whole brain irradiation mediates degradation of ECM, the expression levels of collagen type IV, one of the major ECM components of BBB basement membrane, in brains were visualized by immunofluorescence staining. As illustrated in Fig. 4, a significant reduction in collagen type IV immunoreactivity was detected in brains 24 h after irradiation compared with sham-irradiated control brains. In contrast, the expression levels of collagen type IV were not significantly changed in rat brains 4 and 8 h after irradiation.
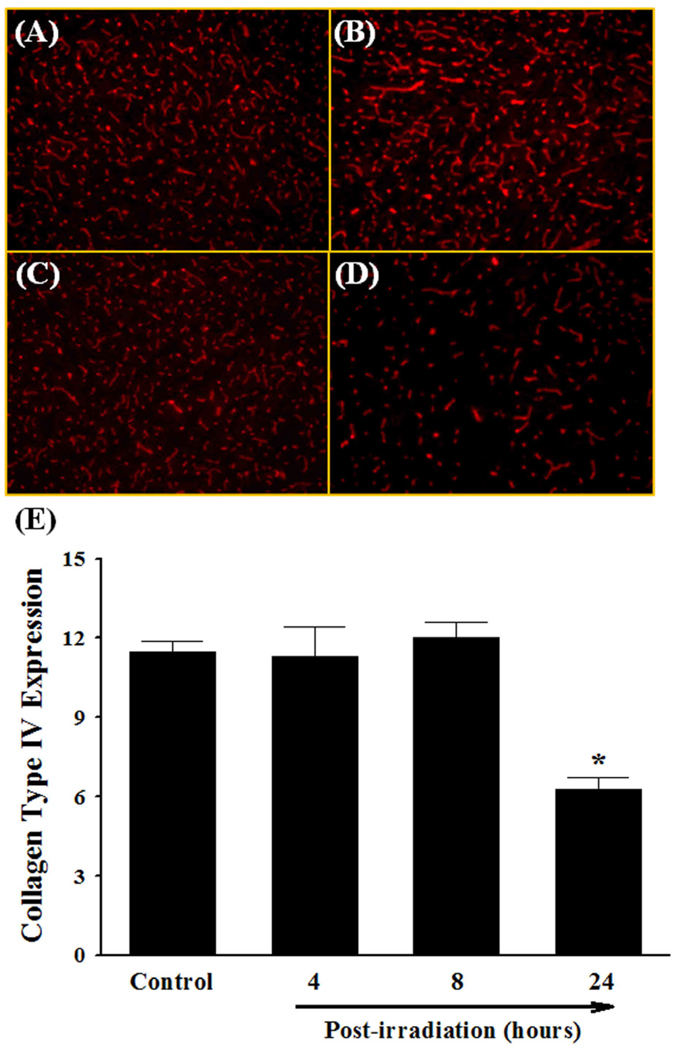
Fig. 4.
Effect of a single dose of whole brain irradiation on collagen type IV expression in rat brain. Immunoreactivity of collagen type IV was visualized in rat brain (A–D). A single dose of whole brain irradiation markedly and significantly reduced the protein expression levels of collagen type IV in rat brains 24 h after irradiation (E). Images (A–D) are as viewed under microscope at 100×. Data shown are mean ± SEM for each group (n=4). *Statistically significant from control (p<0.05). A: sham-irradiation (Control); B: 4 h post-irradiation; C: 8 h post-irradiation; D: 24 h post-irradiation; E: quantitative analysis of fluorescence intensity.
Fractionated whole brain irradiation mediates induction of MMP-2 mRNA expression, increase in gelatinase activity, and degradation of ECM in mouse brain
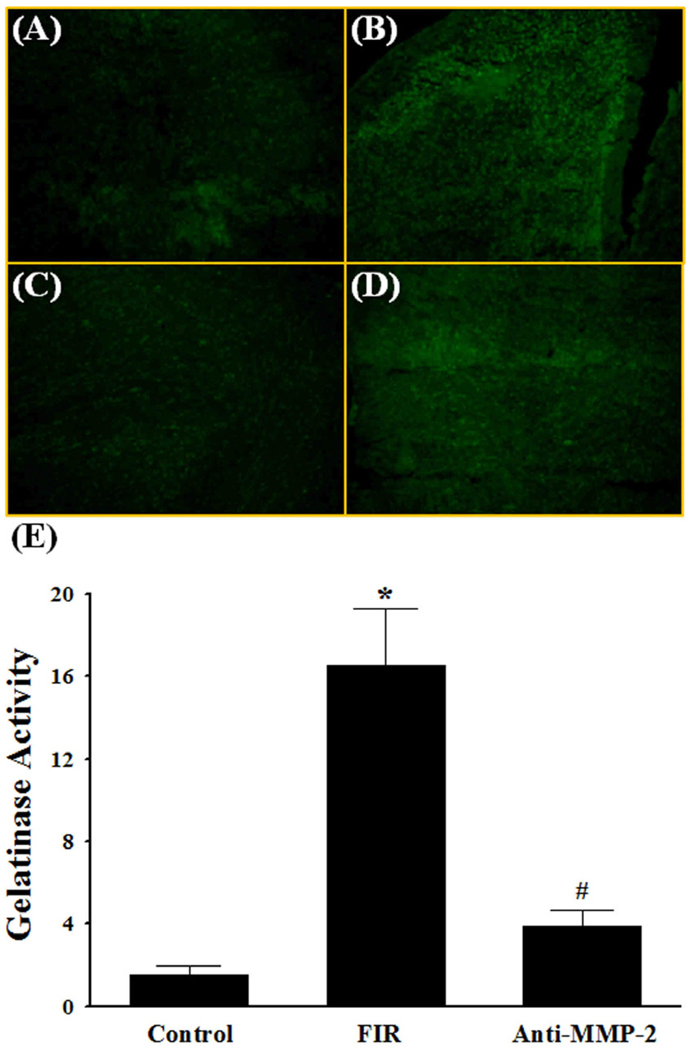
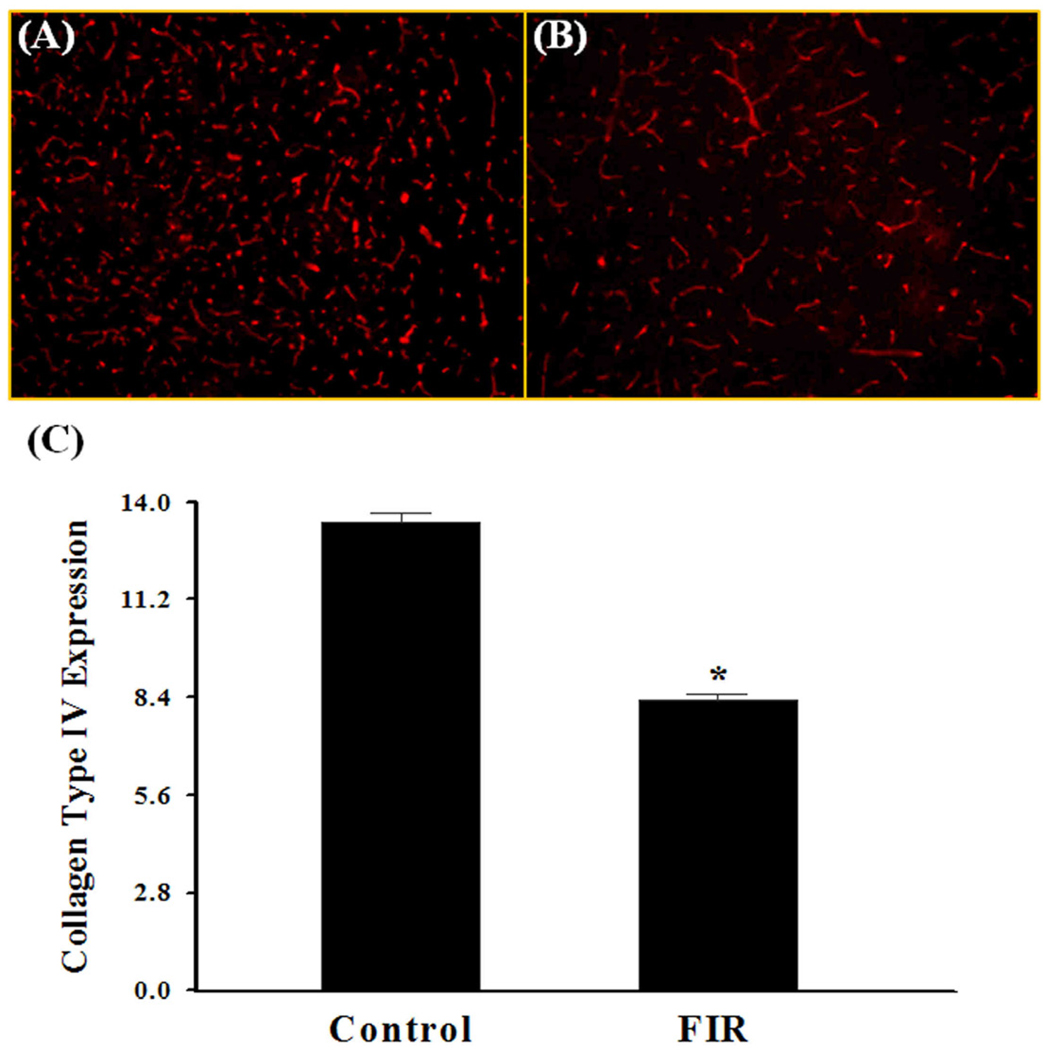
In addition to a single dose of whole brain irradiation, the present study also examined the effect of a clinically relevant regimen of fractionated whole brain irradiation on MMP-2 expression and ECM degradation in brains from mice. Quantitative real-time RT-PCR showed that irradiation resulted in a significant up-regulation of MMP-2 mRNA expression in mouse brains at 4, 8, and 24 h after the completion of a fractionated dose of whole brain irradiation. Specifically, the mRNA expression levels of MMP-2 in mouse brains were increased by 2.1-fold at 4 h, 2.2-fold at 8 h, and 2.1-fold at 24 h after irradiation (Fig. 5A). In contrast, fractionated whole brain irradiation did not affect mRNA expression levels of TIMP-2 (Fig. 5B). Fractionated whole brain irradiation also significantly increased gelatinase activity by 16.5-fold at 24 h post-irradiation compared to the sham-irradiated control brains (Fig. 6A, 6B and 6E). In contrast, radiation-mediated increase in gelatinase activity in the brain was significantly attenuated in the presence of a neutralizing antibody against MMP-2 (Fig. 6C and 6E). The negative control experiment with normal non-immune IgG did not show any significant effect on gelatinolytic activity in irradiated brain (Fig. 6D). We further examined the effect of fractionated whole brain irradiation on ECM degradation in mouse brain. As depicted in Fig. 7, a significantly reduced collagen type IV immunoreactivity was observed in mouse brain at 24 h after fractionated whole brain irradiation compared with those determined in sham-irradiated controls.
Fig. 5.
Effect of fractionated whole brain irradiation on mRNA expression of MMP-2 and TIMP-2 in mouse brain. Compared with sham-irradiated controls, fractionated whole brain irradiation up-regulated mRNA expression of MMP-2 (A) in mouse brain, while the expression of TIMP-2 was not affected by irradiation (B). Data shown are mean ± SEM for each group (n=4). *Statistically significant from control (p<0.05).
Fig. 6.
Effect of MMP-2 neutralizing antibody on radiation-induced gelatinase activity in mouse brain. The localization of green fluorescence demonstrates the gelatinolytic activities of MMPs in mouse brain (A–D). A significant increase in gelatinase activity in brains 24 h after fractionated whole brain irradiation (FIR) was markedly and significantly attenuated in the presence of MMP-2 neutralizing antibody (Anti-MMP-2) (E). Images (A–D) are as viewed under microscope at 200×. Data shown are mean ± SEM for each group (n=4). *Statistically significant from control (p<0.05). #Statistically significant from the irradiated brain (p<0.05). A: sham-irradiation (Control); B: 24 h post-irradiation; C: 24 h post-irradiation with anti-MMP-2 antibody; D: 24 h post-irradiation with normal non-immune IgG; E: quantitative analysis of fluorescence intensity.
Fig. 7.
Effect of fractionated whole brain irradiation on collagen type IV expression in mouse brain. Immunoreactivity of collagen type IV was visualized in mouse brain (A and B). Fractionated whole brain irradiation significantly reduced the protein expression levels of collagen type IV in brains 24 h after irradiation (FIR) (C). Images (A and B) are as viewed under microscope at 100×. Data shown are mean ± SEM for each group (n=4). *Statistically significant from control (p<0.05). A: sham-irradiation (Control); B: 24 h post-irradiation; C: quantitative analysis of fluorescence intensity.
DISCUSSION
The present study provides compelling evidence that whole brain irradiation induces an imbalance between MMPs and TIMPs expression, increases gelatinase activity, and degrades collagen type IV in the brain. Initially, an animal model of whole brain irradiation with a single dose of 10 Gy was selected because it is the lowest dose to have a clear radiation effect and is under the threshold for vascular changes, demyelination, or radionecrosis (12). Studies were also conducted after a fractionated dose of whole brain irradiation to assess a clinical relevance (12, 15). A significant and marked decrease in collagen type IV immunoreactivity as well as a strong gelatinase activity was detected in the brain 24 h after irradiation. In addition, an enhanced gelatinolytic activity mediated by irradiation was significantly attenuated in the presence of anti-MMP-2 antibody, highlighting the contribution of MMP-2 to the radiation-induced alterations of ECM components in the brain. To our knowledge, these results demonstrated for the first time that a radiation-induced imbalance between MMP-2 and TIMP-2 expression may have an important role in the pathogenesis of brain injury by degrading ECM components of the BBB basement membrane.
In a variety of physiological and pathophysiological conditions, MMPs become activated and play a key role in degradation of the ECM proteins (16). Recent evidence from in vivo and in vitro studies has identified that ionizing irradiation up-regulates MMPs expression in various tissues. For example, enhanced activity of MMP-2 and MMP-9 was observed in lung after thoracic irradiation (9). Araya et al. (10) have reported a significant elevation of MMP-2 production in human airway epithelial cells after irradiation. Additionally, previous clinical studies showed that use of pelvic radiation therapy for prostate cancer patients resulted in significant increases in MMP-2 and MMP-9 activity in rectal mucosa (11). It was also found that abdominal irradiation led to a significant elevation in MMP-2 and MMP-14 levels in rat ileum (8). These findings are also supported by in vitro study from Nirmala et al. (17) demonstrating that radiation-induced increased expression levels of MMP-2 and MMP-9 may be involved in the alteration of the CNS microvasculature by regulating glial-endothelial cells interaction. Furthermore, our previous study showed that whole brain irradiation significantly up-regulated MMP-9 mRNA expression for all age groups in rat brain (18). Alterations in the levels of other MMPs after irradiation in normal brain, however, remain to be further investigated. In the present study, we examined effects of whole brain irradiation on various MMPs in brain. MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, and MMP-12 were chosen because previous studies have demonstrated the pivotal role of these MMPs in ECM degradation of the BBB basement membrane including collagen type IV, laminin, and fibronectin (16).
The enzymatic activity of MMPs is inactivated by TIMPs, the endogenous inhibitors with a higher affinity for specific MMPs (19). For example, TIMP-1 forms a specific complex with MMP-9, whereas MMP-2 is bound by TIMP-2. Therefore, the balance between MMPs and TIMPs is considered necessary for normal homeostasis during periods of development and plasticity in the CNS (7). Since our data demonstrated the selective induction of MMP-2 and MMP-9 in irradiated brains, we further examined the effects of whole brain irradiation on TIMP-1 and TIMP-2 expression in the brain. TIMP-1 mRNA expression was found to be up-regulated in the irradiated rat colon while the mRNA level of TIMP-2 was unchanged (20). In lung epithelial cells, radiation increased MMP-2 mRNA but had no effect on TIMP-2 indicating that the balance between the MMP-2 and TIMP-2 was in favor of MMP-2 promoting proteolysis (10). Our own data indicate that whole brain irradiation resulted in a significant increase in TIMP-1 mRNA expression in the brain at 4, 8, and 24 h after irradiation. We could speculate that radiation-mediated up-regulation of TIMP-1 expression was counterbalanced by overexpression of MMP-9 in irradiated brain suggesting that the balance of MMP-9/TIMP-1 levels remains unchanged. In contrast, mRNA expression of TIMP-2 was not affected by either a single dose or fractionated whole brain irradiation in the brain. It is possible that the balance in the relative ratio of MMP-2 to TIMP-2 levels was altered in favor of a persistent enzymatic activity of MMP-2. Our data from neutralizing antibody experiment further supports the critical role of MMP-2 in radiation-mediated increase in gelatinase activity.
Radiation-induced vascular damage, in the form of subintimal fibrosis, hyalinization of vessels, increased vascular permeability, and edema may be responsible for tissue injury. For example, irradiation mediates disruption of the BBB by altering the structural and functional integrity of the microvasculature in brain (21). In addition, damage to the ECM is one of the important components of radiation-induced tissue injury (22, 23). In fact, irradiation induces immediate damage to ECM in connective tissue through depolymerization of hyaluronic acid in synovial fluid (24). Brinkman et al. (25) also demonstrated that radiation-induced degradation of hyaluronic acid is mediated by hydroxyl radicals from water hydrolysis. Furthermore, several studies showed that irradiation causes loss of proteoglycans of the vascular basement membrane, resulting in edema and transudation of proteins into the parenchyma (21, 23, 26).
In addition, previous studies have demonstrated an essential role for collagen type IV degradation in BBB disruption and brain injury. Collagen type IV is the major ECM constituent of the BBB basement membrane and a known substrate of MMP-2 and MMP-9 (10). Scholler et al. (27) have shown that a significant decrease in microvascular collagen type IV immunoreactivity following subarachnoid hemorrhage is correlated with increased BBB permeability and may be, at least in part, responsible for ischemic brain edema. Additionally, acute loss of basal lamina antigens including collagen type IV during cerebral ischemia/reperfusion injury may offer an explanation for understanding decreased microvascular integrity as well as development of hemorrhagic complications of acute stroke (28). Even though the importance of collagen type IV degradation in BBB disruption and brain injury was suggested previously, the mechanism by which proteases are involved in compromising the integrity of the BBB has not yet been fully understood. Studies from Rosenberg et al. (6) provide evidence suggesting the potential role of MMP-2 and collagen type IV in BBB breakdown. These investigators showed that direct intracerebral administration of MMP-2 resulted in an increase in collagen type IV degradation and BBB permeability, and inhibition of MMP-2 (by injection of TIMP-2) prevented BBB disruption. These results support our observations that collagen type IV degradation in the irradiated brain may be mediated through enhanced MMP-2 expression and activity. However, the causal relationship between MMP-2 activity and ECM degradation remains unclear and needs to be further investigated. Selective MMP-2 inhibitors (29) and MMP-2 knockout mice (30) could be employed as in-depth pharmacological and genetic approaches to elucidate a mechanistic link among MMP-2 expression, collagen type IV degradation, and BBB disruption in brain after irradiation.
In conclusion, we provide the first direct evidence indicating that whole brain irradiation mediates degradation of collagen type IV, a major ECM component of the BBB basement membrane, by altering the balance of MMP-2 and TIMP-2 levels in the brain. These findings may contribute to defining a new cellular and molecular basis for radiation-mediated BBB disruption and subsequent brain injury that will lead to new opportunities for preventive and therapeutic interventions for brain tumor patients who are undergoing radiotherapy.
Acknowledgment
The project described was supported by Grant Number R01NS056218 from the National Institute of Neurological Disorders and Stroke.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None.
Conflict of Interest Notification: None
REFERENCES
- 1.Kantor G, Laprie A, Huchet A, et al. Radiation therapy for glial tumors: technical aspects and clinical indications. Cancer Radiother. 2008;12:687–694. doi: 10.1016/j.canrad.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 3.Denham JW, Hauer-Jensen M. The radiotherapeutic injury-a complex 'wound'. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27:93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg GA, Estrada E, Kelley RO, et al. Bacterial collagenase disrupts extracellular matrix and opens blood-brain barrier in rat. Neurosci Lett. 1993;160:117–119. doi: 10.1016/0304-3940(93)90927-d. [DOI] [PubMed] [Google Scholar]
- 7.Sellner J, Simon F, Meyding-Lamade U, et al. Herpes-simplex virus encephalitis is characterized by an early MMP-9 increase and collagen type IV degradation. Brain Res. 2006;1125:155–162. doi: 10.1016/j.brainres.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 8.Strup-Perrot C, Vozenin-Brotons MC, Vandamme M, et al. Expression of matrix metalloproteinases and tissue inhibitor metalloproteinases increases in X-irradiated rat ileum despite the disappearance of CD8a T cells. World J Gastroenterol. 2005;11:6312–6321. doi: 10.3748/wjg.v11.i40.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang K, Liu L, Zhang T, et al. TGF-betal transgenic mouse model of thoracic irradiation: modulation of MMP-2 and MMP-9 in the lung tissue. J Huazhong Univ Sci Technolog Med Sci. 2006;26:301–304. doi: 10.1007/BF02829557. [DOI] [PubMed] [Google Scholar]
- 10.Araya J, Maruyama M, Sassa K, et al. Ionizing radiation enhances matrix metalloproteinase-2 production in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L30–L38. doi: 10.1152/ajplung.2001.280.1.L30. [DOI] [PubMed] [Google Scholar]
- 11.Hovdenak N, Wang J, Sung CC, et al. Clinical significance of increased gelatinolytic activity in the rectal mucosa during external beam radiation therapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:919–927. doi: 10.1016/s0360-3016(02)02808-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee WH, Sonntag WE, Mitschelen M, et al. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 2010;86:132–144. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conner KR, Payne VS, Forbes ME, et al. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res. 2010;173:49–61. doi: 10.1667/RR1821.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh LY, Larsen PH, Krekoski CA, et al. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler JF. Brief summary of radiobiological principles in fractionated radiotherapy. Sem Radia Oncol. 1992;2:16–21. [Google Scholar]
- 16.Planas AM, Sole S, Justicia C. Expression and activation of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis. 2001;8:834–846. doi: 10.1006/nbdi.2001.0435. [DOI] [PubMed] [Google Scholar]
- 17.Nirmala C, Jasti SL, Sawaya R, et al. Effects of radiation on the levels of MMP-2, MMP- 9 and TIMP-1 during morphogenic glial-endothelial cell interactions. Int J Cancer. 2000;88:766–771. doi: 10.1002/1097-0215(20001201)88:5<766::aid-ijc13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Lee WH, Sonntag WE, Lee YW. Aging attenuates radiation-induced expression of pro-inflammatory mediators in rat brain. Neurosci Lett. 2010;476:89–93. doi: 10.1016/j.neulet.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukes A, Mun-Bryce S, Lukes M, et al. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol. 1999;19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- 20.Strup-Perrot C, Vozenin-Brotons MC, Vandamme M, et al. Expression and activation of MMP -2, -3, -9, -14 are induced in rat colon after abdominal X-irradiation. Scand J Gastroenterol. 2006;41:60–70. doi: 10.1080/00365520510023963. [DOI] [PubMed] [Google Scholar]
- 21.Baker DG, Krochak RJ. The response of the microvascular system to radiation: a review. Cancer Invest. 1989;7:287–294. doi: 10.3109/07357908909039849. [DOI] [PubMed] [Google Scholar]
- 22.Thomlinson RH. The implications of radiobiology for radiotherapists. Clin Radiol. 1974;25:471–474. doi: 10.1016/s0009-9260(74)80127-3. [DOI] [PubMed] [Google Scholar]
- 23.Law MP. Radiation-induced vascular injury and its relation to late effects in normal tissues. Adv in Radiat Biol. 1981;9:37–73. [Google Scholar]
- 24.Aarnoudse MW, Lamberts HB. Depolymerization of mucopolysaccharides by X-rays and fast neutrons. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;20:437–445. doi: 10.1080/09553007114551341. [DOI] [PubMed] [Google Scholar]
- 25.Brinkman R, Lamberts HB. Current topics in Radiation Research. Vol II 1966. Radiopathology of extracellular structures. [Google Scholar]
- 26.Phillips TL. An ultrastructural study of the development of radiation injury in the lung. Radiology. 1966;87:49–54. doi: 10.1148/87.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Scholler K, Trinkl A, Klopotowski M, et al. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Hamann GF, Okada Y, Fitridge R, et al. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki Y, Xu ZZ, Wang X, et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corry DB, Rishi K, Kanellis J, et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–353. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]