Abstract
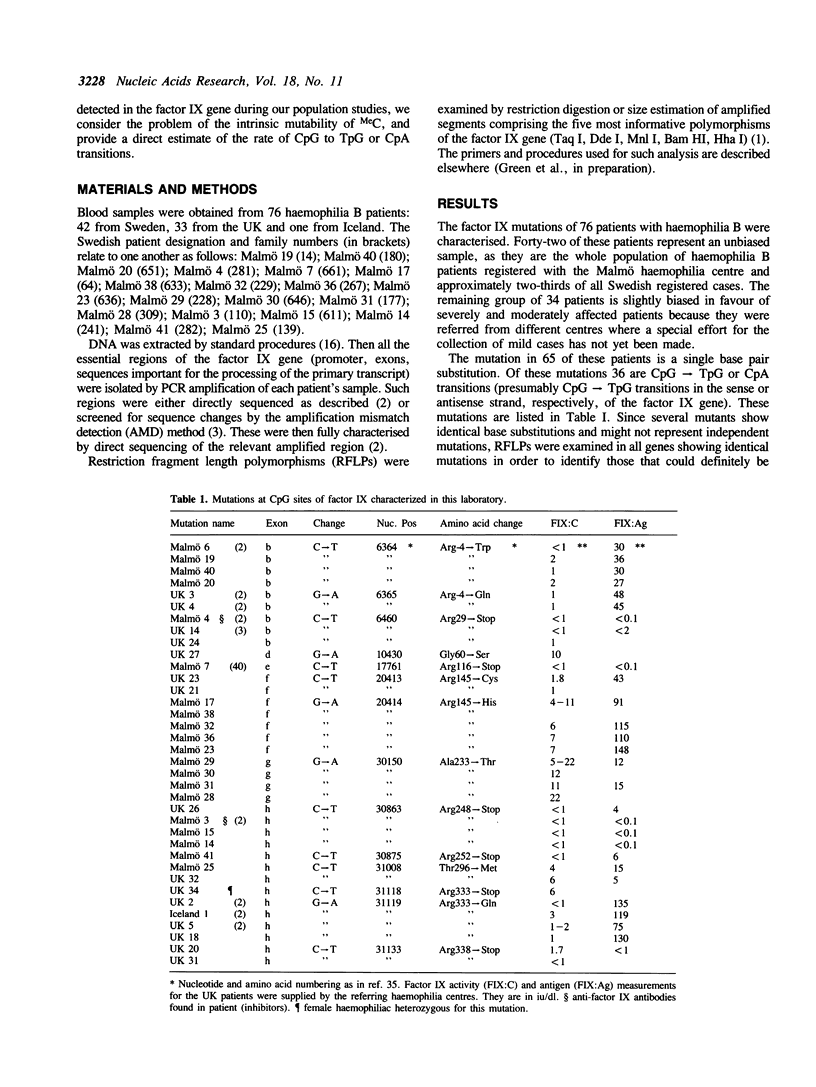
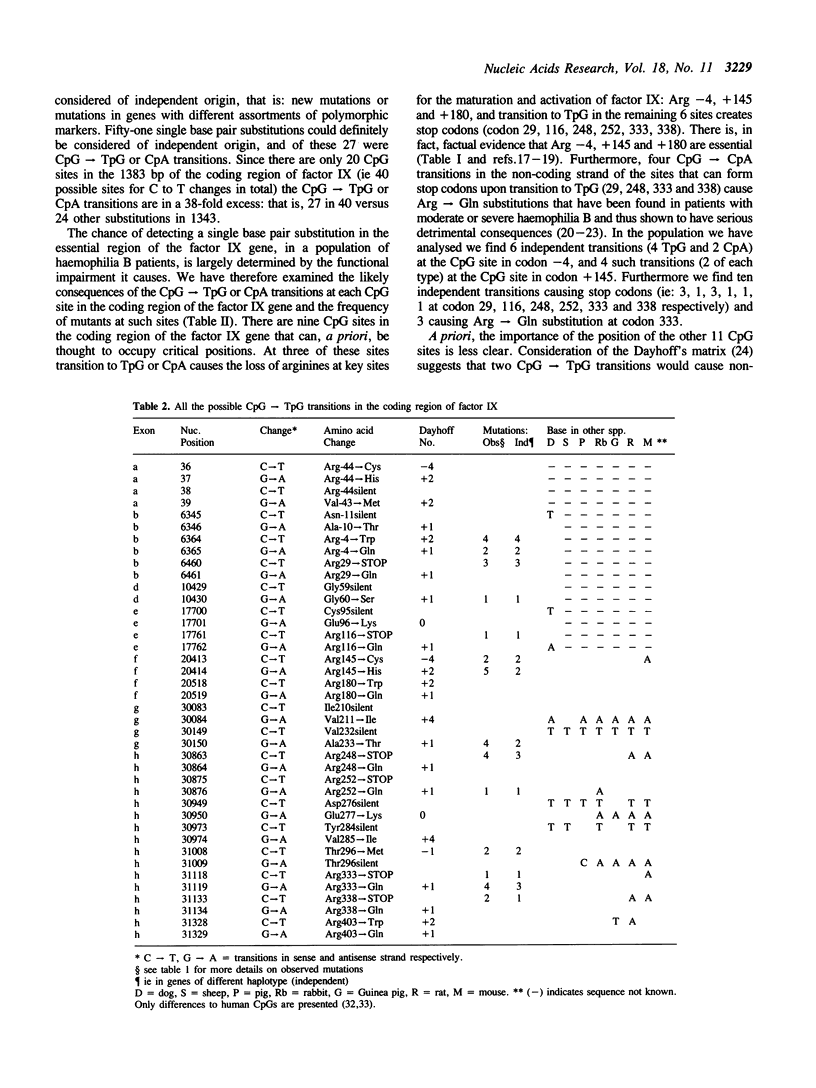
The mutations of 76 haemophilia B patients representing the whole population registered with the Malmö haemophilia centre (42) and referrals from the UK, were characterised. RFLP haplotype analysis of the defective genes indicated that 51 single base pair substitutions were definitely of independent origin and 27 of these were CpG----TpG or CpA transitions. This represents a 38-fold excess over other single-base changes. Most of such transitions (82%) occur at 9 CpG sites occupying critical positions (transitions at 3 sites substitute essential arginines, while at 6 sites transition to TpG creates stop codons). Sixteen of the 18 possible transitions at these 9 sites cause clear haemophilia B and should be fully ascertained in our haemophilia B population. This allowed the direct estimate of the rate of CpG transitions. This is 1.05 x 10(-7) substitutions per base per gamete per generation. The marked excess of CpG transitions in haemophilia B appears partly due to the high proportion of CpG sites at critical positions (at least 9 out of 20). We propose that this follows from the fact that male hemizygosity makes X-linked genes particularly susceptible to selective forces that tend to fix CpG sites arising at critical positions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anson D. S., Choo K. H., Rees D. J., Giannelli F., Gould K., Huddleston J. A., Brownlee G. G. The gene structure of human anti-haemophilic factor IX. EMBO J. 1984 May;3(5):1053–1060. doi: 10.1002/j.1460-2075.1984.tb01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Bentley A. K., Rees D. J., Rizza C., Brownlee G. G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986 May 9;45(3):343–348. doi: 10.1016/0092-8674(86)90319-3. [DOI] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. C., Jiricny J. Different base/base mispairs are corrected with different efficiencies and specificities in monkey kidney cells. Cell. 1988 Aug 26;54(5):705–711. doi: 10.1016/s0092-8674(88)80015-1. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Thompson A. R., Zhang M., Scott C. R. Three point mutations in the factor IX genes of five hemophilia B patients. Identification strategy using localization by altered epitopes in their hemophilic proteins. J Clin Invest. 1989 Jul;84(1):113–118. doi: 10.1172/JCI114130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. N., Krawczak M. Cytosine methylation and the fate of CpG dinucleotides in vertebrate genomes. Hum Genet. 1989 Sep;83(2):181–188. doi: 10.1007/BF00286715. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- Evans J. P., Brinkhous K. M., Brayer G. D., Reisner H. M., High K. A. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. P., Watzke H. H., Ware J. L., Stafford D. W., High K. A. Molecular cloning of a cDNA encoding canine factor IX. Blood. 1989 Jul;74(1):207–212. [PubMed] [Google Scholar]
- Foster D. C., Yoshitake S., Davie E. W. The nucleotide sequence of the gene for human protein C. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4673–4677. doi: 10.1073/pnas.82.14.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F. Factor IX. Baillieres Clin Haematol. 1989 Oct;2(4):821–848. doi: 10.1016/s0950-3536(89)80048-4. [DOI] [PubMed] [Google Scholar]
- Green P. M., Bentley D. R., Mibashan R. S., Nilsson I. M., Giannelli F. Molecular pathology of haemophilia B. EMBO J. 1989 Apr;8(4):1067–1072. doi: 10.1002/j.1460-2075.1989.tb03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. N., Kasper C. K., Roberts H. R., Stafford D. W., High K. A. Molecular defect in factor IXHilo, a hemophilia Bm variant: Arg----Gln at the carboxyterminal cleavage site of the activation peptide. Blood. 1989 Feb 15;73(3):718–721. [PubMed] [Google Scholar]
- Koeberl D. D., Bottema C. D., Buerstedde J. M., Sommer S. S. Functionally important regions of the factor IX gene have a low rate of polymorphism and a high rate of mutation in the dinucleotide CpG. Am J Hum Genet. 1989 Sep;45(3):448–457. [PMC free article] [PubMed] [Google Scholar]
- Koop B. F., Goodman M., Xu P., Chan K., Slightom J. L. Primate eta-globin DNA sequences and man's place among the great apes. Nature. 1986 Jan 16;319(6050):234–238. doi: 10.1038/319234a0. [DOI] [PubMed] [Google Scholar]
- Leytus S. P., Chung D. W., Kisiel W., Kurachi K., Davie E. W. Characterization of a cDNA coding for human factor X. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3699–3702. doi: 10.1073/pnas.81.12.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Karlström O. Heat-induced depyrimidination of deoxyribonucleic acid in neutral solution. Biochemistry. 1973 Dec 4;12(25):5151–5154. doi: 10.1021/bi00749a020. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Heat-induced deamination of cytosine residues in deoxyribonucleic acid. Biochemistry. 1974 Jul 30;13(16):3405–3410. doi: 10.1021/bi00713a035. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. M., Koop B. F., Slightom J. L., Goodman M., Tennant M. R. Molecular systematics of higher primates: genealogical relations and classification. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7627–7631. doi: 10.1073/pnas.85.20.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Giannelli F., Bentley D. R. Direct detection of point mutations by mismatch analysis: application to haemophilia B. Nucleic Acids Res. 1989 May 11;17(9):3347–3358. doi: 10.1093/nar/17.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes C. M., Griffith M. J., Roberts H. R., Lundblad R. L. Identification of the molecular defect in factor IX Chapel Hill: substitution of histidine for arginine at position 145. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4200–4202. doi: 10.1073/pnas.80.14.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara P. J., Grant F. J., Haldeman B. A., Gray C. L., Insley M. Y., Hagen F. S., Murray M. J. Nucleotide sequence of the gene coding for human factor VII, a vitamin K-dependent protein participating in blood coagulation. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5158–5162. doi: 10.1073/pnas.84.15.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Koeberl D. D., Sommer S. S. Direct sequencing of the activation peptide and the catalytic domain of the factor IX gene in six species. Genomics. 1990 Jan;6(1):133–143. doi: 10.1016/0888-7543(90)90458-7. [DOI] [PubMed] [Google Scholar]
- Suehiro K., Kawabata S., Miyata T., Takeya H., Takamatsu J., Ogata K., Kamiya T., Saito H., Niho Y., Iwanaga S. Blood clotting factor IX BM Nagoya. Substitution of arginine 180 by tryptophan and its activation by alpha-chymotrypsin and rat mast cell chymase. J Biol Chem. 1989 Dec 15;264(35):21257–21265. [PubMed] [Google Scholar]
- Tsang T. C., Bentley D. R., Mibashan R. S., Giannelli F. A factor IX mutation, verified by direct genomic sequencing, causes haemophilia B by a novel mechanism. EMBO J. 1988 Oct;7(10):3009–3015. doi: 10.1002/j.1460-2075.1988.tb03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. Y., Kuo K. C., Gehrke C. W., Huang L. H., Ehrlich M. Heat- and alkali-induced deamination of 5-methylcytosine and cytosine residues in DNA. Biochim Biophys Acta. 1982 Jun 30;697(3):371–377. doi: 10.1016/0167-4781(82)90101-4. [DOI] [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]
- Zell R., Fritz H. J. DNA mismatch-repair in Escherichia coli counteracting the hydrolytic deamination of 5-methyl-cytosine residues. EMBO J. 1987 Jun;6(6):1809–1815. doi: 10.1002/j.1460-2075.1987.tb02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


