Abstract
This article investigates in vitro light transmission through the human cornea in the ultraviolet (UV) portion of the electromagnetic spectrum as a function of position across the cornea from center to periphery. Spectrophotometry was used to measure UV transmission in the wavelength range 310–400 nm, from the central cornea to its periphery. UV transmission decreases away from the center, and this is attributed to scattering and absorbance. Corneal endothelial cells, which line the back of the cornea and are more numerous in the periphery, therefore receive a lower dose of UV than do those in the central cornea. This is consistent with the recent observation that endothelial cells in the corneal periphery exhibit less nuclear oxidative DNA damage than those in the central cornea.
Introduction
The cornea is the transparent, collagen-rich tissue that forms the front of the eye. In humans it is just over 10 mm in diameter and at its edge merges with the white sclera that forms the rest of the eye's outer tunic. At the cornea's anterior surface is a thin, multilayered epithelium that coats the underlying corneal stroma. The stroma comprises ∼90% of the cornea and is ∼500 μm thick in man (1). It is a connective tissue matrix composed predominantly of modified type-I collagen fibrils, which are arranged parallel to each other in flat ribbon-like layers known as lamellae. Approximately 200–250 lamellae make up the human stroma (2), and these are thinner and more interwoven in the anterior portion of the stroma. Collagen fibrils are embedded in an extrafibrillar substance composed of proteoglycans (proteins to which are attached elongated and highly sulfated long disaccharide chains called glycosaminoglycans that attract and bind water), as well as other collagens, matrix molecules, and inorganic ions. Proteoglycans have an important role in maintaining the hydration of the stroma, and regulating fibril diameter and spacing, which is vital for corneal transparency (3). The extrafibrillar matrix also contains cells called keratocytes; with an estimated density of between 4.6 × 104 and 6.2 × 104 cells/mm3 in humans, with more cells located peripherally and in younger individuals (4). In adult rabbit cornea the percentage keratocyte cell volume within the stroma has been estimated at ∼10% (5). Keratocytes are modified fibroblasts that are responsible for the slow turnover of the stromal tissue and can be activated in a wound healing response. The corneal stroma contains soluble glycoproteins, the most abundant of which are aldehyde dehydrogenases. These are supposed to have a dual role of matching the refractive indices inside and outside the keratocytes, thus minimizing light scatter (5,6), and helping to protect the eye against the damaging effects of ultraviolet (UV) radiation (7).
Corneal collagen fibrils and the extrafibrillar substance have different refractive indices (8). This causes the stroma to scatter a modest proportion of the transmitted light away from the incident direction. Because the fibrils are of very small dimensions compared to the wavelength of light, they act as Rayleigh scatterers (8,9). Although the fibrils have a very small scattering cross section and are thus inefficient as individual scatterers, they are present in stroma in such great numbers that if they were arranged randomly the cornea would not be transparent (8–10). Stromal collagen fibrils are not arranged in a perfect crystallographic lattice, but there is sufficient order to render the stroma transparent to visible light due to interference effects (8–11). Corneal transmission is highest in the long wavelength region of the visible spectrum (600–700 nm), and lowest in the short (i.e., <500 nm) wavelength region (12–16).
Little is known about UV transmission through the cornea, other than the tissue does not transmit significant levels of radiation with wavelengths <∼290 nm (8,12). However, some UV radiation does reach the deeper layers of the cornea inducing light damage (17). Kolozsvári et al. (18) measured UV absorbance at the center of the cornea and showed a wavelength dependence of UVA absorbance in the stroma. 70% of the absorbance was attributed to the stroma, the rest coming from the epithelium and Bowman's layer beneath it. The effects of UV radiation are probably most serious in the posterior cellular lining, a single layer of endothelial cells that have little or no ability to proliferate in vivo (19). These cells are the source of a bicarbonate pump (20–22) that maintains the balance of fluid in the corneal stroma, thereby counteracting the swelling forces due to the negatively charged glycosaminoglycans. When the endothelium is damaged beyond its functional reserve, as happens with certain diseases or as an unintended consequence of intraocular surgery, the cornea swells, light scattering increases, and vision is adversely affected. In the UV region, in addition to Rayleigh scattering of UV wavelengths, there will be a certain amount of light absorption (18). UV has a deleterious effect on cells, and a reduction in UV transmission through the cornea from front to back will presumably serve to protect the endothelial cells from oxidative damage. Nevertheless, it is apparent that human endothelial cells are subject to age-related damage in which a reduction in relative ex vivo proliferative capacity occurs in cells in the central 3 mm of the cornea compared with cells in the peripheral 3–5 mm of the cornea (23,24). In vitro, endothelial cells from the central cornea, particularly from donors over 50 years of age, show a number of senescence-like characteristics, including reduced proliferative capacity, cellular enlargement, and increased oxidative nuclear DNA damage (23,25). Our current hypothesis is that the high metabolic activity of corneal endothelial cells, added to chronic exposure to light causes an age-related and position-dependant increase in oxidative stress that leads to nuclear oxidative DNA damage and results in premature stress-induced cell cycle inhibition. If this is the case, one would expect differences in the amount of UV radiation that reaches the endothelium centrally compared to peripherally.
Recently, we showed that visible light transmission is decreased in the peripheral cornea, and that this decrease is caused primarily by an increase in fibril radius and changes in the relative refractive index contrast between the fibrils and the extrafibrillar substance (14). We now extend these studies to examine the penetration of UV light through the central and peripheral human corneal stroma.
Materials and Methods
The project was approved by the National Health Service Research Ethics Committee (UK) and was carried out in accordance with the tenets of the Declaration of Helsinki, which govern the use of human material for research purposes. Four human corneas (age range: 35–67 years) with an intact scleral rim were obtained from the UK Transplant Service Bristol Eye Bank (Bristol, UK). They were not suitable for transplant due to low endothelial cell count and consent for their use in research projects was forthcoming.
Endothelial and epithelial cell layers were scraped off the corneas using laboratory razor blades and blotting paper. The debrided corneoscleral discs were then placed in a 12–14 kDa cutoff dialysis membrane and equilibrated to physiological hydration using 20 kDa polyethylene glycol as outlined elsewhere (26). In brief, the corneas were dialyzed for 24 h against 5 mM HEPES buffer, pH 7.4 containing 0.154 M sodium chloride, and polyethylene glycol (2.8% w/v). After removal from the dialysis membrane corneas were secured in a custom-built sample chamber, half-filled with silicon oil (Dow Corning 200/5cS). A Pye Unicam (Cambridge, UK) SP8-100 double beam spectrophotometer with detector half-angle acceptance of 3° and a beam size adjusted to 1 × 1 mm was used to determine the transmitted light intensity. The sample cell was placed in the spectrophotometer such that the beam was perpendicular to the cell window at all times. Baseline readings were made from cells filled only with silicon oil, and was recorded before each corneal measurement. Transmission measurements were expressed as a ratio of the baseline reading. The position of the cornea in the beam path was adjusted laterally with the aid of a vernier scale, and intensity measurements were recorded at 1 mm intervals radiating outward from the center of the cornea.
At the conclusion of transmission measurements, a 6 mm button was trephined from the central portion of each cornea. After weighing the buttons were heated at 60°C for a minimum period of 48 h before reweighing. Corneal hydration was then determined from the ratio of the wet and dry weights (Hydration, H = (wet weight of tissue-dry weight of tissue)/dry weight of tissue) to confirm that all corneas used were near physiological hydration (i.e., close to H = 3.2).
The summation of scattered fields method was used to calculate the UV light scatter from the corneal collagen fibrils, as described previously (14). The method uses a statistical approach to sum the scattering from individual collagen fibrils, taking into account their diameters, packing arrangement, and refractive indices (27).
Results and Discussion
UV light transmission through the human corneal stroma (H = 3.0–3.5) in the wavelength range of 310–400 nm indicates that, at all positions, corneal transmission decreases gradually at shorter wavelengths (Fig. 1). At wavelengths shorter than 310 nm, the recorded transmission diminishes rapidly, and transmitted light cannot be reliably measured using our technique (although others have demonstrated a rapid loss of transmission below 300 nm (18)). In the central cornea, we found that transmission decreases from 74% to 27% between wavelengths 400 and 310 nm, whereas 5 mm from the center it decreases from 47% to 18% over the same wavelength range (Table 1). The decreases appear to be approximately linear (Fig. 2), in contrast to the decrease in light transmission in the visible spectrum that falls off as the inverse cube of the wavelength (15). At all wavelengths examined here, UV light propagation decreases from central to peripheral (5 mm) regions through the corneal stroma (Table 2). From a clinical standpoint it is interesting to note that a decrease in UV transmission of almost 20% from the center to the periphery occurs at 370 nm, the wavelength conventionally used for riboflavin/UVA cross-linking of the cornea, which is increasingly used to treat progressive conditions such as keratoconus, a disease in which the cornea becomes weak and misshapen resulting in impaired vision (28).
Figure 1.
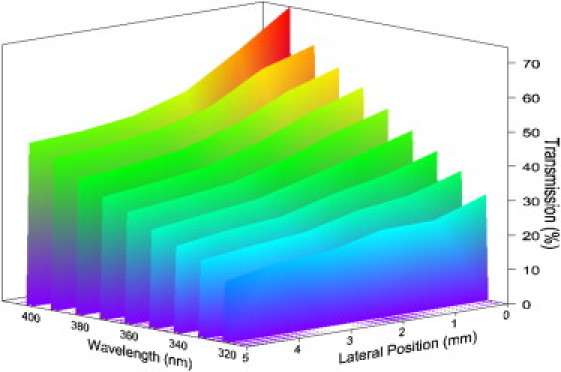
Three-dimensional representation of stromal UV transmission as a function of wavelength and of position from the center of the cornea. The reduction in transmission from center to periphery is apparent at all wavelengths, but is more dramatic at higher wavelengths.
Table 1.
Average transmission data for all four corneas
| Wavelength (nm) | Position (mm) |
|||||
|---|---|---|---|---|---|---|
| 0 mm | 1 mm | 2 mm | 3 mm | 4 mm | 5 mm | |
| 400 | 74.5 (17.0) | 64.2 (14.0) | 54.9 (13.3) | 50.3 (14.9) | 48.3 (11.1) | 47.4 (7.2) |
| 390 | 64.8 (17.9) | 60.3 (14.0) | 51.8 (13.2) | 47.6 (14.6) | 45.5 (11.2) | 44.0 (7.0) |
| 380 | 59.7 (17.1) | 55.0 (13.4) | 47.9 (12.8) | 43.2 (13.5) | 41.0 (10.5) | 39.6 (6.6) |
| 370 | 55.3 (16.4) | 50.5 (13.3) | 44.1 (12.5) | 39.5 (12.9) | 37.4 (10.2) | 35.7 (5.9) |
| 360 | 50.3 (15.5) | 45.9 (12.7) | 40.3 (11.8) | 36.1 (12.2) | 34.2 (9.7) | 32.6 (5.2) |
| 350 | 45.3 (14.1) | 40.8 (11.7) | 36.6 (10.9) | 32.6 (11.3) | 30.8 (8.8) | 28.8 (4.5) |
| 340 | 40.6 (13.0) | 36.1 (11.0) | 33.0 (10.3) | 28.9 (10.3) | 27.6 (8.2) | 25.4 (4.0) |
| 330 | 36.2 (11.0) | 31.6 (9.7) | 29.6 (9.4) | 25.8 (9.2) | 24.4 (7.4) | 22.6 (2.9) |
| 320 | 30.7 (9.6) | 25.6 (8.0) | 25.3 (8.5) | 21.7 (8.1) | 20.7 (6.7) | 17.8 (2.4) |
| 310 | 27.4 (7.4) | 23.7 (7.0) | 24.4 (7.9) | 20.0 (7.5) | 19.5 (6.2) | 17.8 (2.2) |
Standard deviation in parentheses.
Figure 2.
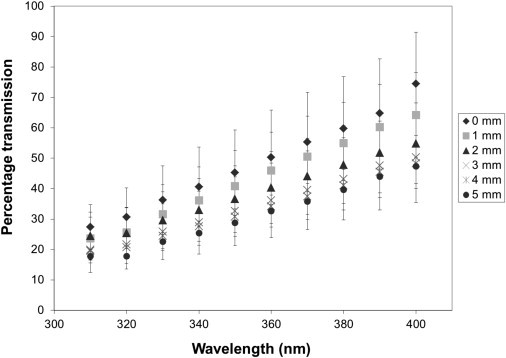
UV transmission through the stroma as a function of wavelength at different positions from the center of the human cornea (n = 4). At all positions from center to periphery there is an approximately linear dependence between transmission and wavelength. The largest reductions in transmission are seen centrally. (Bars represent standard deviations).
Table 2.
Decrease in UV transmission as a function of wavelength from central to 5 mm peripheral stroma
| Wavelength (nm) | Average percentage transmission decrease (± SD) |
|---|---|
| 400 | 27.1 ± 9.2 |
| 390 | 20.8 ± 9.6 |
| 380 | 20.1 ± 9.2 |
| 370 | 19.6 ± 8.7 |
| 360 | 17.7 ± 8.2 |
| 350 | 16.4 ± 7.4 |
| 340 | 15.2 ± 6.8 |
| 330 | 13.7 ± 5.8 |
| 320 | 12.8 ± 4.9 |
| 310 | 9.6 ± 3.8 |
Experimentally, it is difficult to separate the contributions of light scattering and absorption to the measured transmission of UV light through the cornea. However, the contribution of light scattering by the collagen fibrils can be considered theoretically using the direct summation of the scattered fields method (27,29). We have previously published theoretical deductions of visible light transmission as a function of position across the corneal stroma (14). Using the same methods, we have now extended this analysis into the UV region and demonstrated how corneal light transmission is reduced in the peripheral cornea due to the influence of collagen scattering alone (Fig. 3). By accounting for light scattering from collagen alone, corneal transmission is predicted to show a substantial decrease from the central to peripheral regions for all wavelengths. This scattering will contribute to the observed reduction in transmission between the center and periphery, as shown in Figs. 1 and 2. To approximate the relative contributions of scattering and absorption we can consider the flux lost (extinction) as light traverses the cornea as the sum of collagen scattering and other flux loss at each wavelength. The other flux loss is due to absorption as well as to scattering from cellular components. The measured values of extinction can be determined as = 100% − percentage transmission. The theoretical values of extinction due to collagen scattering can be determined from Fig. 3 in a similar way. By subtracting the theoretical extinction values due to collagen scattering from the measured extinction values, we can estimate the expected contribution of the other sources of extinction as a function of wavelength, both at the center and at the periphery of the cornea (Fig. 4). This reveals that at the center of the cornea below ∼380 nm, collagen scatter accounts for about half the total extinction. At 400 nm, absorbtion is negligible and the small amount of noncollagen scatter probably arises from the corneal keratocytes. In contrast, at the periphery of the cornea, a greater proportion of the extinction comes from collagen scatter, with a wavelength independent contribution from absorption and keratocyte scatter.
Figure 3.
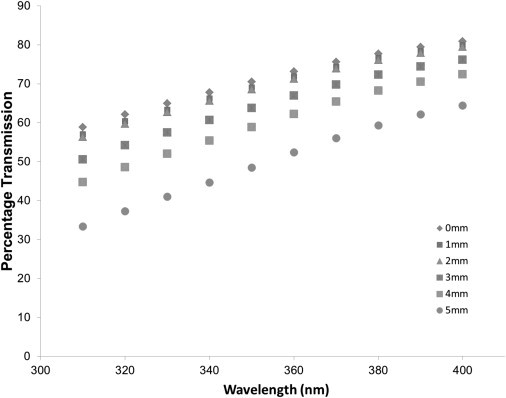
Corneal light transmission as a function of wavelength, from theoretical calculations of light scattering. Theoretical predictions of scatter from the collagen fibrils alone suggest the same trends as observed experimentally from the cornea as a whole, although the numerical dependencies are different to those seen experimentally (in Figure 2).
Figure 4.
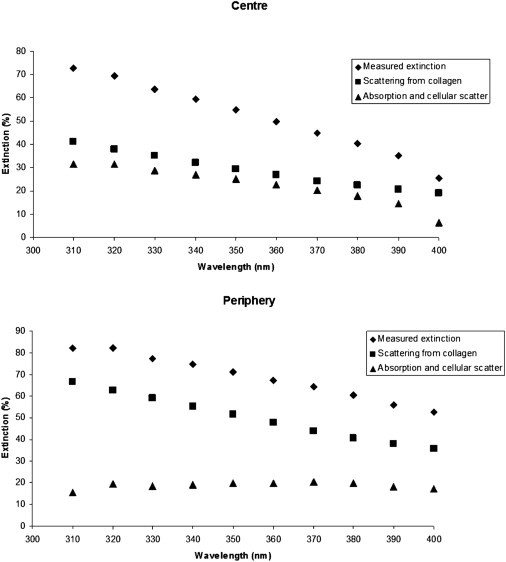
Estimated contribution of scattering (squares) and absorption (triangles) to the measured extinction of UV light (diamonds) at the center and periphery of the cornea. At the corneal periphery, absorption shows negligible wavelength dependence. At the corneal center, however, a negative correlation between absorption and increasing wavelength is apparent so that at the lower limits of the visual spectrum (400 nm) the human cornea absorbs very little light.
The results presented here indicate that in vitro light transmission through the human corneal stroma decreases substantially in the UV portion of the electromagnetic spectrum, and that there are pronounced differences between the central and peripheral cornea. This is expected from theoretical considerations, and has previously been noted in visible light transmission through the human cornea (14). In the visible region, it was demonstrated that corneal light scattering as a function of position across the stroma is predominantly affected by the documented changes in collagen fibril spacing and diameter (30–32). The changes in collagen fibril spacing are expected to alter the local refractive index contrast between the collagen fibrils and extrafibrillar matrix leading to increased scattering (14). Light scattering also depends heavily on the fibril diameter, the scattering cross section of the corneal stroma having fourth-power dependence on fibril diameter (8,15). These two factors act to decrease corneal transmission of visible light peripherally. Any decrease in transmission due to the peripheral increase in corneal thickness appears to be largely negated by a decrease in fibril density at this region of the tissue. In these experiments, it must be noted that the path length traversed by light through the stroma is slightly greater than the actual tissue thickness, which is taken as the thickness perpendicular to the corneal surface. However, by using a simplified model of the corneal stroma, it can be shown that the correction from parallel to perpendicular incident light is quite small. For the theoretical model it was calculated that the difference in transmission between light parallel to the central optical axis and perpendicular to the tissue surface at the far periphery is around 2% for all wavelengths (14).
In the near-UV region we have observed that peripheral transmission is significantly reduced throughout the 310–400 nm UV range. It is likely that this reduction is due to the combined influence of scattering and absorption. It is difficult to ascertain the relative amounts of extinction that can be attributed, independently, to scattering and to absorption. However, theoretical calculations can supply a useful guide, with the caveat that models of corneal light scattering do not account for any scattering contribution from the stromal keratocytes (5,6). Nevertheless, the theoretical and experimental data suggest that, at the center of the cornea, cellular scatter and UV absorption show a continuous increase with decreasing wavelengths. In contrast, in the peripheral cornea, cellular scatter and absorption appear to be constant throughout the wavelength region studied. This may be due to differences in the cellular and macromolecular composition of the tissue centrally and peripherally. Aromatic amino acid residues cause absorption by biological macromolecules of near-UV wavelengths. Collagen, too, is known to absorb radiation shorter than wavelengths of 285 nm (33,34). It is also likely that the glycosaminoglycans in the extrafibrillar matrix will have an effect on the corneal UV absorbance (33).
The spatial distribution of keratocytes within the stroma may also influence both light scattering and absorption. As previously mentioned, keratocytes are more concentrated in the peripheral stroma (4) and this will presumably increase peripheral light scattering and reduce the amount of light reaching the endothelium. In addition, these cells contain corneal crystallins (6), which will also increase UV absorption because they are thought to possess some UV filtering properties. Hence, there are two possible mechanisms—scattering and absorption—by which corneal keratocytes would play an influential role in reducing the amount of UV light that reaches the endothelium in the more peripheral cornea. Thus, when modeling light extinction in the UV spectrum for corneal stroma, one must consider the total extinction cross section; i.e., the sum of the scattering cross section from the collagen fibrils and keratocytes, as well as the absorption cross sections from the biological macromolecules. It can be speculated that the absorption coefficient may show some variation with position across the cornea because the relative volumes of collagen and extrafibrillar substance vary between central and peripheral regions (30–32). However, without more compositional data it is not possible to calculate the extent to which this affects the absorption coefficient.
The reduced UV flux transmitted through the peripheral corneal stroma in humans may have biological implications in situ. The corneal epithelium contains high levels of aldehyde dehydrogenase, however, because the epithelium tends to be of constant thickness as a function of position across the cornea (35), positional changes in epithelial thickness are unlikely to be related to increased UV absorption in the peripheral cornea. The epithelium was removed from the corneas examined here, and the decrease in the transmission of UV light from central to peripheral regions of the stroma alone is significant, with 30% less overall transmission in the far periphery as compared to the stromal center. This is consistent with the observation that human endothelial cells in the peripheral cornea display significantly less nuclear oxidative DNA damage and exhibit greater proliferative capacity than those located centrally (23). Corneal endothelial cells are very metabolically active, because they are responsible for corneal hydration control and transparency (20). In all metabolically active cells, regulation of oxidative stress can be a significant problem. In corneal endothelium, this stress may be increased, particularly in the central area as the result of increased exposure to UV light. In addition, it is possible that the response of corneal endothelial cells to UV may differ between the central and peripheral areas due to topographic differences in the relative expression of various crystallins or antioxidant enzymes, or differences in the relative concentration of antioxidants. Interestingly, recent studies (36) showed an increase in oxidative stress and oxidative DNA damage in the endothelium of patients with Fuchs' dystrophy, a disease that is first observed in the central endothelium. It is not known whether topographical differences in UV absorption play a role in this disease.
Conclusions
The transmission of UV light by human corneal stroma was evaluated as a function of position across the corneal surface. It was determined that transmission is substantially decreased in the peripheral regions, as it is for visible light. The variation in UV flux reaching the endothelial cellular layer therefore varies with position. UV wavelengths presumably exert radiation damage on cells through free radical pathways, and maximum UV transmission is achieved in the central regions of the cornea. This correlates with data showing increased oxidative stress in central endothelial cells compared with those at the periphery.
Acknowledgments
We thank the staff of the Bristol Eye Bank for their continued support of our research and specifically for the provision of human corneas.
This work was supported by Medical Research Council Programme grant G0600755 to K.M.M. and A.J.Q. and a Medical Research Council studentship to J.J.D. K.M.M. is a Royal Society/Wolfson Research Merit Award holder.
References
- 1.Komai Y., Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest. Ophthalmol. Vis. Sci. 1991;32:2244–2258. [PubMed] [Google Scholar]
- 2.Hogan M.J., Alvarado J.A., Weddell J.E. WB Saunders; Philadelphia, PA: 1971. Histology of the Human Eye: An Atlas and Textbook. [Google Scholar]
- 3.Michelacci Y.M. Collagens and proteoglycans of the corneal extracellular matrix. Braz. J. Med. Biol. Res. 2003;36:1037–1046. doi: 10.1590/s0100-879x2003000800009. [DOI] [PubMed] [Google Scholar]
- 4.Møller-Pedersen T., Ehlers N. A three-dimensional study of the human corneal keratocyte density. Curr. Eye Res. 1995;14:459–464. doi: 10.3109/02713689509003756. [DOI] [PubMed] [Google Scholar]
- 5.Jester J.V. Corneal crystallins and the development of cellular transparency. Semin. Cell Dev. Biol. 2008;19:82–93. doi: 10.1016/j.semcdb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jester J.V., Moller-Pedersen T., Piatigorsky J. The cellular basis of corneal transparency: evidence for ‘corneal crystallins’. J. Cell Sci. 1999;112:613–622. doi: 10.1242/jcs.112.5.613. [DOI] [PubMed] [Google Scholar]
- 7.Stagos D., Chen Y., Vasiliou V. Corneal aldehyde dehydrogenases: multiple functions and novel nuclear localization. Brain Res. Bull. 2010;81:211–218. doi: 10.1016/j.brainresbull.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrell R.A., McCally R.L. Corneal transparency. In: Albert D.M., Jakobiec F.A., editors. Principles and Practice of Ophthalmology. WB Saunders; Philadelphia, PA: 2000. pp. 629–643. [Google Scholar]
- 9.Maurice D. The structure and transparency of the cornea. J. Physiol. 1956;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hart R.W., Farrell R.A. Light scattering in the cornea. J. Opt. Soc. Am. 1969;59:766–774. doi: 10.1364/josa.59.000766. [DOI] [PubMed] [Google Scholar]
- 11.Feuk T. On the transparency of the stroma in the mammalian cornea. IEEE Trans. Biomed. Eng. 1970;17:186–190. doi: 10.1109/tbme.1970.4502732. [DOI] [PubMed] [Google Scholar]
- 12.Beems E.M., Van Best J.A. Light transmission of the cornea in whole human eyes. Exp. Eye Res. 1990;50:393–395. doi: 10.1016/0014-4835(90)90140-p. [DOI] [PubMed] [Google Scholar]
- 13.Boettner E.A., Wolter J.R. Transmission of the ocular media. Invest. Ophthalmol. Vis. Sci. 1962;1:776–783. [Google Scholar]
- 14.Doutch J., Quantock A.J., Meek K.M. Light transmission in the human cornea as a function of position across the ocular surface: theoretical and experimental aspects. Biophys. J. 2008;95:5092–5099. doi: 10.1529/biophysj.108.132316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrell R.A., McCally R.L., Tatham P.E. Wave-length dependencies of light scattering in normal and cold swollen rabbit corneas and their structural implications. J. Physiol. 1973;233:589–612. doi: 10.1113/jphysiol.1973.sp010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freegard T.J. The physical basis of transparency of the normal cornea. Eye (Lond.) 1997;11:465–471. doi: 10.1038/eye.1997.127. [DOI] [PubMed] [Google Scholar]
- 17.Zigman S. Ocular light damage. Photochem. Photobiol. 1993;57:1060–1068. doi: 10.1111/j.1751-1097.1993.tb02972.x. [DOI] [PubMed] [Google Scholar]
- 18.Kolozsvári L., Nógrádi A., Bor Z. UV absorbance of the human cornea in the 240- to 400-nm range. Invest. Ophthalmol. Vis. Sci. 2002;43:2165–2168. [PubMed] [Google Scholar]
- 19.Murphy C., Alvarado J., Maglio M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest. Ophthalmol. Vis. Sci. 1984;25:312–322. [PubMed] [Google Scholar]
- 20.Fischbarg J., Maurice D.M. An update on corneal hydration control. Exp. Eye Res. 2004;78:537–541. doi: 10.1016/j.exer.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Sun X.C., Li J., Bonanno J.A. Role of carbonic anhydrase IV in corneal endothelial HCO3- transport. Invest. Ophthalmol. Vis. Sci. 2008;49:1048–1055. doi: 10.1167/iovs.07-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swan J.S., Hodson S.A. Rabbit corneal hydration and the bicarbonate pump. J. Membr. Biol. 2004;201:33–40. doi: 10.1007/s00232-004-0704-7. [DOI] [PubMed] [Google Scholar]
- 23.Joyce N.C., Zhu C.C., Harris D.L. Relationship among oxidative stress, DNA damage, and proliferative capacity in human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2009;50:2116–2122. doi: 10.1167/iovs.08-3007. [DOI] [PubMed] [Google Scholar]
- 24.Mimura T., Joyce N.C. Replication competence and senescence in central and peripheral human corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2006;47:1387–1396. doi: 10.1167/iovs.05-1199. [DOI] [PubMed] [Google Scholar]
- 25.Konomi K., Joyce N.C. Age and topographical comparison of telomere lengths in human corneal endothelial cells. Mol. Vis. 2007;13:1251–1258. [PubMed] [Google Scholar]
- 26.Kostyuk O., Nalovina O., Hodson S.A. Transparency of the bovine corneal stroma at physiological hydration and its dependence on concentration of the ambient anion. J. Physiol. 2002;543:633–642. doi: 10.1113/jphysiol.2002.021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freund D.E., McCally R.L., Farrell R.A. Direct summation of fields for light scattering by fibrils with applications to normal corneas. Appl. Opt. 1986;25:2739–2746. doi: 10.1364/ao.25.002739. [DOI] [PubMed] [Google Scholar]
- 28.Wollensak G., Spoerl E., Seiler T. Riboflavin/ultraviolet-a-induced collagen cross-linking for the treatment of keratoconus. Am. J. Ophthalmol. 2003;135:620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 29.Freund D.E., McCally R.L., Edelhauser H.F. Ultrastructure in anterior and posterior stroma of perfused human and rabbit corneas. Relation to transparency. Invest. Ophthalmol. Vis. Sci. 1995;36:1508–1523. [PubMed] [Google Scholar]
- 30.Boote C., Dennis S., Meek K.M. Collagen fibrils appear more closely packed in the prepupillary cornea: optical and biomechanical implications. Invest. Ophthalmol. Vis. Sci. 2003;44:2941–2948. doi: 10.1167/iovs.03-0131. [DOI] [PubMed] [Google Scholar]
- 31.Borcherding M.S., Blacik L.J., Weinstein H.G. Proteoglycans and collagen fibre organization in human corneoscleral tissue. Exp. Eye Res. 1975;21:59–70. doi: 10.1016/0014-4835(75)90057-3. [DOI] [PubMed] [Google Scholar]
- 32.Boote C., Kamma-Lorger C.S., Meek K.M. Quantification of collagen organization in the peripheral human cornea at micron-scale resolution. Biophys. J. 2011;101:33–42. doi: 10.1016/j.bpj.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lerman S. Biophysical aspects of corneal and lenticular transparency. Curr. Eye Res. 1984;3:3–14. doi: 10.3109/02713688408997182. [DOI] [PubMed] [Google Scholar]
- 34.Sionkowska A. Effects of solar radiation on collagen and chitosan films. J. Photochem. Photobiol. B. 2006;82:9–15. doi: 10.1016/j.jphotobiol.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Reinstein D.Z., Archer T.J., Coleman D.J. Epithelial thickness in the normal cornea: three-dimensional display with Artemis very high-frequency digital ultrasound. J. Refract. Surg. 2008;24:571–581. doi: 10.3928/1081597X-20080601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurkunas U.V., Bitar M.S., Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am. J. Pathol. 2010;177:2278–2289. doi: 10.2353/ajpath.2010.100279. [DOI] [PMC free article] [PubMed] [Google Scholar]


