Summary
Objectives
Hypoplastic left heart syndrome (HLHS) with an intact atrial septum (IAS) is a rare finding, reported in only 1% of pathologic specimens with hypoplasia of the aortic tract complex. In newborns with left heart obstruction, the existence of an interatrial communication is very important for oxygenated blood to be distributed to the body and to prevent pulmonary congestion. The ability to predict prenatally restriction of the atrial defect may allow earlier surgery to be planned.
Methods
We report a case of prenatal diagnosis of HLHS with a complete premature closure of the foramen ovale that was not detected by prenatal echocardiography.
Results and conclusion
The management of neonates with HLHS in the first days of life is crucial to the results of the first stage of the Norwood procedure. We suggest that delivery of the mother close to surgical centre and avoiding neonatal transfer improve the results, but stabilisation with prostaglandins and balancing of the systemic and pulmonary resistances are also important. A restrictive or closed atrial septal defect may contribute to haemodynamic instability in the first days of life. The ability to predict this complication prenatally may help in the immediate postnatal management of the affected infant.
Keywords: hypoplastic left heart syndrome, intact atrial septum, fetal echocardiography
Introduction
Hypoplastic left heart syndrome (HLHS) refers to congenital hypoplasia of the left ventricle that may be associated with mitral atresia, aortic atresia, aortic stenosis and coarctation of the aorta.
This syndrome accounts for 4% of congenital heart defects and it is the cause of 23% of deaths from heart disease in the first year of life (1).
HLHS is potentially detectable on prenatal sonography between 18 and 22 weeks’ gestation with a 4-chamber view of the fetal heart and it is one of the most common cardiac malformation detected in fetal life.
Furthermore, in newborns with left heart obstruction, the existence of an interatrial communication is very important for oxygenated blood to be distributed to the body and to prevent pulmonary congestion. The ability to predict prenatally restriction of the atrial defect may allow earlier surgery to be planned. However, direct evaluation of the atrial septum in the fetus has proved difficult. It is usually easy to see if the foramen is widely patent but conversely, a normally sized defect can be difficult to distinguish from a restrictive defect.
Visualisation of the defect requires good colour penetration and a perpendicular orientation to the atrial defect, which is not always achievable, especially in late pregnancy (2).
Case report
A 30-year-old primigravid woman was referred to our hospital at 21 weeks’ gestation for a detailed cardiac evaluation because a fetal heart defect was suspected on routine ultrasound examination.
Her family history was insignificant and the woman had no particular past medical or surgical history. The villocentesis showed a normal fetal karyotype and obstetric ultrasound at 13 weeks showed no abnormalities, but an hypoplastic left ventricle was detected at 20 weeks.
Nevertheless, sonography showed a normally grown fetus with no extracardiac malformations.
Fetal echocardiography revealed a normal right ventricle and an hypoplastic left ventricle with patent and dysplastic mitral valve. It was difficult to visualize the foramen ovale and the aortic valve, but the ascending aorta appeared very hypoplastic (1.7 mm in diameter). The main pulmonary trunk appeared to arise from the right ventricle.
The parents were informated by the gynaecologists of the poor prognosis of the fetus, but they choose to continue the affected pregnancy. Therefore, we decided for a seriate surveillance of the fetus with ultrasound examinations and we did not find in utero compromise.
The woman gave birth to a male newborn by vaginal spontaneous delivery at 37 weeks and 3 days of gestation, with birth weight of 2880 g and lenght of 50 cm. Due to rapid worsening of the clinical setting, with extreme hypoxemia, hypercapnia and acidosis, orotracheal intubation and mechanical ventilation were immediately required and the newborn was transferred to the pediatric cardiology department.
Postnatal echocardiography confirmed the diagnosis of hypoplastic left heart syndrome and revealed an intact atrial septum (Fig. 1). Thus, cardiac catheterisation, which confirmed that the interatrial communication was closed, was immediately performed, and Rashkind balloon atrial septostomy was successfully attempted (Fig. 2). The newborn was transferred to the cardiac surgery department with intravenous perfusion of dopamine and prostaglandins and the day after he underwent an operation.
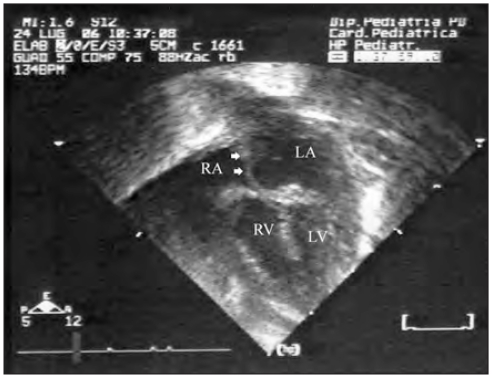
Figure 1.
Hypoplastic left heart syndrome. Echocardiography after birth shows an enlargement of right ventricle (RV) and right atrium (RA). The left ventricle (LV) is very hypoplastic. LA = left atrium. Arrowheads mark the intact atrial septum.
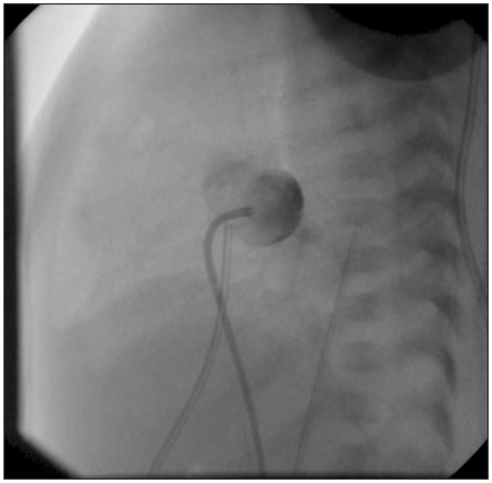
Figure 2.
Hypoplastic left heart syndrome. Cardiac catheterization confirms that the atrial septum is closed. Catheter’s point is in the left atrium and this image shows that there is not communication between the left and the right atrium because there is no crossing of the contrast medium.
With total cardiopulmonary bypass, the main pulmonary branches were isolated and an extremely hypoplastic ascending aorta was recognized (< 1 mm in diameter). Thus, a Goretex pulmonary arterial banding was performed and a pulmonary artery to anonymous artery shunt with tubule prosthesis (3.5 mm in diameter) was carried out. Finally, the baby was transferred to the Intensive Care Unit with Extra Corporeal Membrane Oxigenator (ECMO) and intravenous perfusion of dopamine.
A week later a rapid worsening of clinical setting with ischemic failure of extremities and multi organ failure were recognized; ECMO was stopped and mechanical ventilation and intravenous perfusion of adrenalina were immediately necessary. This was followed by rapid clinical deterioration, with cardiopulmonary arrest, which did not respond to resuscitation maneuvers, and death was recorded at few days of life.
The anatomopathologic examination confirmed the diagnosis of hypoplastic left heart syndrome with intact atrial septum, showed an extensive hypertrophy of the pulmonary veins and a posterior right opening in the diaphragm, with herniated higher pole of right kidney.
Discussion
Hypoplastic left heart syndrome with an intact atrial septum is a rare finding, reported in only 1% of pathologic specimens with hypoplasia of the aortic tract complex. It may be associated with non-immune fetal hydrops and congenital pulmonary cystic lymphangiectasia, indicating that impediment to pulmonary venous drainage in utero may result in prenatal morbidity or mortality in some.
In the normal heart before birth, the left-sided structures fill primarily from the right atrium via the foramen ovale (FO). With prenatal restriction, or complete premature closure of the foramen ovale (i.e., intact atrial septum), flow is diverted away from the left atrium and left ventricle. This has little effects on systemic perfusion in utero, because the right ventricle continues to eject the systemic circulation via the ductus arteriosus and pulmonary blood flow is relatively minimal. However, once separation from placental circulation takes place at birth, pulmonary blood flow increases substantially, as does pulmonary venous return to the left atrium. If left ventricular hypoplasia and an intact atrial septum are present, effective egress from the left atrium is impossible, resulting in marked elevation of pulmonary venous pressure. The combination of single-ventricle physiology and impediment to pulmonary venous egress can result in postnatal hypoxemia to a degree which may incompatible with life (3). Survival of infants with left ventricular hypoplasia is actually possible with early identification of the anomaly and application of a strategy consisting of either heart transplantation or staged surgical reconstruction, starting with the Norwood operation (4). However, preoperative management can be complicated by haemodynamic instability. As the results of the Norwood staged palliation for hypoplastic left heart syndrome have improved, the preoperative status has been recognised as having an important influence on the results. A restrictive atrial septum has been suggested as a risk factor for Norwood palliation.
In our case, anatomopathologic examination showed nor fetal hydrops neither pulmonary lymphangiectasia, but an intact atrial septum and an extensive hypertrophy of pulmonary veins, which were not detected by prenatal echocardiography.
During fetal life, it is essential to study flows through the foramen ovale, particulary in left heart obstruction. In fact, in severe cases, left atrial outflow is severely impaired and the only escape channel for pulmonary venous return is the FO, thus reversing the normal right-to-left flow to left-toright.
If the FO becomes restrictive, left atrial pressure increases, but the atrium does not adapt to this increased and the upstream pressure leads to hypertrophy of the pulmonary veins (5).
These hemodynamic alteration could be detected by Doppler echo study of either the FO itself or the pulmonary veins. In order to examine the atrial septum directly in the fetus, the septum must be imaged with the ultrasound beam perpendicular to it. At this orientation, the colour flow map is optimised for displaying the direction of flow and turbolence, if present. Imaging in this way is often difficult, especially in late pregnancy and particularly in the fetus with the hypoplastic left heart syndrome, when the interatrial communication is often in a high secundum position (2).
Pulmonary venous flow patterns are related to left atrial haemodynamics and have been studied intensively, particularly in the adult. The normal pattern of pulmonary venous blood flow has been described previously in the fetus and shows a similar pattern to that found in postnatal life.
With the application of color Doppler in fetal cardiology, the visualization of fetal pulmonary veins since the second trimester became possible and in the pulmonary veins there will be a systolic peak only, with no diastolic peak, and an inverted wave that corresponds to atrial contraction (6). Reversed pulmonary vein flow may predict infants that will be hypoxic at birth. Finally, atrial morphology appears to be a marker for the degree of severity of pulmonary venous egress. This can be helpful, because evaluating directly for venous anomalies can be difficult with fetal echocardiography; therefore, prenatal characterization of atrial morphology may reliably allow for detection of patients at high risk for severe obstruction and poor prognosis (3).
In our experience, it has become evident that the management of the neonate in the first days of life is crucial to the results of the first stage of the Norwood procedure. Therefore, we suggest that delivery of the mother close to surgical centre and avoiding neonatal transfer improve the results, but stabilisation with prostaglandins and balancing of the systemic and pulmonary resistances are also important. A restrictive or closed atrial septal defect may contribute to haemodynamic instability in the first days of life. The ability to predict this complication prenatally may help in the immediate postnatal management of the affected infant. In addition, urgent cardiac catheterization is required to relieve the septal obstruction and improve oxygenation.
Finally, these fetuses may be candidates for in utero intervention to decompress the left atrium and minimize injury to the pulmonary vascular bed (7).
References
- 1.Allen RH, Benson CB, Haug LW. Pregnancy outcome of fetuses with a diagnosis of hypoplastic left ventricle on prenatal sonography. J Ultrasound Med. 2005;24:1199–1203. doi: 10.7863/jum.2005.24.9.1199. [DOI] [PubMed] [Google Scholar]
- 2.Better DJ, Apfel HD, Zidere V, Allen LD. Pattern of pulmonary venous blood flow in the hypoplastic left heart syndrome in the fetus. Heart. 1999;81:646–649. doi: 10.1136/hrt.81.6.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rychik J, Rome JJ, Collins MH, DeCampli WM, Spray TL. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol. 1999;34(2):554–60. doi: 10.1016/s0735-1097(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 4.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–6. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 5.Rebelo M, Veiga E, Machado AJ, Pinto F, Kaku S. Hypoplastic left heart sindrome with in utero closed foramen ovale: case report. Rev Port Cardiol. 2006 Mar;25(3):331–6. [PubMed] [Google Scholar]
- 6.Lenz F, Machlitt A, Hartung J, Bollmann R, Chaoui R. Fetal pulmonary venous flow pattern is determined by left atrial pressure: report of two cases of left heart hypoplasia, one with patent and the other with closed interatrial communication. Ultrasound Obstet Gynecol. 2002;19:392–395. doi: 10.1046/j.1469-0705.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 7.Donofrio MT, Bremer YA, Moskowitz WB. Diagnosis and management of restricted or closed foramen ovale in fetuses with congenital heart disease. Am J Cardiol. 2004;94:1348–1351. doi: 10.1016/j.amjcard.2004.07.133. [DOI] [PubMed] [Google Scholar]