Case report
Case #1
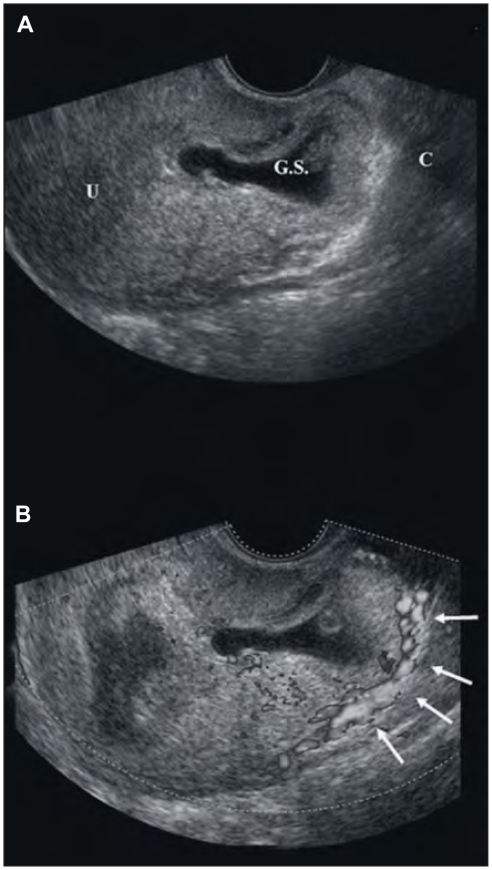
A 36-year-old woman (gravida 2, para 1) come to the Prenatal Diagnosis Center, Artemisia, in Rome referred by another institution for evaluating the presence of a Cesarean Scar Pregnancy (CSP). Woman came at our institution with no symptoms. She had a caesarean section a term of gestation 3 years prior. She was at 9 weeks gestation calculated from the first day of the last menstrual period. Physical examination was negative and no bleeding from the vagina was observed. Serum human corionic gonadotropin level was 7500 mIU/ml. Both transabdominal and transvaginal ultrasound revealed a CSP according to the Jurkovic criteria (6). The gestational sac, containing a yolc sac and a embryonic pole with cardiac motion, was located in the anterior isthmus, in the location of the previous caesarean scar (Fig. 1A). Within the endometrial cavity, above the gestational sac, a fluid collection was observed, but no communications were observed between this collection and the gestational sac. Also sonographic examination with color-flow Doppler imaging was performed to determine whether the pregnancy was implanted in the uterus or was ectopic (Fig. 1B). No normal myometrium was visualized between the bladder and the gestational sac; only 3 mm of thickness separated the sac from the urinary bladder (Fig. 1).
Figure 1.
A gestational sac (GS) within the fibroid tissue of a previous caesarean scar in a sagittal view uf the uterus (U). The cervix (C) is empty (Fig. 1A) and the Doppler imaging with the peripheral vascularization (white arrows) (Fig. 1B).
Termination of pregnancy was suggested and the patient was carefully counselled with the therapeutic options, including laparotomy, laparoscopy, suction evacuation and medical treatment with methotrexate. After a written informed consent was obtained an exploratory laparotomy was performed. When the peritoneal cavity was opened after the mobilization of the bladder, the lower uterine myometrial implantation was confirmed. A hysterotomy was performed but a severe hemorrage ensued and could not be contained by uterotonics and conservative surgical measures; therefore, a decision was made to perform an emergency subtotal hysterectomy. The estimated blood loss was 1500 ml; woman had an uneventful postoperative time and was discharged from the hospital after 6 days with haemoglobin level of 9.1 g/dl. Definitive histologic examination revealed the presence of mature chorionic villi infiltrating the myometrium. Endometriosis was also found in the superficial myometrium. No gestational tissue was found in the entire uterine cavity.
Case #2
A 32-year-old woman coming to our emergency department of Obstetric and Gynecology, at the 2nd University of Naples with vaginal bleeding. She had two previous cesarean sections in 2000 and 2003. She was at 6 weeks gestation calculated from the first day of the last menstrual period and the serum Beta-hCG level was 1800 mIU/ml. Physical examination was unremarkable.
A transvaginal ultrasound exam revealed a gestational sac of 12 mm of diameter with an embryonic pole without cardiac activity implanted within the scar of the two previous cesarean sections. The entire cervical canal was empty and the layer between the sac and the bladder was diminished. After the confirmation of the CSP, woman was informed about the medical and surgical options to treat this condition. After a written informed consent was obtained an operative hysteroscopy was carried out. The cervix was dilated by an Hegar dilator up to number 12. Then the hysteroscope was introduced into the uterus and the entire cavity was visualized to exclude any type of gestational tissue. Then we proceeded to visualize the gestational tissue in the myometrium of the previous cesarean scar moving the hysteroscope outward in the cervix. The gestational tissue was pull out using placenta forceps under sonographic guidance; a curettage of the uterine cavity was carried-out and the bleeding points were stopped with a hysteroscopic rolling ball.
The postoperative period was uneventful and follow-up included daily serial measurements of serum Beta-hCG; woman was discharged when two consecutive measurements fell or reached a level of 10mIU/ml. Then we seen woman every 48 hrs in an ambulatory with serum Beta-hCG and transvaginal evaluation until levels dropping below 5 mIU/ml.
Discussion
Ectopic pregnancy is one of the leading cause of morbidity and mortality among fertile women accounting of 9% of the pregnancies-related deaths (1). Among ectopic pregnancy there is a clinical entity called CSP in which the implantation of the pregnancy is within the fibroid tissue of a previous caesarean scar (2); according some authors CSP is the rarest form of ectopic pregnancy but its incidence is not yet well established (3). From 1978 the incidence is increased probably related to the high percentage of caesarean sections and the use of transvaginal ultrasound early in pregnancy (3, 4). Anyway, because of the early onset in pregnancy of vaginal spotting or low abdominal painful, this condition could be exchanged at least for a spontaneous miscarriage (5).
Due to the limited number of women affected CSP, as reported in literature, information concerning both the natural history and treatment are scarce. Decision concerning the possibility to perform a conservative treatment in women affected by CSP cannot rely on robust and evidencebased data and poses challenging problems to the obstetricians. Indeed, the mode of treatment of CSP is often related to the severity of symptoms, the serum levels of free-Beta-hCG or surgical experience (3).
Hence, in these women issues concerning early detection is of utmost importance and it is the corner-stone to reduce heavy complications related to the CSP. (4). Also in dedicated tertiary referral medical center for early pregnancy, only 1–5 patients per year are diagnosed making their management extremely complex (3, 4, 6). From 1966 to 2004 about 37 case reports and four series of CSP were described in the literature (5). Although the pathophysiology of cesarean scar pregnancy remains to be established, it can be supposed that in the first days of gestation the blastocyst invades the myometrium through a microscopic lesion present in the cesarean scar related to a previous trauma of surgical procedure such as cesarean section, myomectomy, hysteroscopy and even manual removal of the placenta (7–10). Seow et al. (3) supposed also a possible correlation between intrauterine device, pelvic inflammatory disease and CSP. The pathological trophoblastic invasion associated with CSP can lead to severe pregnancy complications such as massive hemorrage, placenta previa, placenta accreta and uterine rupture (11) and due to these problems the termination of pregnancy is recommended (2, 12).
The likelihood of fewer complications and of preserving the reproductive function may assumed to be related to the week of the diagnosis. The failure rate of unsuccessful in women ≥ 7 gestational week subjected to evacuation therapy was indeed 80%, while in cases < 7 gestational week, such a rate was 11% (5). These two cases seem to confirm this hypothesis, but a longer series of cases are needed to validate these observations.
In case #1, a resection of the gestational sac via laparotomy was performed because of the clinical and biochemical features and also because the pregnancy was at an advanced stage. A recent review seems to justify the use of laparotomy or laparoscopy in such selected cases instead of medical or other surgical treatments (3). Profuse hemorrhaging accounted for 12.5% of all complications in the laparotomy approach (3), but this was the first case in which reproductive function was not preserved.
Case #2 is the second case reported in the literature in which CSP was treated by hysteroscopy. In this case, low levels of beta-hCG, small gestational sac, the time of gestation and the experience of the endoscopist suggested the use of hysteroscopy. Moreover, hysteroscopy was suggested because the ectopic gestational sac grows toward the uterine cavity, not yet deeply in the scar. In this case, local MTX would probably also be effective in treatment, but mass regression is very long, ranging from 2 months to 1 year.
Due to the severity of complications, it is important do diagnose scar pregnancy as early and accurately as possible but it is very difficult because there are others clinical entities could be exchanged for CSP (i.e. spon- taneous abortion and/or cervico-isthmic pregnancy) and diagnosis is often not made until uterine rupture (2). Transvaginal sonography is a useful tool for diagnosing CSP; probably in woman whose underwent a previous cesarean section an evaluation of the scar very early in pregnancy, could make an early diagnosis of CSP; in this way a conservative treatment of the uterus and of the reproductive function could be feasible by medical or surgical approach.
In conclusion the management of CSP is not well established, but according our experience a conservative treatment of the uterus is feasible early in pregnancy, probably before 7 weeks of gestation. However, a larger series of women are necessary to validate this hypothesis. An accurate selection of patients, an informed consent to the conservative treatment and a strict adherence to the follow-up program are mandatory. We also suggest an evaluation of the scar in women who underwent a previous Caesarean section very early on in the pregnancy. Due to the extreme rarity of disease in pregnant women, centralization of cure in tertiary centers should be firmly pursued.
References
- 1.From the Centers for Disease Control and Prevention. Ectopic pregnancy–United States, 1990–1992. JAMA. 1995 Feb 15;273(7):533. [PubMed] [Google Scholar]
- 2.Fylstra DL, Pound-Chang T, Miller MG, Cooper A, Miller KM. Ectopic pregnancy within a cesarean delivery scar: a case report. Am J Obstet Gynecol. 2002 Aug;187(2):302–4. doi: 10.1067/mob.2002.125998. [DOI] [PubMed] [Google Scholar]
- 3.Seow KM, Huang LW, Lin YH, Lin MY, Tsai YL, Hwang JL. Cesarean scar pregnancy: issues in management. Ultrasound Obstet Gynecol. 2004 Mar;23(3):247–53. doi: 10.1002/uog.974. [DOI] [PubMed] [Google Scholar]
- 4.Maymon R, Halperin R, Mendlovic S, Schneider D, Vaknin Z, Herman A, et al. Ectopic pregnancies in Caesarean section scars: the 8 year experience of one medical centre. Hum Reprod. 2004 Feb;19(2):278–84. doi: 10.1093/humrep/deh060. [DOI] [PubMed] [Google Scholar]
- 5.Wang CB, Tseng CJ. Primary evacuation therapy for Cesarean scar pregnancy: three new cases and review. Ultrasound Obstet Gynecol. 2006 Feb;27(2):222–6. doi: 10.1002/uog.2644. [DOI] [PubMed] [Google Scholar]
- 6.Jurkovic D, Hillaby K, Woelfer B, Lawrence A, Salim R, Elson CJ. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol. 2003 Mar;21(3):220–7. doi: 10.1002/uog.56. [DOI] [PubMed] [Google Scholar]
- 7.Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997 Jul;177(1):210–4. doi: 10.1016/s0002-9378(97)70463-0. [DOI] [PubMed] [Google Scholar]
- 8.McGowan L. Intramural pregnancy. JAMA. 1965 May 17;192:637–8. doi: 10.1001/jama.1965.03080200055023. [DOI] [PubMed] [Google Scholar]
- 9.Chuang J, Seow KM, Cheng WC, Tsai YL, Hwang JL. Conservative treatment of ectopic pregnancy in a caesarean section scar. BJOG. 2003 Sep;110(9):869–70. [PubMed] [Google Scholar]
- 10.Godin PA, Bassil S, Donnez J. An ectopic pregnancy developing in a previous caesarian section scar. Fertil Steril. 1997 Feb;67(2):398–400. doi: 10.1016/S0015-0282(97)81930-9. [DOI] [PubMed] [Google Scholar]
- 11.Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985 Jul;66(1):89–92. [PubMed] [Google Scholar]
- 12.Lam PM, Lo KW, Lau TK. Unsuccessful medical treatment of cesarean scar ectopic pregnancy with systemic methotrexate: a report of two cases. Acta Obstet Gynecol Scand. 2004 Jan;83(1):108–11. doi: 10.1111/j.1600-0412.2004.0033a.x. [DOI] [PubMed] [Google Scholar]