Introduction
Mesenchymal stem cells (MSC) are capable of differentiating into different mesenchymal lineages, including adipose and connective tissue, bone and cartilage. The most common source of MSCs for clinical use is human adult bone marrow. Because the frequency and differentiating capacity of MSCs are decreasing with age, different fetal tissues have been studied as an alternative source for MSCs. Recently, in ‘t Anker et al. (2003) and Tsai et al. (2004) reported that second-trimester amniotic fluid (AF) is a novel and rich source of fetal MSCs useful for clinical applications. AF contains a heterogeneous population of cells from fetal origin. The fetal MSCs in AF are probably deriving from the amnion membrane or other embryonic and extra-embryonic tissues during the process of fetal development and growth (in ‘t Anker et al., 2004).
Aim of the study
The purpose of this study was to investigate whether we were able to isolate and cultivate MSC from fresh second- trimester amniotic fluid samples.
Materials and methods
Amniotic fluid was collected from 8 second-trimester pregnancies (mean gestational age, 16 weeks [range 16–18 weeks]). Four mL of the AF samples were centrifugated for 10 minutes at 300g. Pellets were resuspended and cultured in DMEM with low glucose supplemented with 15% Fetal bovine serum and antibiotics. Culture flasks were incubated at 37°C, 5% CO2 and 100% humidity. Medium was changed every 3–4 days until day 14. At that moment, treatment with trypsin/EDTA was applied to detach the cells from the bottom of the flasks (Passage 0). Cell number and vitality was determined using a Bürker counting chamber combined with a trypan blue stain. After cell count, cells were recultured and further passaged to determine the population doubling level of the AF derived MSCs. Mesenchymal stem cell characteristics were analysed by flow cytometry using specific CD73+/CD44+/CD45- cell surface markers.
Results
6156 ± 9056 (mean ± SD) flat, spindle shaped cells were found attached to the bottom of the plate after a 14 day culture period out of 4mL of fresh AF (n = 8). The results varied strongly among the 8 different test samples (minimum = 2083 cells, maximum = 52083 cells), 1 sample (gestational week 16) could not give any MSC. In Passage 1 (after 2 trypsine treatments) 75 ± 11% (mean ± SD) of the cells showed MSC surface markers whereas in Passage 2 this percentage increased to 83± 7% (mean ± SD).
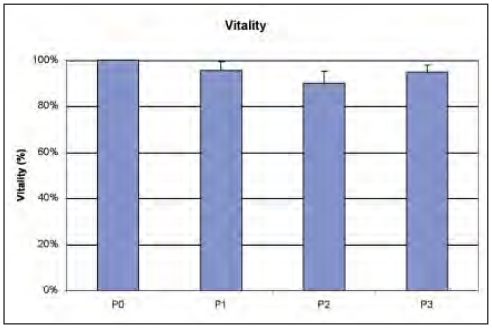
The vitality of the MSCs remained high (>90%) over the different passages (Fig. 1).
Figure 1.
Vitality of AFMSCs.
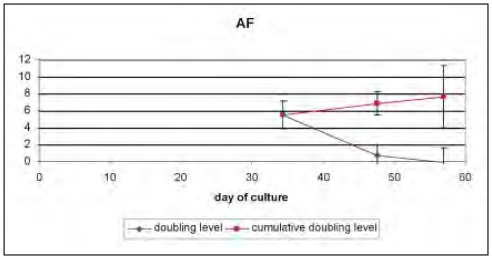
In Figure 2, the doubling levels over the different passages in time are depicted.
Figure 2.
Doubling population level of AFMSCs.
Whereas from passage 0 to passage 1 the MSCs, derived from the AF, possessed a high expansion potency with a doubling level of 5.5, this growth rate decreased drastically to passage 2 with a remaining doubling level of 0.7 while decreasing futher to the next passage.
After 55 days, the initial population would be doubled 8 times, from 6156 cells at the start to 1.5 × 106 cells.
Conclusion
Amniotic fluid may be an alternative fetal stem cell source. The expansion potency is extremely high in the first passage (a doubling level of 5.5 compared with our findings for umbilical cord and adipose tissue derived MSCs showing a doubling level of 3). Whereas the MSCs in these latter tissues keep their growth characteristics in time, the amniotic fluid derived MSCs lose their expansion potency drastically. Furhter studies are needed to overcome this problem (e.g. changing media composition).
Studies for an optimal cryopreservation of amniotic fluid are undergoing.
References
- 1.Kim BG, Hwang DH, Lee SI, Kim EJ, Kim SU. Stem cell-based cell therapy for spinal cord injury. Cell Transplant. 2007;16:355–364. doi: 10.3727/000000007783464885. [DOI] [PubMed] [Google Scholar]
- 2.Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev. 2001;122:713–714. doi: 10.1016/s0047-6374(01)00224-x. Review. [DOI] [PubMed] [Google Scholar]
- 3.Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood. 2003;121:368–374. doi: 10.1182/blood-2003-04-1291. [DOI] [PubMed] [Google Scholar]
- 4.Tsai MS, Lee JL, Chang YJ, Hwang SM. Isolation of human multipotent mesenchymal stem cells from second-tri-master amniotic fluid using a novel two-stage culture protocol. Human Reproduction. 2004;19:145–1456. doi: 10.1093/humrep/deh279. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Lee Y, Hwang KJ, et al. Human amniotic fluid-derived stem cells have characteristic of multipotent stem cells. Cell Prolif. 2007;40:75–90. doi: 10.1111/j.1365-2184.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338– 1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 7.Kunisaki SM, Armant M, Kao GS, et al. Tissue engineering from human mesenchymal amniocytes: a prelude to clinical trials. J Pediatr Surg. 2007;42:974–979. doi: 10.1016/j.jpedsurg.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol. 2007 doi: 10.1007/s10735-007-9118-1. in press. [DOI] [PubMed] [Google Scholar]