Abstract
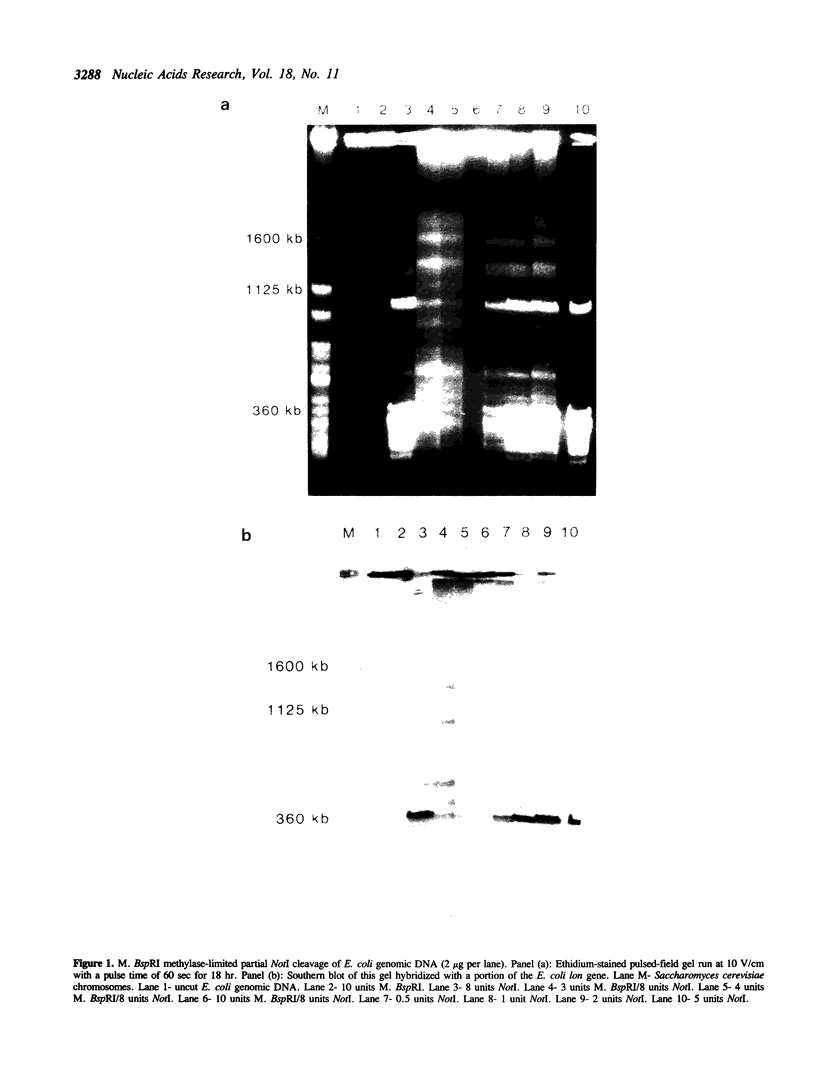
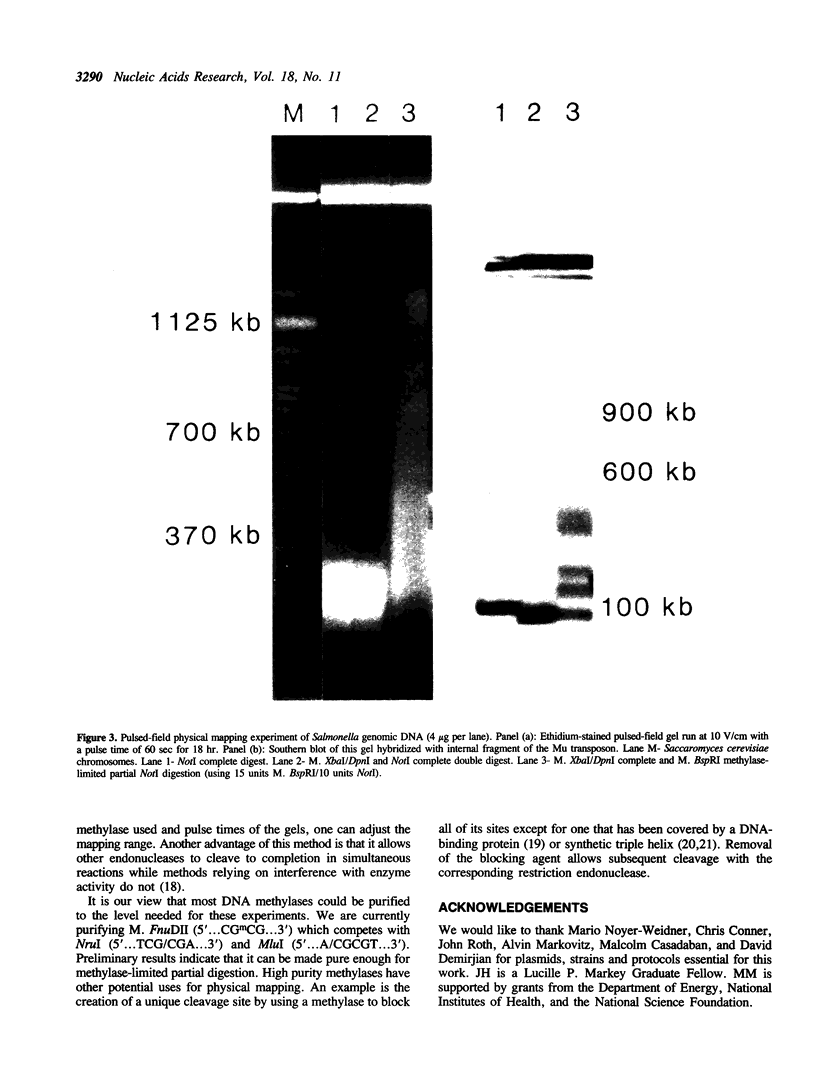
Partial cleavage of DNA with the restriction endonuclease NotI (5'...GC/GGCCGC...3') is an important technique for genomic mapping. However, partial genomic cleavage with this enzyme is impaired by the agarose matrix in which the DNA must be suspended. To solve this problem we have purified the blocking methylase M. BspRI (5'...GGmCC...3') for competition digests with NotI. The resulting methylase-limited partial DNA cleavage is shown to be superior to standard techniques on bacterial genomic DNA. Abbreviations: bp, base-pair; kb, one thousand base-pairs; Mb, one million base-pairs; Tris, Tris(hydroxy-methyl)aminomethane; EDTA, (ethylenedinitrilo)tetraacetic; beta-ME, beta-mercaptoethanol; PMSF, phenyl methyl-sulfonyl fluoride; PEG, polyethyleneglycol (MW = 8000); 3H, tritium; SAM, S-adenosylmethionine; KGB, potassium glutamate buffer; DTT, dithiothreitol; IPTG, isopropyl-beta-D-thiogalactopyranoside; BSA, bovine serum albumin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsen H. M., Le Paslier D., Abderrahim H., Dausset J., Cann H., Cohen D. Improved control of partial DNA restriction enzyme digest in agarose using limiting concentrations of Mg++. Nucleic Acids Res. 1989 Jan 25;17(2):808–808. doi: 10.1093/nar/17.2.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R. Molecular cloning of human telomeres in yeast. Nature. 1989 Apr 27;338(6218):774–776. doi: 10.1038/338774a0. [DOI] [PubMed] [Google Scholar]
- Cheng J. F., Smith C. L., Cantor C. R. Isolation and characterization of a human telomere. Nucleic Acids Res. 1989 Aug 11;17(15):6109–6127. doi: 10.1093/nar/17.15.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. H., Allshire R. C., McKay S. J., McGill N. I., Cooke H. J. Cloning of human telomeres by complementation in yeast. Nature. 1989 Apr 27;338(6218):771–774. doi: 10.1038/338771a0. [DOI] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Hanish J., McClelland M. Activity of DNA modification and restriction enzymes in KGB, a potassium glutamate buffer. Gene Anal Tech. 1988 Sep-Oct;5(5):105–107. doi: 10.1016/0735-0651(88)90005-2. [DOI] [PubMed] [Google Scholar]
- Hanish J., McClelland M. Controlled partial restriction digestions of DNA by competition with modification methyltransferases. Anal Biochem. 1989 Jun;179(2):357–360. doi: 10.1016/0003-2697(89)90144-9. [DOI] [PubMed] [Google Scholar]
- Koob M., Grimes E., Szybalski W. Conferring operator specificity on restriction endonucleases. Science. 1988 Aug 26;241(4869):1084–1086. doi: 10.1126/science.2842862. [DOI] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Wold B., Dervan P. B. Inhibition of DNA binding proteins by oligonucleotide-directed triple helix formation. Science. 1989 Aug 18;245(4919):725–730. doi: 10.1126/science.2549631. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Pósfai G., Kiss A., Erdei S., Pósfai J., Venetianer P. Structure of the Bacillus sphaericus R modification methylase gene. J Mol Biol. 1983 Nov 5;170(3):597–610. doi: 10.1016/s0022-2836(83)80123-5. [DOI] [PubMed] [Google Scholar]
- Ratet P., Richaud F. Construction and uses of a new transposable element whose insertion is able to produce gene fusions with the neomycin-phosphotransferase-coding region of Tn903. Gene. 1986;42(2):185–192. doi: 10.1016/0378-1119(86)90295-7. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storts D. R., Markovitz A. Construction and characterization of mutations in hupB, the gene encoding HU-beta (HU-1) in Escherichia coli K-12. J Bacteriol. 1988 Apr;170(4):1541–1547. doi: 10.1128/jb.170.4.1541-1547.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szomolányi E., Kiss A., Venetianer P. Cloning the modification methylase gene of Bacillus sphaericus R in Escherichia coli. Gene. 1980 Aug;10(3):219–225. doi: 10.1016/0378-1119(80)90051-7. [DOI] [PubMed] [Google Scholar]
- Weil M. D., McClelland M. Enzymatic cleavage of a bacterial genome at a 10-base-pair recognition site. Proc Natl Acad Sci U S A. 1989 Jan;86(1):51–55. doi: 10.1073/pnas.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y. N., Van Etten J. L. DNA methyltransferase induced by PBCV-1 virus infection of a Chlorella-like green alga. Mol Cell Biol. 1986 May;6(5):1440–1445. doi: 10.1128/mcb.6.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]





