Abstract
The impact of 10 years of annual foot trampling on soil biocrusts was examined in replicated field experiments at three cold desert sites of the Colorado Plateau, USA. Trampling detrimentally impacted lichens and mosses, and the keystone cyanobacterium, Microcoleus vaginatus, resulting in increased soil erosion and reduced C and N concentrations in surface soils. Trampled biocrusts contained approximately half as much extractable DNA and 20–52% less chlorophyll a when compared with intact biocrusts at each site. Two of the three sites also showed a decline in scytonemin-containing, diazotrophic cyanobacteria in trampled biocrusts. 16S rRNA gene sequence and terminal restriction fragment length polymorphism (T-RFLP) analyses of soil bacteria from untrampled and trampled biocrusts demonstrated a reduced proportion (23–65% reduction) of M. vaginatus and other Cyanobacteria in trampled plots. In parallel, other soil bacterial species that are natural residents of biocrusts, specifically members of the Actinobacteria, Chloroflexi and Bacteroidetes, became more readily detected in trampled than in untrampled biocrusts. Replicate 16S rRNA T-RFLP profiles from trampled biocrusts at all three sites contained significantly more fragments (n=17) than those of untrampled biocrusts (n⩽6) and exhibited much higher variability among field replicates, indicating transition to an unstable disturbed state. Despite the dramatic negative impacts of trampling on biocrust physical structure and composition, M. vaginatus could still be detected in surface soils after 10 years of annual trampling, suggesting the potential for biocrust re-formation over time. Physical damage of biocrusts, in concert with changing temperature and precipitation patterns, has potential to alter performance of dryland ecosystems for decades.
Keywords: chlorophyll a, cyanobacteria, Microcoleus vaginatus, soil biocrust, scytonemin, 16S rRNA
Introduction
Over 35% of the Earth's land mass is considered dryland regions that include hyperarid, semiarid, arid, alpine and polar regions (Belnap, 2006). Biological soil crusts (hereafter termed biocrusts) are prominent surface features in all of Earth's natural drylands (Belnap and Lange, 2003). Biocrusts are photosynthetic, diazotrophic communities of bacteria, fungi, algae, lichens and mosses that colonize the surfaces of dryland soils. In the Western US, over 40% of the 100 million hectares of dryland grazing lands are covered by biocrusts (Garcia-Pichel et al., 2003).
Biocrusts are important to the stability and productivity of dryland ecosystems where plants are typically sparse. The physical structure stabilizes the soil against wind and water erosion (Belnap and Gillette, 1998; Warren, 2001). Biocrusts typical of the Colorado Plateau, USA, have been shown to increase soil fertility and soil moisture retention (Belnap and Lange, 2001; Belnap, 2003a), and thus influence the germination, survival and nutritional status of the widely spaced vascular plants (Belnap et al., 2001). Additionally, dark cyanobacterial and lichen pigments in biocrusts decrease surface albedo, affecting local and regional temperatures (Belnap, 1995).
Biocrust formation is a successional process, beginning with surface soil colonization by the cyanobacterium Microcoleus vaginatus (Büdel et al., 2009; Zaady et al., 2010). Polysaccharides produced by M. vaginatus effectively ‘glue' soil particles together, dramatically increasing soil stability and moisture-holding capacity of dry soils (Mazor et al., 1996). Over time, diazotrophic cyanobacteria, fungi, lichens and mosses colonize the M. vaginatus biocrust.
Globally, drylands are increasingly being used for agriculture, animal grazing and recreational activities that physically disrupt soil surfaces. These activities have been shown to negatively impact dryland ecosystems by causing erosion, reduced soil fertility and negative effects on plant health and productivity (Barger et al., 2006; Belnap and Sherrod, 2009). Although the impacts of physical disturbance on surface biocrusts are readily seen from a distance (Table 1), the response and resilience of M. vaginatus and other biocrust bacteria to chronic physical disturbances is unknown. Collectively, the biocrust Cyanobacteria and associated heterotrophs contribute significant amounts of C and N to dryland soils, and their loss from the system has potential to affect long-term soil fertility and ecosystem performance in addition to the physical stability of the surface.
Table 1. Characteristics of the three arid shrubland field sites.
We examined the impact of 10 years of annual foot trampling on the bacterial community of the biocrust, in replicated field experiments conducted at three cold desert sites in the Colorado Plateau. Using a combination of pigment analyses, 16S rRNA gene sequencing and terminal restriction length polymorphism (T-RFLP) pattern comparisons, we measured the impact of trampling on biocrust biomass and bacterial community composition, with particular emphasis on the keystone cyanobacterium, M. vaginatus. As trampling destroys biocrust physical structure, we hypothesized that the effects on the biocrust bacterial community would be severe, and that trampling effects would be similar across different shrubland sites.
Materials and methods
Experimental design and sample collection
Three field sites, termed Arches (Arches National Park, UT), ISKY-1 and ISKY-2 (ISKY=Island in the Sky district of Canyonlands National Park, UT) were established in the Spring of 1994 (WGS84: Arches 625317–625427 m, ISKY-1 602530–602619 m, ISKY-2 602171–602185 m). The dominant vegetation at all three sites was blackbrush (Coleogyne ramosissima), but the sites differed in soil depth, as well as blackbrush size and spacing (Table 1). Individual plants were larger and more densely distributed at the Arches site, intermediate in size and spacing at ISKY-1 and smaller and further apart at ISKY-2, presumably due to a parallel gradient in soil depth (that is, Arches>ISKY-1>ISKY-2). Experimental plots at the Arches site also contained abundant prickly pear (Opuntia phaeocantha).
At each of the three sites, 10 plots, each about 10 m2 in size, were delineated in a randomized complete block design. Beginning in May 1995, five plots were trampled annually and five were left undisturbed (untrampled control plots). Trampling was conducted once a year in May, and was implemented as two gentle, flat-footsteps over the entire plot to disrupt the biocrust while minimizing soil compaction or mixing. Plots were accessed for sampling along established trails within the plots.
In April 2004, approximately 11 months after the 2003 trampling event (hereafter termed ‘1 year') and 10 years after the initial disturbance, soil samples were collected for soil chemical, pigment and DNA-based analyses. Additional surface soil samples were collected in May 2004, 1 month after the April 2004 trampling event. The percent coverage of lichens, mosses and rock in the control and trampled plots was assessed visually at the time soil samples were collected, using Daubenmire cover classes in 10 frames in each plot (Daubenmire, 1959). For analysis of bacterial community composition, a sterile spatula was used to remove ∼5 g of surface soil (0–1 cm) (containing either intact biocrust or trampled surface soil). Sub-biocrust soils were collected by removing the biocrust and scooping 5 g of sample at 5 cm depth. After preliminary analysis by T-RFLP and 16S rRNA gene sequencing showed little variability in biocrust biomass or composition across the five 10-m2 plots within each trampling treatment, DNA-based analyses were conducted on one of the five 10-m2 plots for each treatment in each field site, with four replicate samples collected within that plot (n=24). Soil samples for DNA-based analyses were collected into sterile 50-ml Falcon tubes, immediately placed on dry ice and transported back to the laboratory where they were stored at −70 °C. All samples were collected from the plant interspaces, avoiding plant debris and root systems.
Pigment and soil N analysis
Chlorophyll a was used as a relative measure of cyanobacterial biomass in the biocrust. In addition, soil scytonemin concentration was used to measure the relative abundance of the scytonemin-producing cyanobacteria at these study sites, typically the heterocystous, diazotrophic Nostoc, Scytonema and Tolypothrix species (Redfield et al., 2002; Gundlapally and Garcia-Pichel, 2006; Yeager et al., 2007). For biocrust pigment analysis at each site, ten 2-g scoops of surface biocrust (0–0.5 cm depth) were collected at random locations from each of the 10 plots. The ten 2-g scoops were pooled to generate a 20-g sample for each of the ten plots, and were processed immediately. Chlorophyll a and scytonemin content in each soil sample was measured using high-performance liquid chromatography after acetone extraction (Bowker et al., 2002). Thirty soil samples were collected at 0–10 cm depth, composited and subsampled for soil chemical analysis. Soil N fractions were determined colorimetrically after 2 KCl extraction (Bremner and Keeney, 1966).
DNA extraction
Total biocrust or soil DNA was extracted using a bead mill method described previously (Kuske et al., 1998) and cleaned of PCR-inhibiting impurities using N-phenacylthiazolium bromide (Dunbar and Kuske, 2006; Kuske et al., 2006). DNA extracts were quantified using the Quant-It PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol.
16S rRNA gene clone/sequence library analysis
Partial 16S rRNA genes were amplified by PCR from each soil sample using bacterial primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) (Lane, 1990) and 787Rb (5′-GGACTACNRGGGTATCTAAT-3′) (Kuske et al., 2006). Each 50-μl reaction was carried out in triplicate, and contained the following: 15 ng template DNA, 10 μl 0.1 trehalose (Sigma-Aldrich, St Louis, MO, USA) 5 μl GeneAmp 10 × PCR buffer (Applied Biosystems, Foster City, CA, USA), 4 μl 10 m dNTPs (Applied Biosystems), 1 μl of each primer at 50 μ, 0.5 μl 20 mg ml−1 bovine serum albumin (Roche, Indiapolis, IN, USA), and 2.5 U AmpliTaq DNA Polymerase LD (Applied Biosystems). Reactions were carried out in a PTC-200 thermal cycler (MJ Research, Waltham, MA, USA) as follows: 5 min at 95 °C, 35 cycles of 45 s at 55 °C, 90 s at 72 °C and 30 s at 95 °C, followed by a final extension step of 72 °C for 10 min.
The 16S rRNA gene clone/sequence libraries (96 clones in size before sequence processing) were generated to estimate the proportion of soil bacterial represented by Cyanobacteria in trampled and untrampled biocrusts, and to identify peaks in the T-RFLP profiles (described in more detail below). Clone libraries were constructed using the TOPO-TA system (Invitrogen) and individual clones were sequenced using Sanger technology. Sequence data was assembled and edited in Sequencher v4.7 (GeneCodes, Ann Arbor, MI, USA), oriented and manually edited in BioEdit (Hall, 1999). Chimeric sequences were removed using the Bellerophon program (Huber et al., 2004), and sequences were assigned to phylum-level taxa using RDPquery (Dyszynski and Sheldon, 2007). Resulting libraries each contained 41–84 double-pass sequences of 600–800 bp each. A clone library was constructed from two field replicates in untrampled plots at each of the three sites (n=6 libraries, 359 final sequences), one field replicate from a trampled plot at each of the three sites (n=3 libraries, 170 final sequences) and one sub-biocrust sample from control plots at each of the three sites (n=3 libraries, 180 final sequences). The composition of non-cyanobacterial sequences in the untrampled, trampled and sub-crust soils was compared using the Library Compare tool within RDP (Cole et al., 2009). Representative 16S rRNA sequences from this study have been deposited in GenBank under accession numbers JN795522–JN795891.
Bacterial community structure (T-RFLP) analysis
PCR amplifications for T-RFLP analysis were as described above, except using a 27F primer labeled with 6-carboxyfluorescein and the unlabeled 787Rb primer. After amplification, the triplicate reactions were pooled, concentrated via vacuum centrifugation and gel purified using the QiaQuick Spin Kit (Qiagen, Valencia, CA, USA). Amplicons (50 ng) were digested in 50-μl reactions containing 5 U RsaI (Promega Corporation, Madison, WI, USA) and 5 μl buffer C (Promega) for 4.5 h at 35 °C. Digested products were desalted by standard ethanol precipitation and stored at −20 °C until analysis. T-RFLP analysis was carried out on an Applied Biosystems 3130XL Genetic Analyzer using the POP-7 polymer. Profiles were examined using GeneMapper (Applied Biosystems) and T-RFLP fragments of size 50–700 bp were used for comparative analysis. T-RFLP profiles were normalized to percent fluorescence in each profile.
To compare clone library sequences with the T-RFLP profiles, in silico RsaI restriction digestion was performed on each soil clone sequence (T-DistinctiEnz in silico T-RFLP tool; http://www.bioinformatics.org/~docreza/cgi-bin/restriction/t_DistinctEnz.pl).
Statistical analyses
Two-sample t-tests and multiple comparisons were conducted using one-way analysis of variance and Tukey-HSD analyses, after checking for a normal distribution. The relative abundance (fluorescence) of T-RFLP fragments of size 50–700 bp, representing >1% of the total fluorescence, were compared using a Manhattan distance matrix and sample relationships visualized using 2D nonmetric multidimensional scaling plots generated in the R program software (version 2.11.1; http://www.r-project.org).
Results
Cross-site comparisons of untrampled biocrusts
Soil and biocrust characteristics
The soils were sandy, alkaline (pH 8.1–8.2), not horizonated and contained less than 1% organic matter. The Arches and ISKY-1 soils were similar in texture and were at least 25 cm deep; the ISKY-2 site was only 10 cm deep and twice as high in clay content (Table 1). At the ISKY-2 site, 19% of the surface was bare rock. Soil N concentration was numerically higher in surface soils at the ISKY-1 and ISKY-2 sites than at the Arches site (29% and 143% higher, respectively: Table 2), and was statistically higher at the ISKY-2 site than the Arches site (analysis of variance P=0.03, Tukey's HSD mean separation ISKY-2 a, ISKY-1 ab, Arches c). The amount of exposed rock was highest at the ISKY-2 site. Other soil chemical measures were similar across the three sites (P, K and the cations Ca, Mg, Na; data not shown).
Table 2. Soil nitrogen measures at three field sites.
|
Arches |
ISKY-1 |
ISKY-2 |
||||
|---|---|---|---|---|---|---|
| Untrampled | Trampled | Untrampled | Trampled | Untrampled | Trampled | |
| Total N | 86 (6) | 66 (6) | 111 (5)* | 89 (5)* | 209 (32) | 159 (32) |
| NH4+ | 0.4 (0.1) | 0.4 (0.1) | 0.3 (0.05)* | 0.1 (0.02)* | 0.7 (0.1) | 0.7 (0.2) |
| NO3− | 0.7 (0.8) | 0.6 (0.1) | 0.9 (0.1)* | 0.6 (0.2)* | 1.5 (0.2) | 1.0 (0.2) |
The means and s.e. (n=2 for total N, n=5 for NH4+ and NO3−) of μg-nutrient g−1 soil are shown. The * denotes significant difference between control and trampled values within each site, by two-tailed pair-wise t-test (P<0.05 for NH4+ and NO3−, and P<0.08 for total N).
Biocrust characteristics and developmental stage varied among the three sites. Lichen and moss cover was higher on the deeper soils at the Arches site (17%) than the two ISKY sites (4–7%) (Table 1). The biocrusts were most well developed at the ISKY sites, where they were dark and pinnacled (Table 1).
Biocrust biomass and relative abundance of cyanobacteria
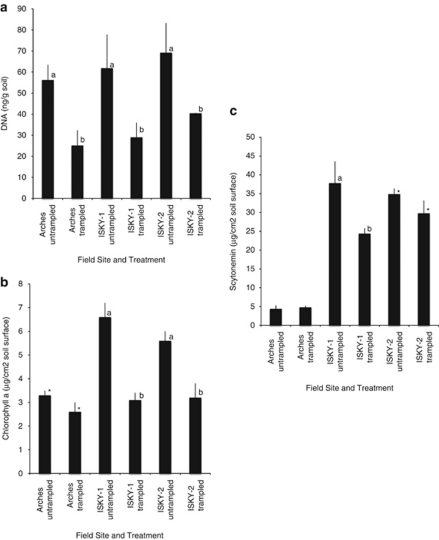
Extracted soil DNA concentrations were similar in biocrust samples collected across the three sites (Figure 1a). However, relative abundance of Cyanobacteria in untrampled crusts, measured as biocrust chlorophyll a concentration, was significantly higher at the ISKY sites compared with the Arches site. Chlorophyll a concentrations were 1.7–2-fold higher in biocrusts at the ISKY sites than at the Arches site (Figure 1b; P<0.0005). Similarly, the relative abundance of scytonemin was 8.0–8.6-fold higher at the ISKY sites than and the Arches site (Figure 1c; P<0.0001).
Figure 1.
Relative abundance of (a) DNA, (b) chlorophyll a, and (c) scytonemin extracted from trampled and untrampled plots sampled 1 year after trampling. Values are means (s.e.) for n=5 samples per treatment. Different letters denote a significant effect of trampling between pairs of untreated and trampled soils within each field site (two-tailed pair-wise t-test P<0.05 or smaller). The * denotes pairs that were significantly different by one-tailed pair-wise t-test at P<0.10.
Biocrust bacterial community
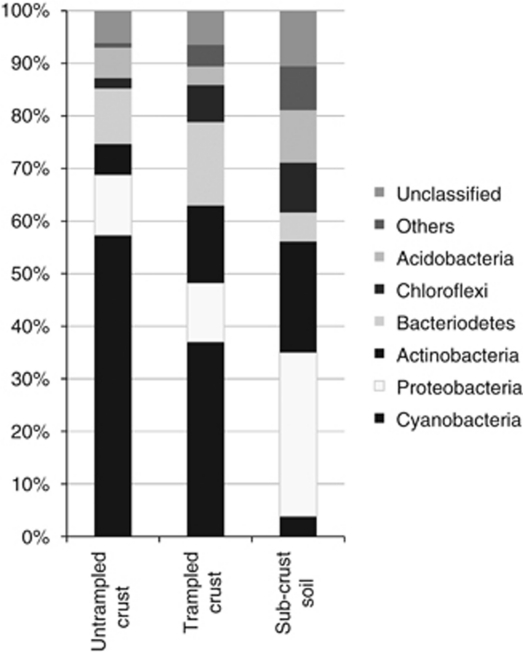
The composition of the total soil bacterial community, measured by sequence analysis of 16S rRNA gene clone libraries, illustrated the dominance of Cyanobacteria in both trampled and untrampled biocrusts compared with sub-biocrust soil in each of the three study sites (pooled data in Figure 2, results by individual sample in Supplementary Figure S1). Cyanobacteria sequences were the most highly represented phylum in biocrusts from duplicate clone libraries at all three sites, comprising 32 (±1)%, 59 (±2)% and 68 (±5)% of the total from Arches, ISKY-1 and ISKY-2, respectively. M. vaginatus was the dominant member of the cyanobacteria sequences, comprising 77–95% of the cyanobacteria sequences in the libraries. Other phyla represented in the biocrust clone libraries included Bacteroidetes (6–17% of the total sequences per library), Proteobacteria (3–24%), Actinobacteria (0–17%), Chloroflexi (0–4%), Acidobacteria (3–8%) and other/unclassified sequence types (2–20%). In contrast, there were no cyanobacteria sequences (Arches and ISKY-1) or few (7%, ISKY-2) in sub-biocrust soils at 5 cm depth. These libraries were comprised of Actinobacteria (13–29%), Bacteroidetes (1–14%), Proteobacteria (26–60%), Acidobacteria (5–14%), Chloroflexi (4–9%) and other phyla or unclassified sequence types (10–26%).
Figure 2.
Major bacterial phyla in 16S rRNA gene clone/sequence libraries from untrampled biocrusts (0–2 cm depth), trampled soils (0–2 cm depth) and sub-biocrust soils (5 cm depth). Sequences from clone libraries generated from all three field sites (Arches, ISKY-1 and ISKY-2) were pooled to generate the bars depicted in the figure. The untrampled biocrust bar depicts the pooled composition of sequences from six clone libraries (two field replicates for each site, total 385 sequences). Trampled biocrust and sub-biocrust soil bars each represent the pooled sequence composition of three clone libraries (one field replicate for each site, total 180 and 220 sequences, respectively). Individual clone library results are shown in Supplementary Figure 1.
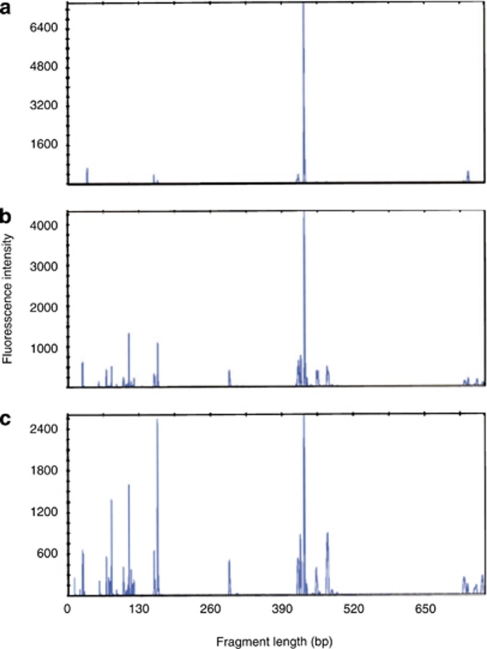
Replicate T-RFLP profiles from untrampled biocrusts at each of the three sites were characterized by few fragments (n=3–7) and the most abundant terminal restriction fragment (TRF; 430 bp in length), accounted for an average of 60%, 85% and 88% of the total fluorescence in Arches, ISKY-1 and ISKY-2 profiles, respectively (Figure 3a and Table 3). By establishing restriction fragment sizes of the 16S rRNA sequences in the clone libraries, we determined that the 430-bp TRF represented M. vaginatus and potentially a few other cyanobacterial species within the Oscillatoriales order (not detected in the sequence dataset but identified in public databases). The majority of the remaining fluorescence signal detected in untrampled biocrust profiles was associated with TRFs (417–424 bp) that most likely represent Cyanobacteria or α-Proteobacteria, based on in silico restriction analysis of the 16S rRNA sequences in the clone libraries (Table 3).
Figure 3.
T-RFLP profiles of biocrusts from the Arches site. (a) Untrampled biocrust, trampled biocrust collected (b) 1 year after trampling or (c) trampled biocrust collected 1 month after trampling. The dominant M. vaginatus peak is visible in the profiles at ∼420 bp in length.
Table 3. Number of T-RFLP fragments and % of fluorescence contributed by major bacterial phyla (for which TRFs could be assigned).
| Number of Fragments | % Cyanobacteria (pk 430) | % Actinobacteria (pk 76-79, 162-164) | % Chloroflexi (pk 100-101, 156) | % Bacteriodetes (pk 69, 110, 293) | % alpha-Proteobacteria (pk 415-416, 417-418) | |
|---|---|---|---|---|---|---|
| (A) Arches | ||||||
| Untrampled control | 4 (1) a | 60 (5.1) a | 0 (0) a | 0 (0) a | 0 (0) a | 19 (4.7) a |
| Trampled (1 month ago) | 16 (2.1) b | 46 (7.3) a | 19 (6.0) b | 5 (0.5) b | 2 (0.3) b | 13 (1.2) a |
| Trampled (1 year ago) | 13 (2.3) b | 40 (5.9) a | 11 (5.4) b | 4 (2.4) b | 2 (0.5) b | 13 (4.6) a |
| F-test result, P-value | 0.0002 | 0.0982 | 0.0038 | 0.0099 | 0.0002 | 0.1500 |
| (B) ISKY-1 | ||||||
| Untrampled control | 3 (1.6) a | 85 (2.8) a | 0 (0) a | 3 (1.3) a | 0 (0) a | 0 (0) a |
| Trampled (1 month ago) | 14 (1.6) b | 28 (24.5) b | 19 (5.5) b | 7 (1.3) a | 3 (0.7) b | 7 (2.0) b |
| Trampled (1 year ago) | ND | ND | ND | ND | ND | ND |
| T-test result, P-values | 0.0033 | 0.0037 | 0.052 | 0.075 | 0.030 | 0.041 |
| (C) ISKY-2 | ||||||
| Untrampled control | 7 (1.5) a | 88 (5.1) a | 2 (1.5) b | 1 (0.7) a | 1 (0.5) a | 3 (1.7) a |
| Trampled (1 month ago) | 17 (7) b | 31 (11.0) b | 17 (8.7) a | 8 (2.8) b | 7 (2.9) b | 10 (11.6) a |
| Trampled (1 year ago) | 17 (1) b | 52 (5.6) c | 10 (2.8) ab | 7 (3.3) b | 4 (1.1) b | 11 (6.5) a |
| F-test result, P-value | 0.0068 | 0.0001 | 0.0093 | 0.0116 | 0.0023 | 0.3508 |
Abbreviations: ND, not determined; pk, T-RFLP peak or fragment length.
P-values are from one-way analysis of variance with three treatments (for Arches, ISKY-2 sites), or with two treatments (for ISKY-1 site). Values in bold text followed by different letters were significantly different (alpha =0.05 or less) by Tukey's HSD mean separation procedure (Arches, ISKY-2 sites, or by pair-wise t-test (ISKY-1 site).
Effects of yearly trampling on biocrusts at the three sites
Visual biocrust assessment and soil chemistry
Visual biocrust assessment and soil chemistry analyses were conducted approximately 1 year after the last trampling. After 10 years of annual trampling, the biocrust physical structure had been destroyed and the surface alterations were readily visible (Table 1 photographs). The trampled biocrusts were not pinnacled and were lighter in color than the untrampled biocrusts. The percent coverage of mosses and lichens was significantly reduced at all three sites: from 17% to zero at Arches, 4% to zero at ISKY-1, and 7% to 0.1% at ISKY-2 (P<0.05 for each pair-wise comparison). At the ISKY sites, trampling resulted in a significant increase in rock cover, where repeated trampling of the shallow soils at these sites resulted in erosion and exposure of the underlying rock (P<0.05 for each pair-wise comparison). At ISKY-1, rock cover increased from 0.2% to 1.6%, and at ISKY-2, rock cover increased from 19% to 29% of the surface. The trampled soil at the ISKY-1 site contained significantly less total N, NH4 and NO3 than plots with biocrust (Table 2). The other two sites followed this same trend although the replicate values were not significantly different. Soil pH and concentrations of P, K and cations (Ca, Mg, Na) did not differ between the untrampled and trampled soils at any of the three sites (data not shown).
Biocrust microbial and cyanobacterial biomass
Total biocrust microbial biomass, measured as total extracted DNA, was significantly reduced in the trampled plots, with a 42–55% reduction compared with the untrampled plots at all three sites, 1 year after trampling (Figure 1a). Soil chlorophyll a concentration was also reduced by about one half in trampled plots compared with untrampled plots at ISKY-1 and ISKY-2 (52% and 42%, respectively; pair-wise t-test, P<0.05, Figure 1b). The effect of trampling on soil chlorophyll a levels at the Arches site was less extreme (20% reduction, P<0.10). Soil scytonemin concentrations were high in the untrampled biocrusts at the ISKY sites, and trampling reduced this concentration by 15–35% (Figure 1c). In contrast, soil scytonemin concentration was not significantly different between the untrampled and trampled plots at the Arches site, where soil concentrations of the pigment were low in both treatments.
Biocrust bacterial community composition
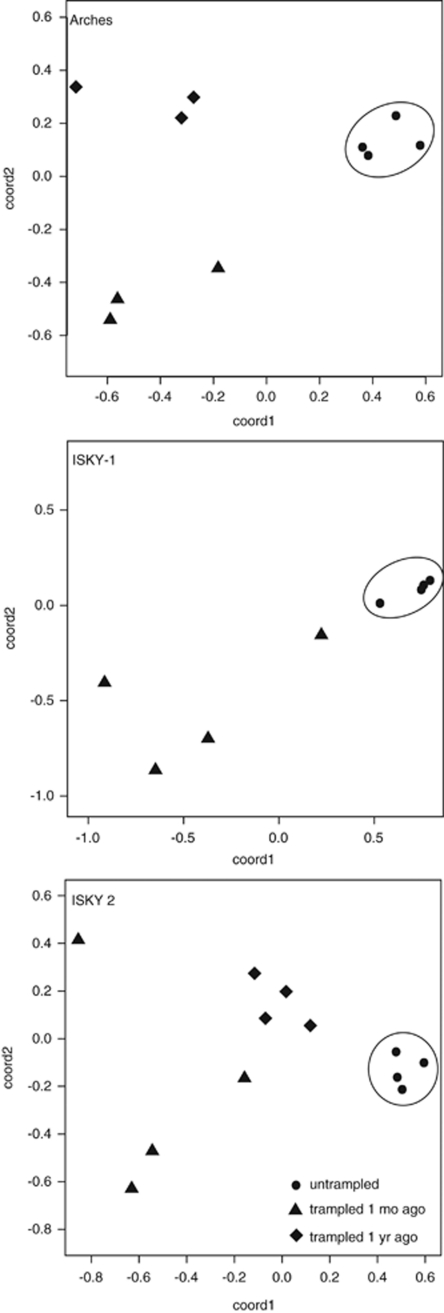
Comparison of replicate 16S rRNA T-RFLP profiles was used to determine if trampling caused major shifts in bacterial community composition. Trampled soils collected from all three sites, either 1 month or 1 year after the most recent trampling event, contained significantly more fragments (n=13–17) than found in untrampled soils (n=3–7; Figure 3 and Table 3). The bacterial community composition differed dramatically between the intact and trampled biocrusts (Figure 3, T-RFLP profile example, and Figure 4, MDS plot). The T-RFLP profiles from the untrampled crusts at all three sites were dominated by the 430-bp cyanobacteria peak, and were very similar in replicate soil samples, as illustrated by the tight clustering pattern for these samples in the MDS plots (Figure 4). In contrast, the bacterial composition of the trampled soils was variable among the replicate field samples for each of the two post-trampling sampling dates, and bacterial community composition was dissimilar between the two time points. The trend of highly uniform composition changing to highly variable composition was consistent across all three of the field sites (Figure 4).
Figure 4.
Nonmetric multidimentional scaling plots derived from Manhattan distance matrices of 16S rRNA T-RFLP profiles show the uniformity of the bacterial community composition in replicate field samples from untrampled biocrusts (circles) at three different field sites (labeled within plot boxes). Bacterial community composition in trampled plots differed dramatically from the untrampled plots 1 month (triangles) or 1 year (diamonds) after a trampling event, and was highly variable across the field replicates.
Using the 430-bp TRF as an indicator of Cyanobacteria relative abundance, we found a significantly lower proportion of cyanobacteria rRNA genes in trampled soils than in untrampled biocrusts from both of the ISKY sites (Tables 3B and C). At the Arches site, the proportion of Cyanobacteria in the trampled soils was also lower than in the untrampled biocrusts (60%, untrampled; 46%, 1 month post trampling; 40%, 1 year post trampling) but the difference was not statistically significant (P⩽0.05) (Table 3A).
As the proportion of the cyanobacterial TRF decreased, fragments representing three other bacterial phyla, the Actinobacteria, Chloroflexi and Bacteriodetes, were more readily detected in T-RFLP profiles in trampled biocrusts. The TRFs assigned to represent actinobacteria sequences (76–79 and 162–164 bp fragments; ∼50% represent Rubrobacter species) were more frequently detected in profiles from trampled soils at all three sites. They comprised 0–2% of the profile fluorescence from untrampled biocrusts, but 10–19% of the profile fluorescence from trampled soils (Table 3). Individual TRFs could also be assigned to the Chloroflexi and Bacteriodetes phyla (primarily Chitinophagaceae family, 109–110 bp) and the α-Proteobacteria (Table 3). Individually, these phyla represented less than 8% of the profiles and showed small positive changes in response to trampling.
Only one TRF trended differently among the three sites when comparing relative abundance between the untrampled and trampled soils. The 417–418-bp TRF, which was assigned to the Sphingomonas genus of the α-Proteobacteria, represented a small portion of the untrampled biocrust T-RFLP profiles at the ISKY sites (0–2%), but increased to 7–11% in trampled soil profiles of those sites. In contrast, this TRF comprised a greater percentage of the total T-RFLP profile for untrampled (19%) than trampled (11–13%) biocrusts in the Arches site.
The 16S rRNA sequence data (n=6 libraries for untrampled and n=3 libraries for trampled plots), pooled across the three field sites, provided an observational comparison that supports the trends observed in the replicated T-RFLP profiles (Figure 2). The reduction in relative abundance of cyanobacteria sequences (from an average of 52% to 35% of the sequences) was accompanied by an increase in actinobacteria and bacteriodetes sequences in the trampled soils relative to untrampled biocrusts (Figure 2).
Pair-wise comparisons of the 16S rRNA gene surveys at the class level were conducted to determine if the non-cyanobacterial species in the trampled biocrust were natural biocrust residents that were more readily detected in trampled biocrusts once cyanobacteria became depleted, or were members of the sub-crust soil moving into the surface. The composition of dominant, non-cyanobacterial bacterial sequences was very similar between the untrampled and trampled biocrusts, but class-level composition in each of the two biocrusts differed greatly with the sub-crust soil. Members of the Sphingobacteria and α-Proteobacteria were the two dominant classes in the untrampled (24% and 26%, respectively) and trampled (25% and 17%, respectively) biocrusts, but were not common in the sub-crust soil (3% and 7.5%, respectively). In contrast, the γ-Proteobacteria comprised 12% of the sub-crust sequences but were not detected at all in the untrampled or trampled biocrust sequence datasets. Pair-wise comparisons for these three classes were not significantly different between the untrampled and trampled biocrusts, but were each significant at P<0.0001 in comparisons between either biocrust and the sub-crust soil. This suggests that after trampling, the non-cyanobacterial biocrust colonists remain the same and are not recruited from the sub-crust soil.
Discussion
Trampling clearly destroys the structural integrity of the biocrust (Belnap, 2003a; Eldridge et al., 2006). Consistent with previous studies on biocrusts, our three-site experiment in the Colorado Plateau showed that most lichens and mosses were completely destroyed by trampling, with no evidence of re-colonization after a year. Annual trampling also resulted in increased soil erosion, as evidenced by the greater proportion of bare rock at the two shallow soil sites (ISKY-1 and ISKY-2) and a significant reduction of total N, ammonium and nitrate at ISKY-1. The other sites showed a similar, but not significantly different, trend in these three variables. The observed reduction in soil N could be due to loss of the dominant diazotrophic cyanobacteria, Nostoc, Tolypothrix and Scytonema species, and/or increased gaseous N loss from processes associated with microbial cell death or water erosion (Belnap, 2002, 2003b; Barger et al., 2006).
Annual trampling resulted in a significant reduction of biocrust biomass in all three of the shrubland field sites. Biocrust DNA concentrations in trampled plots were half, or less than half, of the amount measured in untrampled biocrusts. Biocrusts at these study sites and of the Colorado Plateau region in general, are dominated by the cyanobacterium M. vaginatus and multiple heterocystous cyanobacteria (Redfield et al., 2002; Gundlapally and Garcia-Pichel, 2006). Fungi, algae, archaea and eukaryotic microfauna, collectively account for less than 20% of the biocrust biomass (Bates and Garcia-Pichel, 2009; Soule et al., 2009). The cyanobacterial dominance within the bacterial community can be seen from both the T-RFLP (Figure 3, Table 3) and 16S rRNA sequence analyses (approximately 32–59%, depending on the site; Figure 2, Supplementary Figure S1). In all three shrubland sites, the Cyanobacteria were especially impacted by annual trampling, as demonstrated by the 20–52% reduction in chlorophyll a concentrations in the trampled plots compared with the control plots. In addition, the T-RFLP and sequence analyses showed a significant reduction in the proportion of Cyanobacteria sequences relative to other bacteria with trampling. The impacts of trampling on the Cyanobacteria were most dramatic at the ISKY-1 and ISKY-2 sites, where biomass of the untrampled biocrust was highest and where the biocrusts were well developed, pinnacled and darkly pigmented. At these two sites, the scytonemin-producing cyanobacteria were also negatively affected. Overall, the results suggest that the reduction in soil biomass of the Colorado Plateau biocrusts was primarily due to decreases in the abundance of the biocrust cyanobacterial community and, in particular, M. vaginatus. The decrease in proportion of cyanobacterial sequences was accompanied by an increased proportion of other soil bacterial species, specifically members of the Actinobacteria, Chloroflexi and Bacteroidetes.
Class-level comparisons of the 16S rRNA gene clone libraries provided evidence that the dominant, non-cyanobacterial bacterial taxa in untrampled and trampled biocrusts are very similar and both are very different from the sub-crust soil. This suggests that the dominant Actinobacteria, Chloroflexi and Bacteriodetes members of the trampled biocrusts either remained in place after trampling or were recruited from neighboring biocrusts (via wind or water dispersal), but were not likely recruited from the sub-crust bacterial community. It is not known whether the non-cyanobacterial biocrust constituents were unaffected or positively influenced by the conditions in the trampled soils, or were simply more readily detected in the sampled communities because the cyanobacterial sequences were absent from the DNA samples. Trampling depleted the UV-protective scytonemin layer on the soil surface, destroyed the microscale topography, decreased N availability and likely altered soil moisture retention and soil surface albedo. It is possible that damage to and death of the dominant cyanobacteria liberated a nutritional source for the resident heterotrophs, or that the loss of biocrust structure altered physio-chemical attributes that favored colonization of the other species.
After disturbance ceases, biocrust recovery relies on the colonization of the surface soil by filamentous cyanobacteria such as M. vaginatus to re-form the biocrust structure and photosynthetic capacity (Belnap and Lange, 2003; Chen et al., 2006; Wang et al., 2009). Our results show that even though cyanobacterial biomass was significantly reduced in annually trampled soils, the pioneer species M. vaginatus remained the dominant bacterial component (25–30% of the bacterial community) in the surface soils 1 month and 1 year after a trampling event. Unfortunately, the formation of a mature biocrust structure has been shown to be an extremely slow process that requires decades (Belnap and Gillette, 1998; Eldridge and Ferris, 1999; Belnap and Eldridge, 2003).
Recent studies have provided evidence that, in addition to M. vaginatus, other bacteria play a role in early biocrust formation (Reddy and Garcia-Pichel, 2007; Wu et al., 2010; Zheng et al., 2011). The majority (74%) of Actinobacteria sequences identified in this study belong to the deepest branch of the phylum, the Rubrobacteridae, which are common in arid soils and on rock surfaces worldwide and are extremely resistant to desiccation and UV stress (Holmes et al., 2000; Rainey et al., 2005). The involvement of Rubrobacteridae in soil aggregate formation and calcite precipitation suggests that they may also be involved in engineering the soil surface structure during biocrust formation (Mummey and Stahl, 2004; Rainey et al., 2005; Mummey et al., 2006; Pellerin et al., 2009). Filamentous Chloroflexi bacteria have been visualized within sheaths of Microcoleus chthonoplastes in hypersaline microbial mats and co-located with M. vaginatus in biocrusts of a high elevation site in Colorado, but whether the relationship between the two species is symbiotic or antagonistic is not known (Ley et al., 2006; Freeman et al., 2009). Sequences belonging to the Bacteroidetes phylum belonged almost exclusively to the Chitinophagaceae and Cytophagaceae genera. These microorganisms are known for their ability to degrade complex polysaccharides and aquatic species are known to play an important role in the turnover of algal and cyanobacterial exo-polysaccharides in marine and freshwater aggregates (Kirchman, 2002; Bauer et al., 2006). Regardless of the niche that these species occupy in trampled soils or the role that they play in crust reformation, our pigment and DNA-based surveys suggest that the surface soils in trampled plots certainly retained propagules of M. vaginatus and other native biocrust species that would be required for biocrust re-establishment.
Untrampled biocrusts at all three sites were characterized by a highly skewed community composition with Cyanobacteria as the dominant members (One dominant peak in T-RFLP profile, Figure 3a). This cyanobacteria-dominated pattern was uniform across each field site in untrampled biocrusts (Figure 4). The sub-crust soil in this region is very diverse and T-RFLP patterns typically contain 100–130 fragments (Kuske et al. 2002). The compositional uniformity the biocrust at each site was erased by repeated physical disruption, as evidenced by the increased diversity in the T-RFLP profiles and 16S rRNA gene clone libraries. Repeated physical disturbance also resulted in a dispersive pattern and lack of clustering among the replicate field samples (Figure 4) that suggests a chaotic response. This dispersive pattern was evident in all three study sites, 1 month or 1 year after the most recent trampling event. Community composition differed between the two time points. In sum, the trampled bacterial community was not the same as the original untrampled community after 1 year. The trampled community could still be changing, but not necessarily be moving towards the original untrampled state. The dispersive pattern illustrated in Figure 4 suggests that multiple components of the bacterial community are locally responsive to this type of disturbance, and that the outcome in different microsites varies considerably. Monitoring the soil bacterial communities in trampled plots where the disruption is halted should provide information on the spatio-temporal characteristics of biocrust re-assembly that could aid in predicting mature biocrust recovery and stability.
Soil disturbance alters both the physical characteristics (surface temperature, albedo, moisture dynamics) and nutritional resource distribution in desert soils (Belnap and Eldridge, 2003; Su et al., 2006). Spatial distribution of soil nutrients, in part, controls the distribution of bacterial communities across multiple scales (Franklin and Mills, 2009). Our study documents that the physical attributes of the soil surface, the UV protective pigments, the soil N status, and biocrust and cyanobacterial biomass are greatly altered by physical disturbance, and that the bacterial community remains in a chaotic state at least 1 year after the disturbance. The specific mechanisms underlying this dispersive response, and those operating to re-establish a stable cyanobacterial biocrust, are unknown. Employing assays that measure abundance of members of these phyla, and that address microbial activity, may reveal the factors that underpin our observations.
Chronic physical disturbance of soil surfaces is a major factor in desertification of Earth's drylands, and is increasing throughout the western US due to activities such as energy exploration/development, ex-urbanization and off-road vehicles. Physical disturbance is but one of multiple forces that are currently shaping dryland ecosystems and their biocrusts (for example, precipitation; Büdel et al., 2009; Zaady et al., 2010). Changes in regional weather that alter precipitation and temperature patterns have caused losses of mosses, lichens and cyanobacteria from the surface biocrust that display many parallels to the effects of physical disturbance shown here. For example, multiple years of low rainfall and high temperatures has resulted in a large decline in lichen cover in the Colorado Plateau region (Belnap, 2006; Belnap and Troxler (2006)), and even short-term alterations in summer precipitation pattern have been shown to result in dramatic biocrust decline, with loss of both mosses and cyanobacteria (Belnap et al., 2004; Belnap et al., unpublished data; Johnson, Kuske and Belnap, unpublished data). Soil in stability, and removal of the primary soil C and N inputs conferred by biocrusts, whether instigated by physical disturbance or physiological responses to altered weather conditions, is predicted to have cascading effects on soil erosion and soil biogeochemical cycles that control soil nutrition, and ultimately influence plant establishment and performance in these fragile ecosystems.
Acknowledgments
This study was funded by US Geological Survey; the National Park Service; the US Department of Energy, Program for Ecosystem Research and a Los Alamos National Laboratory postdoctoral research fellowship to CY. We thank the DOE JGI for providing Sanger sequencing of the clone libraries generated in this study, and several people for annual trampling of plots.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Barger N, Herrick J, Van Zee J, Belnap J. Impacts of biological soil crust disturbance and composition on C and N loss from water erosion. Biogeochemistry. 2006;77:247–263. [Google Scholar]
- Bates S, Garcia-Pichel F. A culture-independent study of free-living fungi in biological soil crusts of the Colorado Plateau: their diversity and relative contribution to microbial biomass. Environ Microbiol. 2009;11:56–67. doi: 10.1111/j.1462-2920.2008.01738.x. [DOI] [PubMed] [Google Scholar]
- Bauer M, Kube M, Teeling H, Richter M, Lombardot T, Allers E, et al. Whole genome analysis of the marine Bacteroidetes ‘Gramella forsetii' reveals adaptations to degradation of polymeric organic matter. Environ Microbiol. 2006;8:2201–2213. doi: 10.1111/j.1462-2920.2006.01152.x. [DOI] [PubMed] [Google Scholar]
- Belnap J. Surface disturbances: their role in accelerating desertification. Environ Monit Assess. 1995;37:1–19. doi: 10.1007/BF00546879. [DOI] [PubMed] [Google Scholar]
- Belnap J. Impacts of off-road vehicles on nitrogen cycles in biological soil crusts: resistance in different US deserts. J Arid Environ. 2002;52:155–165. [Google Scholar]
- Belnap J. Biological soil crusts in deserts: a short review of their role in soil fertility, stabilization, and water relations. Algol Stud. 2003a;109:113–126. [Google Scholar]
- Belnap J.2003bFactors influencing nitrogen fixation and nitrogen release in biological soil crustsIn: Belnap J, Lange OL (eds).Biological Soil Crusts: Structure, Function and Management Springer-Verlag: Berlin; 241–261. [Google Scholar]
- Belnap J. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol Process. 2006;20:3159–3178. [Google Scholar]
- Belnap J, Eldridge DJ.2003Disturbance and recovery of biological soil crustsIn: Belnap J, Lange OL (eds).Biological Soil Crusts: Structure, Function, and Management Springer-Verlag: Berlin; 364–383. [Google Scholar]
- Belnap J, Gillette DA. Vulnerability of desert biological soil crusts to wind erosion: the influences of crust development, soil texture, and disturbance. J Arid Environ. 1998;39:133–142. [Google Scholar]
- Belnap J, Lange OL.2001Structure and functioning of biological soil crusts: a synthesisIn: Belnap J, Lange OL (eds).Biological Soil Crusts: Structure, Function and Management Springer-Verlag: Berlin; 471–479. [Google Scholar]
- Belnap J, Lange OL.(eds) (2003Biological Soil Crusts: Structure, Function and Management Springer-Verlag: Berlin; 503pp. [Google Scholar]
- Belnap J, Phillips SL, Miller ME. Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia. 2004;141:306–316. doi: 10.1007/s00442-003-1438-6. [DOI] [PubMed] [Google Scholar]
- Belnap J, Prasse R, Harper KT.2001Influence of biological soil crusts on soil environments and vascular plantsIn: Belnap J, Lange OL (eds).Biological Siol Crusts: Structure, Function and Management Springer-Verlag: Berlin; 281–300. [Google Scholar]
- Belnap J, Sherrod SK. Soil amendment effects on the exotic annual grass Bromus tectorum L. and facilitation of its growth by the native perennial grass Hilaria jamesii (Torr.) Benth. Plant Ecol. 2009;201:709–721. [Google Scholar]
- Belnap J, Troxler T. Soil lichen and moss cover and species richness can be highly dynamic: the effects of invasion by the annual exotic grass Bromus tectorum and the effects of climate on biological soil crusts. Appl Soil Ecol. 2006;32:63–76. [Google Scholar]
- Bowker MA, Reed SC, Belnap J, Phillips SL. Temporal variation in community composition, pigmentation, and Fv/Fm of desert cyanobacterial soil crusts. Microb Ecol. 2002;43:13–25. doi: 10.1007/s00248-001-1013-9. [DOI] [PubMed] [Google Scholar]
- Bremner JM, Keeney DR. Determination and isotope-ratio analysis of different forms of nitrogen in soils. Exchangable ammonium, nitrate, and nitrite by extraction-distillation methods. Soil Soc Am Proc. 1966;30:577–582. [Google Scholar]
- Büdel B, Darienko T, Deutschewitz K, Dojani S, Friedl T, Mohr K, et al. Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb Ecol. 2009;57:229–247. doi: 10.1007/s00248-008-9449-9. [DOI] [PubMed] [Google Scholar]
- Chen L, Xie Z, Hu C, Li D, Wang G, Liu Y. Man-made desert algal crusts as affected by environmental factors in Inner Mongolia, China. J Arid Environ. 2006;67:521–527. [Google Scholar]
- Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmire R. Canopy coverage method of vegetation analysis. Northwest Sci. 1959;33:43–64. [Google Scholar]
- Dunbar JM, Kuske CR. Official Gazette of the United States Patent and Trademark Office Patents. The Regents of the University of California: Santa Fe, NM, USA; 2006. [Google Scholar]
- Dyszynski G, Sheldon WM.2007. A Java program from the Sapelo Program Microbial Observatory for automatic classification of bacteral 16S rRNA sequences based on Ribosomal Database Project taxonomy and Smith-Waterman alignment ( ( http://simo.marsci.uga.edu/public_dp/rdp_query.htm ).
- Eldridge DJ, Ferris JM. Recovery of populations of the soil lichen Psora Crenata after disturbance in arid South Australia. Rangeland J. 1999;21:194–198. [Google Scholar]
- Eldridge DJ, Freudenberger D, Koen KB. Diversity and abundance of biological soil crust taxa in relation to fine and coarse-scale disturbances in a grassy eucalypt woodland in eastern Australia. Plant Soil. 2006;281:255–268. [Google Scholar]
- Franklin RB, Mills AL. Importance of spatially structured environmental heterogeneity in controlling microbial community composition at small spatial scales in an agricultural field. Soil Biol Biochem. 2009;41:1833–1840. [Google Scholar]
- Freeman KR, Pescador MY, Reed SC, Costello EK, Robeson MS, Schmidt SK. Soil CO2 flux and photoautotrophic community composition in high-elevation, ‘barren' soil. Environ Microbiol. 2009;11:674–686. doi: 10.1111/j.1462-2920.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Pichel F, Belnap J, Neuer S, Schanz F. Estimates of global cyanobacterial biomass and its distribution. Arch Hydrobiol. 2003;109:213–228. [Google Scholar]
- Gundlapally S, Garcia-Pichel F. The community and phylogenetic diversity of biological soil crusts in the Colorado Plateau studied by molecular fingerprinting and intensive cultivation. Microb Ecol. 2006;52:345–357. doi: 10.1007/s00248-006-9011-6. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis programs for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Holmes AJ, Bowyer J, Holley MP, O'Donoghue M, Montgomery M, Gillings MR. Diverse, yet-to-be-cultured members of the Rubrobacter subdivision of the Actinobacteria are widespread in Australian arid soils. FEMS Microbiol Ecol. 2000;33:111–120. doi: 10.1111/j.1574-6941.2000.tb00733.x. [DOI] [PubMed] [Google Scholar]
- Huber T, Faulkner G, Hugenholtz P. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics. 2004;20:2317–2319. doi: 10.1093/bioinformatics/bth226. [DOI] [PubMed] [Google Scholar]
- Kirchman DL. The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol Ecol. 2002;39:91–100. doi: 10.1111/j.1574-6941.2002.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Kuske CR, Banton KL, Adorada DL, Stark PC, Hill KK, Jackson PJ. Small-scale DNA sample preparation method for field PCR detection of microbial cells and spores in soil. Appl Environ Microb. 1998;64:2463–2472. doi: 10.1128/aem.64.7.2463-2472.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuske CR, Barns SM, Grow CC, Merrill L, Dunbar J. Environmental survey for four pathogenic bacteria and closely related species using phylogenetic and functional genes. J Forensic Sci. 2006;51:548–558. doi: 10.1111/j.1556-4029.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- Kuske CR, Ticknor LO, Miller ME, Dunbar JM, Davis JA, Barns SM, et al. Comparison of soil bacterial communities in rhizospheres of three plant species and the interspaces in an arid grassland. Appl Environ Microbiol. 2002;68:1854–1863. doi: 10.1128/AEM.68.4.1854-1863.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DS.199016S and 23S rRNA SequencingIn: Stackebrandt E, Goodfellow M (eds).Nucleic acid techniques in bacterial systematics John Wiley: New York, NY; 115–148. [Google Scholar]
- Ley RE, Harris JK, Wilcox J, Spear JR, Miller SR, Bebout BM, et al. Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Appl Environ Microbiol. 2006;72:3685–3695. doi: 10.1128/AEM.72.5.3685-3695.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazor G, Kidron GJ, Vonshak A, Abeliovich A. The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiol Ecol. 1996;21:121–130. [Google Scholar]
- Mummey D, Holben W, Six J, Stahl P. Spatial stratification of soil bacterial populations in aggregates of diverse soils. Microb Ecol. 2006;51:404–411. doi: 10.1007/s00248-006-9020-5. [DOI] [PubMed] [Google Scholar]
- Mummey DL, Stahl PD. Analysis of soil whole- and inner-microaggregate bacterial communities. Microb Ecol. 2004;48:41–50. doi: 10.1007/s00248-003-1000-4. [DOI] [PubMed] [Google Scholar]
- Pellerin A, Lacelle D, Fortin D, Clark ID, Lauriol B. Microbial diversity in endostromatolites (cf. fissure calcretes) and in the surrounding permafrost landscape, Haughton Impact Structure Region, Devon Island, Canada. Astrobiology. 2009;9:807–822. doi: 10.1089/ast.2008.0302. [DOI] [PubMed] [Google Scholar]
- Rainey FA, Ray K, Ferreira M, Gatz BZ, Nobre MF, Bagaley D, et al. Extensive diversity of ionizing-radiation-resistant bacteria recovered from Sonoran Desert soil and description of nine new species of the genus Deinococcus obtained from a single soil sample. Appl Environ Microbiol. 2005;71:5225–5235. doi: 10.1128/AEM.71.9.5225-5235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GSN, Garcia-Pichel F. Sphingomonas mucosissima sp. nov. and Sphingomonas desiccabilis sp. nov., from biological soil crusts in the Colorado Plateau, USA. Int J Syst Evol Microbiol. 2007;57:1028–1034. doi: 10.1099/ijs.0.64331-0. [DOI] [PubMed] [Google Scholar]
- Redfield E, Barns SM, Belnap J, Daane LL, Kuske CR. Comparative diversity and composition of cyanobacteria in three predominant soil crusts of the Colorado Plateau. FEMS Microbiol Ecol. 2002;40:55–63. doi: 10.1111/j.1574-6941.2002.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Soule T, Anderson IJ, Johnson SL, Bates ST, Garcia-Pichel F. Archaeal populations in biological soil crusts from arid lands in North America. Soil Biol Biochem. 2009;41:2069–2074. [Google Scholar]
- Su YZ, Li YL, Zhao HL. Soil properties and their spatial pattern in a degraded sandy grassland under post-grazing restoration, Inner Mongolia, northern China. Biogeochemistry. 2006;79:297–314. [Google Scholar]
- Wang W, Liu Y, Li D, Hu C, Rao B. Feasibility of cyanobacterial inoculation for biological soil crusts formation in desert area. Soil Biol Biochem. 2009;41:926–929. [Google Scholar]
- Warren SD.2001Synopsis: influence of biological soil crusts on arid land hydrology and soil stabilityIn: Belnap J, Lange OL (eds).Biological Soil Crusts: Structure, Function and Management Springer-Verlag: Berlin; 349–360. [Google Scholar]
- Wu N, Zhang YM, Pan HX, Zhang J. The role of nonphotosynthetic microbes in the recovery of biological soil crusts in the Gurbantunggut Desert, Northwestern China. Arid Land Res Manag. 2010;24:42–56. [Google Scholar]
- Yeager CM, Kornosky JL, Morgan RE, Cain EC, Garcia-Pichel F, Housman DC, et al. Three distinct clades of cultured heterocystous cyanobacteria constitute the dominant N2-fixing members of biological soil crusts of the Colorado Plateau, USA. FEMS Microbiol Ecol. 2007;60:85–97. doi: 10.1111/j.1574-6941.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- Zaady E, Ben-David EA, Sher Y, Tzirkin R, Nejidat A. Inferring biological soil crust successional stage using combined PLFA, DGGE, physical and biophysiological analyses. Soil Biol Biochem. 2010;42:842–849. [Google Scholar]
- Zheng Y, Xu M, Zhao J, Bei S, Hao L. Effects of inoculated Microcoleus vaginatus on the structure and function of biological soil crusts of desert. Biol Fert Soils. 2011;47:473–480. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.