Abstract
The inability to associate with local species may constrain the spread of mutualists arriving to new habitats, but the fates of introduced, microbial mutualists are largely unknown. The deadly poisonous ectomycorrhizal fungus Amanita phalloides (the death cap) is native to Europe and introduced to the East and West Coasts of North America. By cataloging host associations across the two continents, we record dramatic changes in specificity among the three ranges. On the East Coast, where the fungus is restricted in its distribution, it associates almost exclusively with pines, which are rarely hosts of A. phalloides in its native range. In California, where the fungus is widespread and locally abundant, it associates almost exclusively with oaks, mirroring the host associations observed in Europe. The most common host of the death cap in California is the endemic coast live oak (Quercus agrifolia), and the current distribution of A. phalloides appears constrained within the distribution of Q. agrifolia. In California, host shifts to native plants are also associated with a near doubling in the resources allocated to sexual reproduction and a prolonged fruiting period; mushrooms are twice as large as they are elsewhere and mushrooms are found throughout the year. Host and niche shifts are likely to shape the continuing range expansion of A. phalloides and other ectomycorrhizal fungi introduced across the world.
Keywords: Amanita phalloides, ectomycorrhizal fungus, host shifts, microbial mutualism, range expansion, stable isotope
Introduction
As species experience range expansions, individuals encounter novel antagonists and mutualists (Richardson et al., 2000; Torchin and Mitchell, 2004), and changes in the frequency, diversity and function of interactions may influence the persistence of populations in novel ranges (Mitchell et al., 2006). The loss of predators or pathogens may facilitate the spread of a species (Torchin and Mitchell, 2004; Colautti et al., 2004), whereas loss of compatible mutualists may constrain spread (Richardson et al., 2000; Pringle et al., 2009a, 2009b).
Symbionts introduced to novel ranges may bypass the constraints imposed by the lack of hosts from a native range by forming associations with novel hosts (Richardson et al., 2000; Pringle et al., 2009a, 2009b). We define these novel associations as host shifts; a symbiont in a new range forms a functional symbiosis with a species it never encountered in its original range. A vast literature on microbial pathogens suggests that host shifts are common during range expansions (Anderson et al., 2004; Woolhouse et al., 2005; Slippers et al., 2005; Stukenbrock and McDonald, 2008). In contrast to pathogens, very little is know about the potential for microbial mutualists to shift hosts as they spread, even though they are commonly introduced to new ranges (van der Putten et al., 2007; Vellinga et al., 2009). Host shifts and range expansions may be widespread, but go unnoticed because most microbial mutualists are difficult to observe and the effects of mutualists on hosts are less apparent than pathogens (Litchman, 2010).
The extent to which a mutualist will shift to novel hosts and expand its range after introduction to a novel habitat depends on (1) the level of specificity evolved between the mutualist and its hosts in native ranges and (2) the distribution of compatible hosts within novel ranges (Vellinga et al., 2009). If a mutualist is a generalist in its native range, it may shift to a diversity of novel species in the introduced range. If compatible hosts are widespread, the mutualist may also become widespread. If the mutualist is specific to a few hosts in its native range, it may either fail to establish or be restricted within the geographic distribution of a limited number of compatible hosts. There is some evidence to support the hypothesis that species with the ability to establish and rapidly spread in new ranges are generalists (Vázquez, 2005; Vellinga et al., 2009), but tests of the role of specificity in determining the outcomes of range expansions are rare. Moreover, it is unclear how patterns of specificity will unfold across geographically disjunct range expansions, where the availability of hosts and diversity of endemic mutualist communities may differ.
Host shifts during range expansions may also cause changes in the timing, abundance and composition of resources exchanged between host and symbiont. Changes in the resources provided by hosts to obligate symbionts may affect the fitness and persistence of symbiont populations (Kiers et al., 2003, Kuikka et al., 2003, Markkola et al., 2004, Oono et al., 2009). Data on these phenomena are scarce, and as far as we are aware, niche shifts associated with the range expansions of microbial mutualists, and the functional implications of these niche shifts for the growth of the mutualist, have not been documented in the literature.
To explicitly test for a role of host specificity in constraining or facilitating the range expansion of a microbial mutualist, we focus on an ectomycorrhizal (EM) fungal species, Amanita phalloides. EM fungi form symbiotic associations with the roots of woody plant species, providing soil nutrients in exchange for carbon from host plants. EM fungi generally function as mutualists, but may occasionally function as parasites (Karst et al., 2008). Associations of EM fungi with hosts can be described using two different metrics at regional and local scales: (1) host specificity describes the richness of all potential hosts across a geographic range of a species of EM fungus (‘host range' in Molina et al., 1992), whereas (2) host selectivity describes the frequency with which an EM fungal species associates with a particular host plant (or plants) in a specific local environment (similar to ‘ecological specificity' in Molina et al., 1992). A given species of EM fungus might have low host specificity (and be a generalist) across the breadth of its distribution, but in a particular forest might be highly selective and associate with only a few of the available host species, either because of abiotic or biotic controls. We are currently unaware of other studies that have clearly documented geographic variation in host selectivity of an EM fungal species, but this phenomenon has been observed in the lichen symbiosis (for example, Yahr et al., 2006) as well as with antagonistic interactions between plants and insects (Fox and Morrow, 1981).
A. phalloides is an EM fungus native to Europe and introduced to North America (Pringle and Vellinga 2006; Pringle et al., 2009a, 2009b). This mushroom-forming basidiomycete is deadly poisonous, and since its introduction to North America, it has been responsible for numerous poisonings including several fatalities (Beug et al., 2006). It has established on both the East and West Coasts of North America, with different patterns of distribution and abundance within each of these ranges (Wolfe et al., 2010). On the West Coast, A. phalloides is widespread in California, and is less frequently found in disturbed habitats throughout the Pacific Northwest (Oregon, Washington and British Columbia). On the East Coast, A. phalloides is less abundant and is generally restricted to forestry plantations and trees planted in disturbed habitats. Anecdotal reports suggest that in Europe A. phalloides most frequently associates with Quercus spp. or other species of the Fagaceae, but also infrequently associates with Pinaceae (Courtecuisse and Duhem, 1994). However, to date, there have been no systematic surveys of associations between A. phalloides and its hosts in either Europe or North America.
To understand whether changes in host specificity or host selectivity are associated with the range expansions of A. phalloides, we first compared the host associations of the fungus in its native and introduced ranges. We then measured host selectivity within individual forests, and tested whether host selectivity will constrain the spread of A. phalloides in California. We also asked whether shifts to novel hosts cause changes in autecology or niche, as measured by stable isotopes of carbon and nitrogen, reproductive output and phenology.
In the aggregate, our data document a mutualism in flux; the different approaches to probing specificity, selectivity and changes in autecology provide a synergistic portrait of a species reaching new ranges, selectively associating with endemic plants and behaving differently in association with its novel hosts. Moreover, and to the best of our knowledge, our study provides the first data on the potential role of hosts in shaping the outcomes of invasions by microbial mutualists at an intercontinental scale.
Materials and methods
Host specificity across Europe and North America
To document the host associations of A. phalloides in its native and introduced ranges, we used data collected from herbarium specimens (positively identified as A. phalloides) or collection reports that clearly noted which species or genus of tree was found in association with an A. phalloides mushroom (Supplementary Table S1). Most data are from specimens collected or observed within the past 20 years, and were taken from sources that covered the entire range of each of the three ranges included in this study (Europe, East Coast of North America and the West Coast of North America). If an individual data source from a particular region had a very large number of records over the past 20 years (for example, the Fungal Records Database of Britain and Ireland), we only used the past 5 years worth of available data to avoid including a disproportionate number of records from any country or region.
Because of the large geographic scale of this study, herbarium records are the only available proxy for host associations. Herbarium records have limitations for determining host associations. Hosts of EM fungi are usually identified by noting the species of tree closest to where the mushroom was collected. The hosts that are apparent to a collector of a mushroom might not be the hosts forming EM fungal root tips belowground. Quantitative estimates of potential discrepancies between perceived aboveground host associations and belowground host associations are lacking. However, any bias or limitation in herbarium records should be similar across the multiple biogeographic regions that we target; differences among biogeographic regions are likely to reflect differences in biology.
We used χ2-tests to test for significant differences in the frequency of host genera among three major regions: Europe, the East Coast of North America and the West Coast of North America. We also calculated generic richness and Shannon's diversity index for host genera of the three regions, to make qualitative comparisons of host composition. We focused on generic diversity because many host identifications were only made to the level of genus. We then quantified the number of host shifts experienced by A. phalloides by determining the number of endemic North American plant species found to associate with the fungus. In this analysis, we restricted our data set to only those records where hosts were identified to species.
Stable isotope measurements of niche shifts
Associations between host shifts and changes in the nutritional dynamics of A. phalloides were determined by quantifying the stable isotope composition of nitrogen and carbon in mushroom tissue across each of the ranges of A. phalloides (Supplementary Table S2). We specifically measured these isotopes because changes in nitrogen and carbon within mushroom tissues can reflect differences in host associations (Högberg et al., 1999; Taylor et al., 2003), although other variables can also influence these kinds of data (see Discussion). Homogenized samples of dried gill tissue were analyzed for carbon and nitrogen composition using standard protocols (Hobbie et al., 2001; see Supplementary Methods). To determine the statistical significance of differences in stable isotope data from the three regions (Europe, East Coast and California), we used Kruskal–Wallis nonparametric tests. For post hoc comparisons, we used Mann-Whitney U-tests. A Bonferroni correction was used to correct for multiple comparisons.
Mushroom biomass and phenology
We obtained mushroom biomass and phenology data from the same set of mushrooms that we used to infer host associations. Phenology data were supplemented with additional records from Mushroom Observer (http://www.mushroomobserver.org), additional herbarium specimens that did not contain host information, and our own observations in the field. We only used Mushroom Observer records where it was clear from photographic evidence that the mushroom collected was A. phalloides.
To obtain biomass data, individual mushrooms that were fully intact and fully mature (each cap was completely open, with all gills fully exposed) were weighed after being dried for 24 h at 37 ° C. Here, we define a population as a set of mushrooms occurring within a forest stand, not larger than 50 m × 50 m that are clearly clustered together and separated from another set of mushrooms by at least 1 km. Individual genets of A. phalloides are small (generally less than 0.5 m), and often individual mushrooms collected near to one another are unique genetic individuals (Pringle, unpublished data). For each population, we calculated mean biomass per mushroom, and used these population-level data for our statistical analyses. Phenology data were collected by determining the time of collection of a mushroom for a unique population. The date collected was converted to day of year (1 to 365) to standardize collection dates across years. To test for statistical significance of differences in mushroom biomass and phenology between the four biogeographic regions (Europe, East Coast, California and the Pacific Northwest), we used the same statistical analysis used with the stable isotope data.
To confirm that mushroom biomass is correlated with reproductive output in A. phalloides, we assessed the correlation between total mushroom biomass and potential reproductive output (biomass of spore producing tissue) using 34 mushrooms from across three populations within Point Reyes National Seashore (Heart's Desire, Drake's Landing and Horse's Trail). We dried the gill tissue (spore bearing structures) separately from the pileus, stipe, bulb and annulus tissue. We specifically collected mature mushrooms of different sizes so that we could capture the range of mushroom biomass variation.
Host selectivity within Californian forests
To assess host selectivity at a local scale within mixed forests, we sampled EM root tips from under A. phalloides mushrooms at three sites on the Point Reyes Peninsula. The three sites, Heart's Desire, Drake's Landing and Horse's Trail, are separated from each other by at least 2.5 km. Each site has a different composition of EM hosts, as described in Supplementary Material.
EM root tips were extracted from soil samples taken directly under mushrooms of A. phalloides at all three sites and also from randomly sampled soil cores at Heart's Desire and Drake's Landing (as part of another study within these same plots; Wolfe et al., 2010). We also collected aboveground tissues (leaves/needles) of potential EM hosts at each site. The host tissue present in each root tip colonized by A. phalloides was identified by comparing root tissue DNA sequences with aboveground tissue sequences (see Supplementary Methods).
Previous work from similar forests (Kennedy et al., 2003) and our own preliminary observations suggested that conifer roots are abundant in soil cores collected at these sites, whereas angiosperm tree roots are less frequently encountered. We confirmed this observation by surveying the abundance of tree roots in the three plots where we also sampled root tips (see Supplementary Methods). The total number of roots that were successfully amplified by PCR at each site varied: Heart's Desire=30, Drake's Landing=25 and Horse's Trail=29.
To test for host selectivity of A. phalloides at these three sites, we used a statistical approach described by Gilbert et al. (2008). We calculated the binomial probabilities of finding equivalent or greater numbers of root tips of A. phalloides associated with a particular host given the available abundance of host roots. We focused our statistical analyses on Quercus agrifolia, because mushroom observations suggested that it was the most frequent host of A. phalloides at Point Reyes (see results below). Binomial probabilities were calculated in JMP version 5.0.1a (SAS Institute Inc., Cary, NC, USA).
Testing for constraints on the distribution of A. phalloides in California
To determine whether the distribution of A. phalloides in California is constrained within the geographic ranges of its available hosts, we used a Monte Carlo randomization approach modified from Brown et al. (2009). We used a subset of the A. phalloides occurrence data reported in Wolfe et al. (2010); this subset comprised 75 recent, confirmed occurrences of A. phalloides associating with native hosts throughout California. Host distributions were obtained from United States Geological Survey (2006). We assessed overlap between A. phalloides and those species of trees that we determined to be hosts of A. phalloides in California (see Results); these hosts included Q. agrifolia, Q. kelloggii, Q. wislizenii, Lithocarpus densiflorus, Corylus cornuta var. californica, Pseudotsuga menziesii and Pinus muricata. We first determined the number of occurrences of A. phalloides inside the range of each host species, from the 75 total occurrences. To create a null distribution, we randomly chose 75 samples without replacement from a pool of 500 random points located throughout the range of A. phalloides in California, delineated by a minimum convex polygon around observed occurrence data. For these 75 random samples, we next determined whether they occurred within the range of the particular host species. The Monte Carlo randomization tested whether the probability of A. phalloides occurring within a potential host range was significantly greater than the probability of randomly placed sampling points across the current range of A. phalloides. The Monte Carlo simulation was run with 1000 replicates for each host species.
Results
Host specificity of A. phalloides is geographically structured
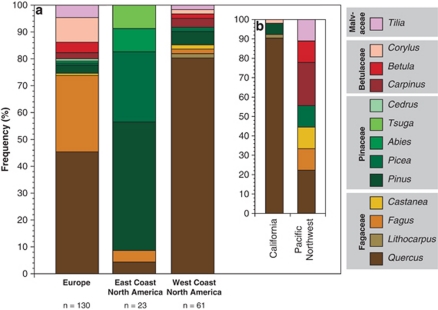
Surveys of host associations among more than 200 populations of A. phalloides across native and introduced ranges show that A. phalloides is not a generalist, and instead exhibits geographically structured host specificity (Figure 1). In Europe, A. phalloides associates most frequently with oaks and other species of the Fagaceae. On the East Coast of North America, where A. phalloides is restricted in its distribution as is rarely found in native forests, it associates most frequently with pines, which are rarely hosts of the fungus in Europe. In contrast, on the West Coast of North America, where A. phalloides is widespread and commonly found in native forests, host associations are more similar to the host associations documented for European populations, with oaks as the most frequent hosts. In California, A. phalloides associates almost exclusively with the endemic coast live oak (Q. agrifolia); 81% of all California records identified to species list Q. agrifolia as the host (Supplementary Table S1).
Figure 1.
(a) Frequency of host associations across the native (European) and introduced (North American) ranges of A. phalloides. (b) West Coast data are divided into California and the Pacific Northwest to show that in California the dominant hosts are Quercus spp., whereas in the Pacific Northwest dominant hosts are from a mix of introduced angiosperm genera (see text for details). n=number of populations from each region.
The frequencies of associations between A. phalloides and its four most commonly encountered host genera (Quercus, Pinus, Fagus and Corylus) differ significantly across Europe and the East and West Coasts of North America (χ2=115.31, P<0.001), and in North America the fungus has shifted to associate with 11 novel hosts. A novel host is defined as a plant endemic to North America, for example, eastern white pine (P. strobus) and California's hazel (C. cornuta ssp. californica). The richness and diversity of host genera is lower in A. phalloides' introduced ranges, as compared with Europe (Supplementary Table S3), although the Pacific Northwest is an exception. In this region, A. phalloides associates with a diversity of nonnative angiosperm species that have been planted in urban parks and yards, for example, a filbert native to Europe (C. maxima; Figure 1, Supplementary Table S1).
Changes in resource use, reproduction, and phenology
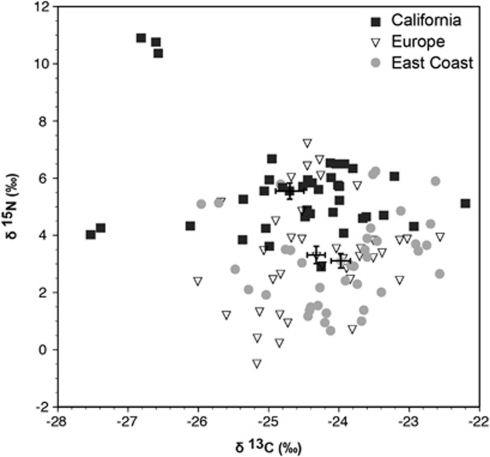
Stable isotopes of carbon and nitrogen from mushrooms collected across the native and introduced ranges correlate host shifts with changes in resource use. On the West Coast, we restricted sampling to California, because A. phalloides is widespread in California and because this state houses the greatest density of North American populations (Wolfe et al., 2010). Stable isotope signatures differed significantly among the three biogeographic regions for both δ13C ‰ (P=0.016) and δ15N ‰ (P<0.001; Figure 2). Mushrooms from California were more variable for both isotopes, with some mushrooms falling well outside of the range of δ13C and δ15N observed for both Europe and the East Coast. East Coast mushrooms were significantly less depleted in 13C compared with mushrooms from Europe (Mann–Whitney U test, P=0.049) and California (P=0.0061). The mean δ13C value of mushrooms from California was more negative than European mushrooms, but this difference was not significant (P=0.3632). Californian mushrooms were significantly enriched in 15N compared with both East Coast (P <0.001) and European (P<0.001) mushrooms. There was no difference in δ15N between East Coast and European mushrooms (P =0.5353).
Figure 2.
δ13C ‰ and δ15N ‰ of mushrooms collected in Europe, the East Coast of North America and California. Each data point represents a single mushroom. Values with standard error bars represent mean values for all mushrooms from each region (±1 s.e.).
A total of 5 mushrooms from California, including the only 3 mushrooms sampled from one site (Albion) and 2 of 14 mushrooms sampled from sites in Tomales Bay State Park, had stable isotope values that were well outside the range of all other observed values (Figure 2). We removed these samples from our data set and repeated statistical analyses. The differences in δ13C were no longer statistically significant (P=0.085), but differences in δ15N were still significant (P<0.001). Interestingly, the three sporocarps from Albion, among the most depleted in 13C and the most enriched in 15N, were the only mushroom collections associated with L. densiflorus.
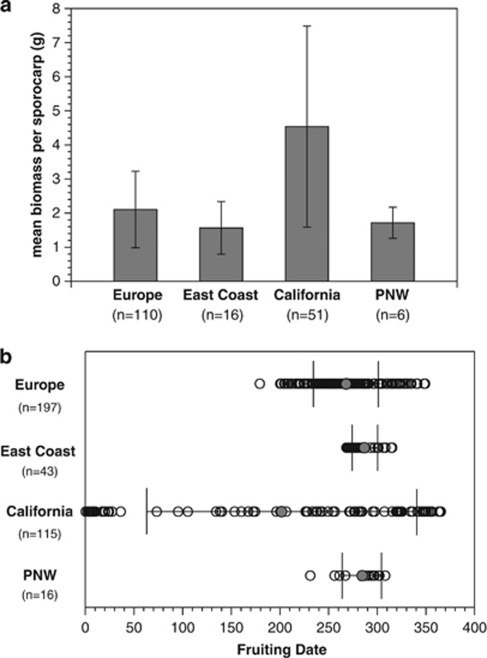
Changes in host associations are also associated with changes in reproductive allocation and phenology. In California, mushrooms are almost twice as large as they are anywhere else (P<0.001, Figure 3a). The timing of reproduction has also changed in California (P<0.001), and mushrooms appear throughout the year. In contrast, mushrooms appear only in late summer or fall in Europe, the East Coast and Pacific Northwest (Figure 3b).
Figure 3.
(a) Mean mushroom biomass and (b) timing of reproduction of A. phalloides across four biogeographic regions; n=number of populations from each region. Error bars represent ±1 s.d. from the mean. In b, individual gray circles represent the mean value. Sampling intensity varies across different regions, but is proportional to the number of known populations in each region (Wolfe et al., 2010). PNW, Pacific Northwest region of North America. Fruiting date indicates the day of the year (1 to 365).
A. phalloides selectively associates with coast live oak in Californian forests
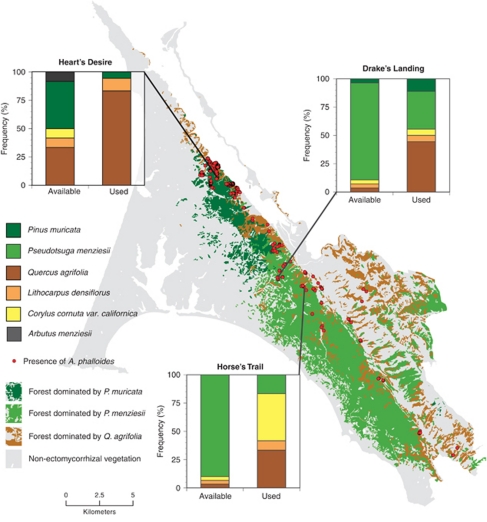
Molecular techniques used to identify the plant in EM root tips colonized by A. phalloides confirm that A. phalloides selectively associates with a subset of potential hosts within mixed forest communities. Root tips are the physical site of a symbiosis between an EM fungus and host. Across three sites within the Point Reyes Peninsula (Marin County, CA, USA), A. phalloides selectively associated with Q. agrifolia, even though the available root community was dominated by conifers, particularly P. muricata and P. menziesii (Figure 4). Q. agrifolia was identified as the host in 56% of the 48 EM root tip samples colonized by A. phalloides. In contrast, only 9% of the 70 available roots sampled across the three sites were Quercus roots; P. muricata and P. menziesii made up 88% of the rest. The binomial probabilities of finding these observed or greater numbers of A. phalloides root tips associated with Q. agrifolia, given the frequency of Q. agrifolia roots, were very low at each site (Heart's Desire: P<0.001; Drake's Landing: P<0.001; and Horse's Trail: P<0.001), confirming that A. phalloides shows selectivity for Q. agrifolia.
Figure 4.
Host selectivity of A. phalloides at three sites on the Point Reyes Peninsula in Marin County, California, USA. Map shows peninsula shaded with distributions of the dominant EM host species (Q. agrifolia, P. muricata and Pseudotsuga menziesii). Graphs show the available root community and the percent of the total number of A. phalloides root tips where each species was detected as a host.
A. phalloides appears constrained within the range of coast live oak in California
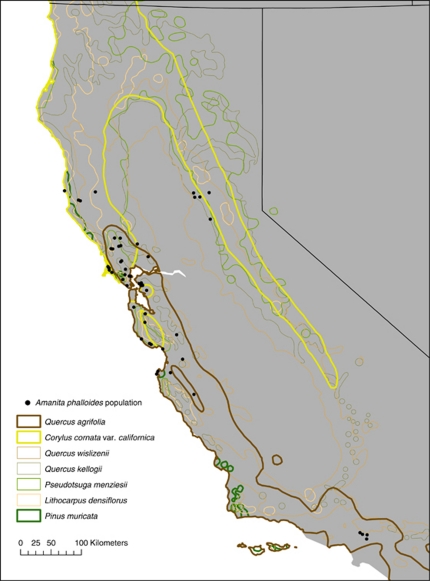
The coast live oak is the dominant host of A. phalloides across California, and selectivity may constrain the death cap to spread within and track the range of Q. agrifolia. To determine whether the distribution of A. phalloides in California is constrained within the geographic ranges of its available hosts, we used a Monte Carlo randomization approach to test whether the probability of A. phalloides occurring within a potential host range was significantly greater than the probability of randomly placed sampling points across the current range of A. phalloides. The distribution of A. phalloides in California was most significantly associated with the distribution of Q. agrifolia (Figure 5, Supplementary Table S4), and 68% of A. phalloides occurrences are recorded within the geographic range of Q. agrifolia (P<0.001). Significant associations were also found for C. cornuta var. californica and P. muricata, but not for the other Quercus species that are hosts of A. phalloides in California. The statistical significance of the overlap between A. phalloides and P. muricata may be an artifact caused by the highly restricted and spatially aggregated range of P. muricata and the high density of A. phalloides in one small part of the range of P. muricata (the Point Reyes Peninsula). In fact, at Point Reyes, A. phalloides actually occurs most frequently in association with Q. agrifolia, as described above. Moreover, only 9% of A. phalloides records occurred within the range of P. muricata.
Figure 5.
Distribution of A. phalloides throughout California and the geographic ranges of associated host plants. A significant association between the distribution of A. phalloides and a host range is marked by the thicker borders around Q. agrifolia, C. cornuta var. californica, and P. muricata. The distribution of A. phalloides is largely within the distribution of its most frequent host, Q. agrifolia. See Supplementary Table S4 for statistics.
Discussion
Host specificity and selectivity of A. phalloides in North America
Shifts by introduced microbial mutualists to endemic hosts have rarely been demonstrated. Introduced microbial mutualists may become abundant in novel ranges, but they generally persist with their introduced hosts and rarely establish functional symbioses with novel hosts (Diez, 2005; Vellinga et al., 2009; Dickie et al., 2010; Jairus et al. 2011). Our work with A. phalloides provides a sharp contrast to these previous studies and demonstrates that this European fungal symbiont can shift to associate with multiple hosts that are endemic to North America. Host shifts are not uniform across ranges, causing a strong geographic structure in patterns of host specificity across North America. On the East Coast, the species associates almost exclusively with conifers, although these are rarely hosts of A. phalloides in its native range. On the West Coast, in California, A. phalloides associates most frequently with oaks, similar to the pattern of host associations observed in its native range. In the Pacific Northwest, A. phalloides is primarily associated with European hosts planted in parks and yards, and is not yet known to associate with native hosts.
Both the evolutionary history of interactions between A. phalloides and its hosts in the native range, and the distribution of compatible hosts in novel ranges, may explain the geographically structured host specificity that we observed in North America. Different symbiont lineages may adapt to specialize on subsets of hosts (Thompson, 1994). Cryptic genetic species specific to particular hosts have been observed within other morphological species of fungi (Sato et al., 2007). However, genetic surveys of A. phalloides in both Europe and North America provide no evidence of cryptic species within A. phalloides (Pringle et al., 2009a). Nonetheless, on the West Coast, A. phalloides may have been introduced with cork oak imported from Europe (Saylor, 1984), and on the East Coast, A. phalloides may have been introduced on European conifer seedlings (Tanghe and Simons, 1973; Tanghe, 1983). If these different introductions included different genotypes of A. phalloides adapted to either oaks or conifers, observed host specificities could be the result of different genetic bottlenecks. Although this hypothesis remains untested, ongoing sequencing of Amanita genomes may soon provide the tools needed to identify the genetic basis of specificity.
The availability and geographic distribution of compatible species in the Fagaceae, the most frequent hosts of A. phalloides in Europe, could also have a role in creating geographically structured host specificity. If A. phalloides were specific to a particular phylogenetic lineage within the Fagaceae, and if these lineages were more common on one coast of North America, then observed patterns of host specificity would be the result of differences in the availability of compatible hosts. However, in Europe, A. phalloides most commonly associates with Q. robur, which is closely related to both the most common oak in the Northeast of North America, Q. rubra, rarely a host of A. phalloides, and to Q. agrifolia, the most common host of A. phalloides on the West Coast (Manos et al., 2001). Moreover, other North American species of Fagaceae and Betulaceae, including Fagus grandifolia, Corylus americana and Betula lenta, are closely related to European species of Fagaceae and Betulaceae and are often found within forests on the East Coast where A. phalloides is found. Yet, we rarely observe A. phalloides associating with these hosts on the East Coast.
Differences in the diversity of available and colonized roots clearly demonstrate that in the mixed forests of Point Reyes A. phalloides is selecting Q. agrifolia as a primary host. The most abundant roots available for colonization at two of the three sites were P. menziesii, but at both sites A. phalloides most frequently associated with Q. agrifolia. Ecological processes are likely to influence the host associations of A. phalloides at these local scales, and competition for the resources provided by a single host may be an especially potent force when multiple symbiont species associate with the host (Saari et al., 2005; Morris et al., 2008). A symbiont may be able to form associations with a wide range of species, but only colonize a host at a particular site when it can establish and persist in the face of competition from other symbionts. Data from the West Coast suggest that EM fungal communities around plants of the Fagaceae are lower in species richness as compared with co-occurring Pinaceae (Massicotte et al., 1999; Kennedy et al., 2003; but see Smith et al., 2009) and these data provide tentative support for our hypothesis.
Changes in niche and reproductive output of A. phalloides in California
Changes in the stable isotope signatures of A. phalloides mushrooms collected from Europe and the East and West Coasts of North America suggest different dynamics of nutrient acquisition and loss across the different ranges; this is the first intercontinental comparison of isotopes taken from a mycorrhizal fungus. The carbon used by EM fungi to build biomass is taken directly from associated plants (Smith and Read, 1997) and the δ13C of mushrooms should reflect the δ13C of host plants. The less depleted δ13C of the East Coast mushrooms compared with the Californian mushrooms provides independent support for a host shift to conifers, because evergreen conifers tend to be less depleted in 13C than evergreen and deciduous angiosperms (Diefendorf et al., 2010). Data of EM fungi within other forest ecosystems also document significant differences in stable isotope signatures based on host associations (Högberg et al., 1999; Taylor et al., 2003). The δ15N values of EM fungal mushrooms are influenced by the source of N used by the fungus, the dynamics of N transfer from the EM fungus to the host and internal physiological processes independent of N transfer to a host (Hobbie and Colpaert, 2003; Hobbie and Hobbie, 2006); current data can only confirm these processes as different in Californian populations of A. phalloides. However, changes in carbon and nitrogen isotope dynamics are correlated with A. phalloides' associations with Q. agrifolia and may suggest the mutualism of the fungus and this plant functions differently from the mutualisms of A. phalloides in Europe or the East Coast. Although stable isotopes of terrestrial biomes are also influenced by additional factors that vary between biogeographic regions, including differences in climate (Marshall et al., 2007), large-scale meta-analysis and modelling approaches show that broad patterns of the δ15N and δ13C of plants and soils are similar across the three regions that we compared (Amundson et al., 2003; Suits et al., 2005; Diefendorf et al., 2010).
Novel patterns of host use are associated with striking changes in the reproductive output of A. phalloides in California. The mushroom, or sporocarp, is the spore-bearing structure of a basidiomycete fungus. Mushroom size is highly correlated with investment in sexual reproduction in other mushroom-forming fungi (Guidot et al., 2002; Schmit, 2002) and in A. phalloides (Supplementary Figure 1). Mushrooms of Californian A. phalloides are often more than twice as large as European mushrooms. Because larger mushrooms produce more spores, the increase in mushroom biomass increases the potential for the dispersal of spores capable of establishing new populations. Moreover, mushrooms in California can appear at any time of year, whereas in the other ranges A. phalloides is restricted to fruiting during specific times of the year. Shifts in the quantity of carbon taken from a host can alter the production of mushrooms by EM fungi (Kuikka et al., 2003; Andrew and Lilleskov, 2009), and it is likely that changes in the dynamics of carbon exchange among A. phalloides and its Californian hosts are driving shifts in the reproductive output and phenology of this fungus. Shifts in the temporal allocation of carbon may also explain changes in reproductive output. The most frequent host in California, Q. agrifolia, is an evergreen oak. Because this host photosynthesizes throughout the entire year, opportunities to obtain carbon from this host extend throughout the year and may lead to increased opportunities for mushroom production by A. phalloides in California. Although the quantity and timing of carbon supply from hosts is likely the major limiting factor in the production of mushrooms by A. phalloides, we cannot rule out differences in abiotic conditions among these regions also having a role.
Constraints on spread of A. phalloides
Within California, the geographic distribution of a preferred host appears to shape range expansion of A. phalloides. The current distribution of the fungus in California is largely within the range of its most frequent host, Q. agrifolia, and A. phalloides selectively associates with this host in mixed forests. When populations of A. phalloides are found outside of the natural range of Q. agrifolia, they are often growing in association with planted Q. agrifolia trees used for landscaping (Wolfe, personal observation). On the East Coast, host specificity does not shape the distribution of A. phalloides. Although shifts to novel species of Pinaceae are common, the fungus has not spread within the distributions of these plants, and novel interactions are restricted to disturbed or managed habitats (Wolfe et al., 2010).
A comparison of the fungus in its three ranges challenges simple hypotheses linking host specificity or its lack with constraints on spread (Richardson et al., 2000; Vázquez, 2005; Pringle et al., 2009b). A catalog of the fungus's associations across the globe defines it as a generalist, but within individual ranges the fungus preferentially associates with subsets of plants. Our California data are a direct contrast to available evidence taken from pollination mutualisms (Vázquez, 2005), and suggest that specificity in local habitats can influence the success of introduced mutualist species, even when species otherwise appear as generalists. Moreover, the ability to shift hosts does not guarantee spread, and a generalist capable of associating with local symbionts may not invade across the ranges of these species. However, when an introduced mutualist does shift hosts and also spread, the range of the novel hosts will be a critical control on the spread of the mutualist.
Acknowledgments
Darvin Deshazer, Jason Hoeksema, Jason Hollinger, Joel Horman, Susan Hopkins, June Johnston, Marion Kyde, Jan Lindgren, Rich Moll, Dan Nicholson, Ron Pastorino, Bob Peabody, David Rust, Paul Sadowski, Douglas Smith, Walt Sturgeon, Steven Trudell, Rod Tulloss, Debbie Veiss, Else Vellinga, Peter Werner and Nathan Wilson provided collections or assistance with locating A. phalloides populations. Hope Jahren provided support with the stable isotope analysis. Matthew Smith and Noah Whiteman helped with earlier drafts of this manuscript. The study was funded by the New England Botanical Club, The Arnold Arboretum of Harvard University, the Mycological Society of America and the US National Science Foundation.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Amundson R, Austin AT, Schuur EAG, Yoo K, Matzek V, Kendall C, et al. Global patterns of the isotopic composition of soil and plant nitrogen. Global Biogeochem Cycles. 2003;17:1031. [Google Scholar]
- Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004;19:535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Andrew C, Lilleskov EA. Productivity and community structure of ectomycorrhizal fungal sporocarps under increased atmospheric CO2 and O3. Ecol Lett. 2009;12:813–822. doi: 10.1111/j.1461-0248.2009.01334.x. [DOI] [PubMed] [Google Scholar]
- Brown JL, Morales V, Summers K. Home range size and location in relation to reproductive resources in poison frogs (Dendrobatidae): a Monte Carlo approach using GIS data. Anim Behav. 2009;77:547–554. [Google Scholar]
- Beug MW, Shaw M, Cochran KW. Thirty-plus years of mushroom poisoning: summary of the approximately 2,000 reports in the NAMA Case registry. McIlvainea. 2006;16:47–68. [Google Scholar]
- Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis. Ecol Lett. 2004;7:721–733. [Google Scholar]
- Courtecuisse R, Duhem B. Guide des Champignons de France et d'Europe. Delachaux & Niestlé: Lausanne; 1994. [Google Scholar]
- Dickie IA, Bolstridge N, Cooper JA, Peltzer DA. Co-invasion by Pinus and its mycorrhizal fungi. New Phytol. 2010;187:475–484. doi: 10.1111/j.1469-8137.2010.03277.x. [DOI] [PubMed] [Google Scholar]
- Diefendorf AF, Mueller KE, Wing SL, Koch PL, Freeman KH. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc Natl Acad Sci USA. 2010;107:5738–5743. doi: 10.1073/pnas.0910513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez J. Invasion biology of Australian ectomycorrhizal fungi introduced with eucalypt plantations into the Iberian Peninsula. Biol Invasions. 2005;7:3–15. [Google Scholar]
- Fox LR, Morrow PA. Specialization: species property or local phenomenon. Science. 1981;211:887–893. doi: 10.1126/science.211.4485.887. [DOI] [PubMed] [Google Scholar]
- Gilbert GS, Gorospe J, Ryvarden L. Host and habitat preferences of polypore fungi in micronesian tropical flooded forests. Mycol Res. 2008;112:674–680. doi: 10.1016/j.mycres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Guidot A, Gryta H, Gourbiere F, Debaud JC, Marmeisse R. Forest habitat characteristics affect balance between sexual reproduction and clonal propagation of the ectomycorrhizal mushroom Hebeloma cylindrosporum. Oikos. 2002;99:25–36. [Google Scholar]
- Hobbie EA, Colpaert JV. Nitrogen availability and colonization by mycorrhizal fungi correlate with nitrogen isotope patterns in plants. New Phytol. 2003;157:115–126. doi: 10.1046/j.1469-8137.2003.00657.x. [DOI] [PubMed] [Google Scholar]
- Hobbie EA, Weber NS, Trappe JM. Mycorrhizal vs. saprotrophic status of fungi: the isotopic evidence. New Phytol. 2001;150:601–610. [Google Scholar]
- Hobbie JE, Hobbie EA. 15N in symbiotic fungi and plants estimates nitrogen and carbon flux rates in arctic tundra. Ecology. 2006;87:816–822. doi: 10.1890/0012-9658(2006)87[816:nisfap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Högberg P, Plamboeck AH, Taylor AFS, Fransson PMA. Natural 13C abundance reveals trophic status of fungi and host-origin of carbon in mycorrhizal fungi in mixed forests. Proc Natl Acad Sci USA. 1999;96:8534–8539. doi: 10.1073/pnas.96.15.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jairus T, Mpumba R, Chinoya S, Tedersoo L. Invasion potential and host shifts of Australian and African ectomycorrhizal fungi in mixed eucalypt plantations. New Phytol. 2011;192:179–187. doi: 10.1111/j.1469-8137.2011.03775.x. [DOI] [PubMed] [Google Scholar]
- Karst J, Marczak L, Jones MD, Turkington R. The mutualism-parasitism continuum in ectomycorrhizas: a quantitative assessment using meta-analysis. Ecology. 2008;89:1032–1042. doi: 10.1890/07-0823.1. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Izzo AD, Bruns TD. There is high potential for the formation of common mycorrhizal networks between understorey and canopy trees in a mixed evergreen forest. J Ecol. 2003;91:1071–1080. [Google Scholar]
- Kiers ET, Rousseau RA, West SA, Denison RF. Host sanctions and the legume-rhizobium mutualism. Nature. 2003;425:78–81. doi: 10.1038/nature01931. [DOI] [PubMed] [Google Scholar]
- Kuikka K, Härmä E, Markkola A, Rautio P, Roitto M, Saikkonen K, et al. Severe defoliation of Scots pine reduces reproductive investment by ectomycorrhizal symbionts. Ecology. 2003;84:2051–2061. [Google Scholar]
- Litchman E. Invisible invaders: non-pathogenic invasive microbes in aquatic and terrestrial ecosystems. Ecol Lett. 2010;13:1560–1572. doi: 10.1111/j.1461-0248.2010.01544.x. [DOI] [PubMed] [Google Scholar]
- Manos PS, Zhou ZK, Cannon CH. Systematics of Fagaceae: phylogenetic tests of reproductive trait evolution. Int J Plant Sci. 2001;162:1361–1379. [Google Scholar]
- Marshall JD, Brooks JR, Lajtha K.2007. Sources of variation in the stable isotopic composition of plants. In: Michener R, Lajtha K (eds), Stable Isotopes in Ecology and Environmental Science2nd edn. Blackwell Publishing: Malden, MA; 22–60. [Google Scholar]
- Markkola A, Kuikka K, Rautio P, Härmä E, Roitto M, Tuomi J. Defoliation increases carbon limitations in ectomycorrhizal symbiosis of Betula pubescens. Oecologia. 2004;140:234–240. doi: 10.1007/s00442-004-1587-2. [DOI] [PubMed] [Google Scholar]
- Massicotte HB, Molina R, Tackaberry LE, Smith JE, Amaranthus MP. Diversity and host specificity of ectomycorrhizal fungi retrieved from three adjacent forest sites by five host species. Can J Bot. 1999;77:1053–1076. [Google Scholar]
- Mitchell CE, Agrawak AA, Bever JD, Gilbert GS, Hufbauer RA, Klironomos JN, et al. Biotic interactions and plant invasions. Ecol Lett. 2006;9:726–740. doi: 10.1111/j.1461-0248.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- Molina R, Massicotte J, Trappe JM.1992. Specificity phenomena in mycorrhizal symbioses: community-ecological consequences and practical implications. In: Allen A (ed.), Mycorrhizal Functioning Chapman and Hall: New York; 357–420. [Google Scholar]
- Morris MH, Smith ME, Rizzo DM, Rejmanek M, Bledsoe CS. Contrasting ectomycorrhizal fungal communities on the roots of co-occurring oaks (Quercus spp.) in a California woodland. New Phytol. 2008;178:167–176. doi: 10.1111/j.1469-8137.2007.02348.x. [DOI] [PubMed] [Google Scholar]
- Oono R, Denison RF, Kiers ET. Controlling the reproductive fate of rhizobia: how universal are legume sanctions. New Phytol. 2009;183:967–979. doi: 10.1111/j.1469-8137.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- Pringle A, Vellinga EC. Last chance to know? Using literature to explore the biogeography and invasion biology of the death cap mushroom Amanita phalloides (vaill. ex fr.: Fr.) link. Biol Invasions. 2006;8:1131–1144. [Google Scholar]
- Pringle A, Adams RI, Cross HB, Bruns TD. The ectomycorrhizal fungus Amanita phalloides was introduced and is expanding its range on the west coast of North America. Mol Ecol. 2009a;18:817–833. doi: 10.1111/j.1365-294X.2008.04030.x. [DOI] [PubMed] [Google Scholar]
- Pringle A, Bever JD, Gardes M, Parrent JL, Rillig MC, Klironomos JN. Mycorrhizal symbioses and plant invasions. Ann Rev Ecol Evol Syst. 2009b;40:699–715. [Google Scholar]
- Richardson DM, Allsopp N, D'Antonio CM, Milton SJ, Rejmanek M. Plant invasions - the role of mutualisms. Biol Rev Camb Philos Soc. 2000;75:65–93. doi: 10.1017/s0006323199005435. [DOI] [PubMed] [Google Scholar]
- Sato H, Yumoto T, Murakami N. Cryptic species and host specificity in the ectomycorrhizal genus Strobilomyces (Strobilomycetaceae) Am J Bot. 2007;94:1630–1641. doi: 10.3732/ajb.94.10.1630. [DOI] [PubMed] [Google Scholar]
- Saylor HM. A. phalloides in California: this preliminary report suggests that it is a relative newcomer to the state. Mushroom Magazine. 1984;2:40–42. [Google Scholar]
- Saari SK, Campbell CD, Russell J, Alexander IJ, Anderson IC. Pine microsatellite markers allow roots and ectomycorrhizas to be linked to individual trees. New Phytol. 2005;165:295–304. doi: 10.1111/j.1469-8137.2004.01213.x. [DOI] [PubMed] [Google Scholar]
- Schmit JP. Tradeoffs between reproduction and mycelium production in the unit-restricted decomposer Coprinus cinereus. Mycologia. 2002;94:40–48. [PubMed] [Google Scholar]
- Slippers B, Stenlid J, Wingfield MJ. Emerging pathogens: fungal host jumps following anthropogenic introduction. Trends Ecol Evol. 2005;20:420–421. doi: 10.1016/j.tree.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Smith ME, Douhan GW, Fremier AK, Rizzo DM. Are true multihost fungi the exception or the rule? Dominant ectomycorrhizal fungi on Pinus sabiniana differ from those on co-occurring Quercus species. New Phytol. 2009;182:295–299. doi: 10.1111/j.1469-8137.2009.02801.x. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ.1997Mycorrhizal Symbiosis2nd edn. Academic Press: London [Google Scholar]
- Stukenbrock EH, McDonald BA. The origins of plant pathogens in agro-ecosystems. Annu Rev Phytopathol. 2008;46:75–100. doi: 10.1146/annurev.phyto.010708.154114. [DOI] [PubMed] [Google Scholar]
- Suits NS, Denning AS, Berry JA, Still CJ, Kaduk K, Miller JB, Baker IT. Simulation of carbon isotope discrimination of the terrestrial biosphere. Global Biogeochem Cycles. 2005;19:GB1017. [Google Scholar]
- Tanghe LJ. Spread of Amanita phalloides in North America. McIllvainea. 1983;6:4–8. [Google Scholar]
- Tanghe LJ, Simons DM. Amanita phalloides in the Eastern United States. Mycologia. 1973;65:99–108. [PubMed] [Google Scholar]
- Taylor AFS, Fransson PM, Högberg P, Högberg MN, Plamboeck AH. Species level patterns in 13C and 15N abundance of ectomycorrhizal and saprotrophic fungal sporocarps. New Phytol. 2003;159:757–774. doi: 10.1046/j.1469-8137.2003.00838.x. [DOI] [PubMed] [Google Scholar]
- Thompson JN. The Coevolutionary Process. University of Chicago Press: Chicago; 1994. [Google Scholar]
- Torchin ME, Mitchell CE. Parasites, pathogens, and invasions by plants and animals. Front Ecol Environ. 2004;2:183–190. [Google Scholar]
- United States Geological Survey 2006. Digital representations of three species range maps from “Atlas of United States Trees” by Elbert L. Little, Jr. (and other publications).Available at: : http://esp.cr.usgs.gov/data/atlas/little/ . Accessed February 5, 2009.
- van der Putten WH, Klironomos JN, Wardle DA. Microbial ecology of biological invasions. ISME J. 2007;1:28–37. doi: 10.1038/ismej.2007.9. [DOI] [PubMed] [Google Scholar]
- Vázquez DP.2005. Exploring the relationship between niche breadth and invasion success. In: Cadotte MW, McMahon SM, Fukami T (eds), Conceptual Ecology and Invasions Biology: Reciprocal Approaches to Nature Springer: Dordrecht; 307–322. [Google Scholar]
- Vellinga EC, Wolfe BE, Pringle A. Global patterns of ectomycorrhizal introductions. New Phytol. 2009;181:960–973. doi: 10.1111/j.1469-8137.2008.02728.x. [DOI] [PubMed] [Google Scholar]
- Wolfe BE, Richard F, Cross HB, Pringle A. Distribution and abundance of the introduced ectomycorrhizal fungus Amanita phalloides in North America. New Phytol. 2010;185:803–816. doi: 10.1111/j.1469-8137.2009.03097.x. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Haydon DT, Antia R. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol Evol. 2005;20:238–244. doi: 10.1016/j.tree.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahr R, Vilgalys R, DePriest PT. Geographic variation in algal partners of Cladonia subtenuis (Cladoniaceae) highlights the dynamic nature of a lichen symbiosis. New Phytologist. 2006;171:847–860. doi: 10.1111/j.1469-8137.2006.01792.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.