Abstract
Using a phyllosphere model system, we demonstrated that the term ‘carrying capacity', as it is commonly used in microbial ecology, needs to be understood as the sum of many ‘local carrying capacities' in order to better explain and predict the course and outcome of bacterial colonization of an environment. Using a green fluorescent protein-based bioreporter system for the quantification of reproductive success (RS) in individual Erwinia herbicola cells, we were able to reconstruct the contribution of individual immigrants to bacterial population sizes on leaves. Our analysis revealed that plant foliage represents to bacteria an environment where individual fate is determined by the local carrying capacity of the site where an immigrant cell lands. With increasing inoculation densities, the RS of most immigrants declined, suggesting that local carrying capacity under the tested conditions was linked to local nutrient availability. Fitting the observed experimental data to an adapted model of phyllosphere colonization indicated that there might exist three types of sites on leaves, which differ in their frequency of occurrence and local carrying capacity. Specifically, our data were consistent with a leaf environment that is characterized by few sites where individual immigrants can produce high numbers of offspring, whereas the remainder of the leaf offered an equal number of sites with low and medium RS. Our findings contribute to a bottom–up understanding of bacterial colonization of leaf surfaces, which includes a quantifiable role of chance in the experience at the individual level and in the outcome at the population level.
Keywords: individual-based ecology, environmental heterogeneity, bioreporter, phyllosphere, single-cell microbiology, modeling
Introduction
In ecology, the ‘carrying capacity' of an environment is the maximum number of individuals that the environment can support (McArthur, 2006). In phyllosphere microbiology, that is, the study of microorganisms that colonize leaf surfaces, this term is invariably referred to as the number of microbial cells that a leaf is able to sustain (Wilson and Lindow, 1994a, 1994b; Wilson et al., 1995; Woody et al., 2007; Nix et al., 2009). Carrying capacity of leaf surfaces is typically determined through counts of colony-forming units (CFUs) on agar plates. It is a conditional value that depends on several biotic and abiotic factors (Leveau, 2006). For example, different plant species are known to sustain different microbial population sizes (Kinkel et al., 2000; Knief et al., 2010b). Furthermore, bacterial species may vary in the densities which they can achieve on leaf surfaces depending on their ability to deal with the environmental conditions on the leaf surface (Jacques et al., 1995; Jacobs et al., 2005). Water availability on the leaf has frequently been highlighted as another driver for microbial abundance in the phyllosphere (Leben, 1965; Elad and Kirshner, 1993; Wilson and Lindow, 1994a, 1994b).
One key factor in determining foliar carrying capacity is the availability of nutrients. Carbon is a limiting resource during phyllosphere colonization (Mercier and Lindow, 2000; Leveau and Lindow, 2001a, 2001b). The process of leaching (Tukey, 1966) deposits photosynthates, such as the sugars fructose, sucrose and glucose, on the leaf surface where they become readily available for consumption by microbes (Mercier and Lindow, 2000; Leveau and Lindow, 2001a, 2001b; van der Wal and Leveau, 2010). Spraying of a carbon source onto plant leaves (Wilson and Lindow, 1995) or making an additional carbon source available through genetic modification of the plant (Wilson et al., 1995) can also increase the carrying capacity of leaves for bacterial populations.
The use of bacterial bioreporters has confirmed that sugars, such as fructose, are not distributed evenly across the leaf surface (Leveau and Lindow, 2001a, 2001b). It has also been reported that unused sugars can still be detected on leaves that are colonized by bacteria at the apparent carrying capacity (Mercier and Lindow, 2000). These two observations are in line with the hypothesized existence of discrete sites on the leaf surface that contain disparate amounts of resources (for example, sugars) and that may or may not be colonized by bacteria (Kinkel et al., 2002; Lindow and Brandl, 2003). At high inoculation densities, all sites on a leaf can be expected to be colonized by bacteria, and in these cases, carrying capacity will be determined by the amount of nutrients available in all sites combined. At low inoculation densities, some sites will remain uncolonized, including some that contain nutrients. In the latter case, the carrying capacity is not reached and instead becomes a function of how many, and which, sites are actually occupied by bacterial colonizers. This model of leaf colonization was partly verified in experiments with the epiphytic bacterium Pseudomonas syringae (Wilson and Lindow, 1994a, 1994b), revealing that populations derived from low inoculum concentrations never were able to reach the carrying capacity that was realized at high inoculum densities. However, what is still lacking is an experimental, quantitative demonstration of the variation that exists in nutrient availability, or local carrying capacity, between sites on a leaf surface. How big is this variation, and how does it contribute to the course and outcome of leaf colonization? Population-based measurements are not suited to address these questions, as they only examine averages across the population as a whole. In order to tackle these issues properly, an individual-based approach seems necessary.
Here, we used the bacterial bioreporter CUSPER (reverse of REPSUC, for REProductive SUCcess; Remus-Emsermann and Leveau, 2010) to describe how increasing inoculation densities are experienced by individual bacterial colonizers of bean leaf surfaces. From this information, we were able to derive estimates for the variation in local carrying capacity and assess the impact of this variation on achieving final foliar population sizes. The CUSPER bioreporter is based on dilution of green fluorescent protein (GFP) from dividing cells in such a way that GFP fluorescence of a single cell is inversely proportional to the reproductive success (RS) of the original immigrant from which the observed cell was derived. The CUSPER bioreporter was recently used to reveal that in a heterogeneous environment, such as the phyllosphere, individual bacterial immigrants contribute unequally to future population sizes (Remus-Emsermann and Leveau, 2010). In this study, we utilized CUSPER for an individual-based examination of leaf colonization at different inoculation densities in order to gain a quantitative understanding of variation in local carrying capacities in the phyllosphere. Such knowledge is potentially important to general ecological understanding as well as practical applications such as plant pathogen control.
Materials and methods
Bacterial strain, culture conditions and plant experiments
CUSPER is a bacterial bioreporter of RS (Remus-Emsermann and Leveau, 2010). It is based on Erwinia herbicola 299R∷JBA28 (pCPP39; Leveau and Lindow, 2001a, 2001b), which constitutively expresses LacIq from plasmid pCPP39 and carries a chromosomal mini-Tn5-Km insertion that expresses stable GFP from a LacIq-repressible PA1/04/03 promoter fusion to gfpmut3. The transposon confers resistance to kanamycin and the plasmid to tetracycline. The concept of CUSPER lies in the loading of bacterial cells with GFP through derepression of the PA1/04/03 promoter with isopropyl β-D-1-thiogalactopyranoside, followed by release of these GFP-loaded cells into an β-D-1-thiogalactopyranoside-free environment. Without de novo GFP production, GFP is diluted every time a cell divides, so that GFP fluorescence in each cell becomes an inverse measure for RS. CUSPER cells were cultured to mid-exponential phase at 300 r.p.m. and 28 °C in LB broth supplemented with 50 μg kanamycin and 15 μg tetracycline per ml in addition to 1 m β-D-1-thiogalactopyranoside. Bacteria were harvested by centrifugation at 3900 × g for 10 min, and resuspended in 1 × phosphate-buffered saline to approximate densities of 105, 106, 107 and 108 CFU ml−1. Two fully expanded cotyledon leaves of 14-day-old Phaseolus vulgaris plants (green snap bean, variety Blue Lake Bush 274; Burpee, Warminster, PA, USA) were inoculated by evenly spraying the adaxial surface of each leaf with ∼0.5 ml of one of the four bacterial suspensions using an airbrush paint gun (Pilot I Spray gun, Walther Pilot, Wuppertal-Vohwinkel, Germany). Inoculated plants were transferred to closed translucent boxes and incubated at high relative humidity and 21 °C.
Recovery of bacteria and analysis by microscopy
After 0, 3, 5, 7 and 24 h, three leaves were sampled from the plants that had been sprayed with one of the four bacterial suspensions, for a subtotal of 12 leaves per time point and a grand total of 60 leaves. Each leaf was transferred to a 50 ml Falcon tube (BD Biosciences, Breda, The Netherlands) with 20 ml 1 × phosphate-buffered saline buffer, vortexed briefly, sonicated for 7 min and vortexed briefly again. Aliquots (50 μl) from each leaf wash were plated using an Eddy Jet spiral plater (IUL, Barcelona, Spain) onto duplicate LB agar-plates supplemented with 50 μg kanamycin per ml to determine CFUs.
For two of the three leaves per time point and treatment (that is, a total of 40 leaves), the remainder of the leaf wash was filtered over a 0.2-μm Isopore filter (Millipore, Amsterdam, The Netherlands). CUSPER cells were recovered from the filter by vortexing for 15 s in 1 ml 1 × phosphate-buffered saline, fixed as previously described (Leveau and Lindow, 2001a, 2001b) and stored in 1 × phosphate-buffered saline at 4 °C for no longer than 3 days. Cells were analyzed with an Axio Imager M1 epifluorescent microscope (Zeiss, Oberkochen, Germany) using standard phase contrast and Zeiss Filter 38 (BP 470/ 40, FT 495, BP 525/ 50) for the visualization of GFP. Digital images were captured using an EC Plan-Neofluar 100 × /1.30 oil objective (Zeiss) with an AxioCam MRm camera (Zeiss). Single-cell fluorescence was quantified as the mean pixel intensity of individual cells (Leveau and Lindow, 2001a, 2001b) using the AxioVision 2.8 software package (Zeiss, Jena, Germany). Downstream data analysis was performed in Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA). To reconstruct the relative contribution of original leaf immigrants to the final population based upon single-cell green fluorescence measurements, we divided the green fluorescence of every one of the N cells in each sample at t=24 by the average cell's green fluorescence in the inoculum at time t=0. For each cell, this relative GFP content was divided by the sum of all GFP contents within the same sample (ΣGFP) to give a GFP/ΣGFP value for each cell. For each cell, we also calculated the RS of its original progenitor as 2log(1/GFP). Cells were then binned into one of the following RS classes: RS<0.5, 0.5⩽RS<1.5, 1.5⩽RS<2.5, 2.5⩽RS<3.5, 3.5⩽RS<4.5, 4.5⩽RS<5.5 and RS⩾5.5. For each bin, the GFP/ΣGFP values of all cells were summed to give Σ(GFP/ΣGFP). Values of Σ(GFP/ΣGFP) were then plotted as a function of RS in a histogram (see Figures 2b, 3, and 5).
Simulation of phyllosphere colonization using a logistic growth model
We adapted a previously developed logistic-growth model for bacteria in a heterogeneous environment (Kinkel et al., 2002). The model simulates a leaf with S=104 sites that vary in their carrying capacity J, that is, the maximal number of bacteria that a single site can sustain. Bacteria and their offspring were not allowed to move from one site to another. At the beginning of the simulation, bacteria were distributed over the S sites following a Poisson distribution assuming an initial population of 103, 104 or 105 bacteria per leaf. With each iteration n, the number of bacteria N in a site doubled, up to a maximum value N=J. Heterogeneity in J was introduced by assuming a normal or log-normal distribution that was created using a custom script for NetLogo 4.2.2 (Wilensky, 1999). Other distributions were created with a custom Visual Basic Script for MS Excel (http://azzalini.stat.unipd.it/SN/ stephen_gersuk.excel). The trimodal distribution that provided a good fit to the observed experimental data (see Supplementary Information) assumed that 48.5% of the sites had a J value of 2, another 48.5% had a J value of 48, whereas the remaining 3% of sites had a J value of 1152.
Results
Determining the carrying capacity of bean leaves
To determine the bacterial carrying capacity of bean leaves under laboratory conditions, we inoculated four sets of cotyledon leaves with a suspension of E. herbicola strain 299R (Eh299R) CUSPER bacteria to reach four different densities, that is, 4.5 × 103, 6.9 × 104, 5.7 × 105 or 1.2 × 107 CFU per leaf (Figure 1a). Plants were incubated at conditions of high relative humidity, and we monitored changes in the bacterial population sizes by determining CFU counts per leaf at 3, 5, 7 and 24 h after inoculation (Figure 1a). With the two lowest inoculation densities, population sizes increased during the first 7 h of the experiment, albeit at different rates. At the two highest inoculation densities, population sizes either remained constant or decreased during this time period. Carrying capacity was estimated from Figure 1b, which shows a plot of the fold increase in population size over 24 h as a function of the bacterial density at the time of inoculation. The trendline crosses the x axis at 3.4 × 106 CFU per leaf, which represents the inoculation density where no net growth is expected to occur, that is, the total carrying capacity under these conditions.
Figure 1.
Colonization of bean leaf surfaces by Eh299R CUSPER bacteria at different inoculation densities. (a) Changes in population size were monitored as CFUs, normalized per leaf and plotted as a function of time. Inoculation densities (CFUs per leaf) at t=0: (•) 4.5 × 103; (▪) 6.9 × 104; (▴) 5.7 × 105; and (▾) 1.2 × 107. (b) Increase in population size between t=0 and t=24, that is, Nt=24/Nt=0, as a function of the population size at time t=0. The trendline crosses the x axis at 6.535, which back-transforms to 3.4 × 106 CFU per leaf. The error bars represent standard deviations from the mean.
Estimating reproductive success (RS) of CUSPER immigrants from GFP content in offspring
Before introduction into the bean phyllosphere, the CUSPER cells were loaded with stable GFP. This initial amount of GFP gets diluted from cells as they divide (Remus-Emsermann and Leveau, 2010). A hypothetical example is given in Figure 2a to illustrate how GFP content of single CUSPER cell was used to reconstruct the RS of individual immigrants to the leaf surface. In this example, we considered an initial population of N0=8 cells. Four of these did not divide, which equaled an RS of 0 for each of those cells. One cell divided once (RS=1), two divided twice (RS=2) to produce four cells each and one divided three times (RS=3) for an offspring of eight cells, resulting in a final population at time t of Nt=22 (Figure 2a). Thus, at time t, this yielded four cells with a relative GFP content of 1 (that is, these cells did not divide and GFP was not diluted), two cells with a relative GFP content of 0.5 (each divided once, thus diluting the original GFP into two daughter cells), eight cells with a relative GFP content of 0.25 and eight cells with a relative GFP content of 0.125. For each cell from this population at time t, we can calculate two values: (1) GFP/ΣGFP, which is its GFP content divided by the sum of all GFP contents in the same population and (2) 2log(1/GFP), which is the RS of its progenitor. Cells can then be binned into one of the following RS classes: 0, 1, 2 or 3, and for each bin, the GFP/ΣGFP values of all cells are summed to give Σ(GFP/ΣGFP). Figure 2b shows Σ(GFP/ΣGFP) charted as a function of RS in a bar graph. The inset shows a regular histogram, whereas the larger graph offers a representation in which the width of each bar equals 2RS.
Figure 2.
Illustrative example of how GFP content in CUSPER offspring at time t can be retropolated to reveal the RS of bacterial cells at time t=0. Details are provided in the text.
It is important to realize that the histogram at any time t=x is constructed solely on the basis of data from time t=x and on the average GFP content at t=0. Yet, it tells us many things about the fate of individual cells and the increase in population size since t=0. First, the total area covered by all bars in the histogram, that is, Σ(Σ(GFP/ΣGFP)x2RS)=2.75, equals the increase in population size between t=0 and time t, or Nt/N0 (22/8=2.75). This value can also be calculated directly from Nt/ΣGFP, that is, the number of cells at time t divided by the sum of all GFP contents. Second, the graph reveals how successful different fractions of the original population were in producing offspring. For example, it shows that half (that is, Σ(GFP/ΣGFP)=0.5) of the cells in the population at t=0 did not divide (RS=0) and that a quarter of the cells (Σ(GFP/ΣGFP)=0.25) at t=0 divided two times to produce four cells at time t (RS=2). Third, the area of any single bar in the graph divided by the total area covered by all bars in the histogram gives the proportional contribution of cells from the corresponding RS bin to the population size at time t. For example, the area of the third bar (RS=2) is Σ(GFP/ΣGFP) × 2RS=0.25 × 22=1, which, divided by 2.75 (=total area, see above), equals 0.36, meaning that 25% of the cells in the original population divided twice to produce enough offspring to account for 36% of the population at time t.
Single-cell experiences of leaf colonization after inoculation at different densities
Using the analysis illustrated in Figure 2, we retropolated GFP content of leaf-recovered bacteria to the RS of individual bacteria in the t=0 population. Specifically, we analyzed bacterial cells retrieved at the t=24 time point from eight leaves (four inoculation densities, two leaf washings each) from the experiment for which population data are shown in Figure 1. The results are summarized in Figure 3, which shows eight histograms (a–h), that is, two leaves from different plants for each density. These graphs indicate great variation in the fate of individual bacteria after they were introduced onto the leaf surface at different starting densities.
Figure 3.
Variation in RS of CUSPER immigrants to the bean leaf surface, as inferred from GFP content in bacteria that were retrieved from leaves after 24 h of incubation. Cells were retrieved from the same experimental setup that was used to construct Figure 1. For each of the four inoculation densities, cells from two independent leaf samples were analyzed: (a and b) 4.5 × 103 CFU per leaf; (c and d) 6.9 × 104 CFU per leaf; (e and f) 5.7 × 105 CFU per leaf; and (g and h) 1.2 × 107 CFU per leaf. Histograms were fashioned in the same way as was done for the fictional data in Figure 2.
On the two leaves that received the lowest inoculation density (Figures 3a and b), 41% and 70%, respectively, of the initial colonizers divided once after arrival on the bean leaf surface. We interpret this to mean that a substantial portion of the immigrant population landed in a spot that did not support much growth. On the other hand, 59% and 21%, respectively, of the cells at t=0 was very successful, with an RS value of 5 or greater, that is, they produced ⩾32 offspring. These very successful immigrants reproduced to become 98% and 80%, respectively, of the final population size, compared with only 2% and 13%, respectively, for the cells that underwent only one division upon arrival.
With increasing inoculation density, the relative fraction of very successful cells (RS⩽5) diminished (Figure 3), down to 2.4% and 0.7%, respectively, on the two leaves that were sprayed with the highest inoculation density (Figures 3g and h). At this density, 91% and 93%, respectively, of the bacterial immigrants each produced four offspring at most, with a majority not dividing at all or only once. Despite their low initial abundance of 2.4% and 0.7%, the very successful cells contributed a considerable fraction, 31% and 11%, respectively, of the final population size.
It is evident from Figure 3 that with increasing inoculation density, the sum of the area underneath all of the bars (representing N24/N0, as explained in Figure 2) decreases. This decline in fold increase in population size is consistent with our findings in Figure 1b, and can be explained by assuming that at higher inoculation densities, similar amounts of resources need to be shared by more bacteria, resulting in fewer offspring per cell. The notion that there were still few very successful immigrants at the highest inoculation density suggests that a small portion of the leaf surface (2.4% and 0.7% in this case) consisted of sites that allow considerably more reproduction than most other sites. Another trend that is apparent in Figure 3 is the increase in resemblance of the two histograms from each inoculation density, as inoculation density increases. This suggests that the course and outcome of leaf colonization become more predictable at higher inoculation densities.
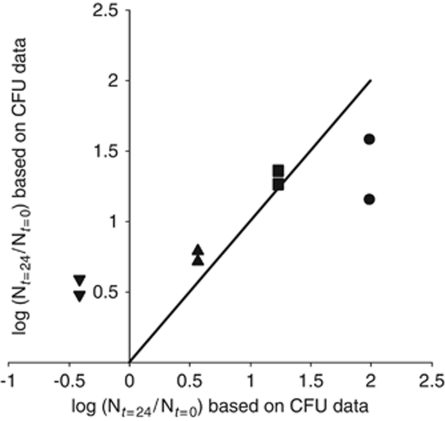
For each of the eight histograms shown in Figure 3, we plotted in Figure 4 the sum of the area underneath all of the bars as a function of the N24/N0 fold increase that was obtained from CFU-based data. The fact that data points did not fall on a line with slope 1 through the origin indicates that GFP- and CFU-based results do not fully agree with each other. At the lowest inoculation density, this discrepancy can be explained by the known limitation of CUSPER (Remus-Emsermann and Leveau, 2010) that after five cell divisions GFP gets diluted to the point where we can no longer reliably detect and quantify it. For the two leaves inoculated at this density, we calculated that in order to get the GFP-based estimates for increase in population size up to the one derived from CFU data, one would have to assume average RS values of 7.4% and 9.0% respectively, for cells that were binned in the RS>5 category in Figure 3. This corresponds to 169 and 512 offspring, respectively, for each of these cells. At the highest inoculation density, the discrepancy between GFP- and CFU-based results may be explained by assuming that a proportion of the cells on the leaf were not able to form a colony on the plate. For the two leaves that were inoculated at this density, we calculated that in order to get the CFU-based estimates for increase in population size to match those derived from GFP data, one would have to assume that 90% and 87%, respectively, of the cells lost their ability to form a colony between t=0 and t=24. Another hypothetical explanation for the discrepancy between CFU- and GFP-based data is that aggregates that were washed from the leaf surface did not break up into single cells during the wash procedure, so that one CFU might represent more than one cell. However, we can exclude this possibility, based on the fact that such aggregates were never observed under the microscope.
Figure 4.
Comparison of estimates for Nt=24/Nt=0, that is, fold increase in bacterial population sizes over 24 h, based on CFU data and GFP data. For the x values of each data point, we used the y axis values shown in Figure 1b. For the y values of each data point, we used the total area under all bars for each corresponding histogram shown in Figure 3. The symbols mark the inoculation density (CFU per leaf) at t=0: (•) 4.5 × 103; (▪) 6.9 × 104; (▴) 5.7 × 105; and (▾) 1.2 × 107, and correspond to the symbols used in Figure 1. If the CFU- and GFP-based estimates for one particular inoculation density were identical, the corresponding data point would fall on the line with slope 1. The deviations from this line at low and high inoculation densities are discussed in the text.
Modeling single-cell experiences in the phyllosphere
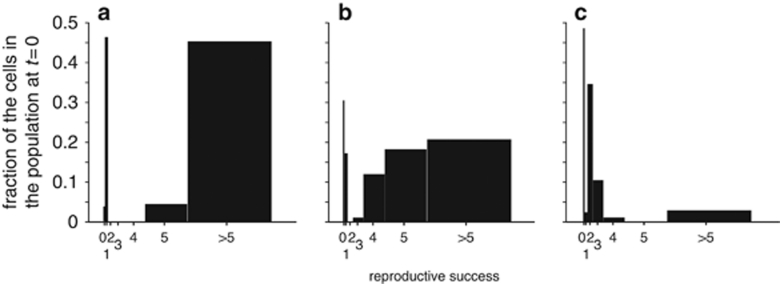
We used our experimental data to test a modified version of a previously formulated logistic growth model for bacterial colonization of a heterogeneous leaf environment (Kinkel et al., 2002). Our model consisted of a virtual leaf that contains 104 independent sites. Leaves were inoculated with 103, 104 or 105 virtual bacteria following a Poisson distribution. For the lowest inoculation density, this means that ∼90% of all sites were devoid of bacteria, whereas at the highest inoculation density each site contained 10 cells on average. In each site, bacteria were allowed to reproduce up to the site's local carrying capacity J. We tested different distributions of J and recorded the RS of individual bacteria. We found that of all the tested scenarios, a trimodal (low, medium and high) distribution of J was the one that best matched the general patterns that we observed for the experimental data (Figure 5), that is, a co-occurrence of very successful and nearly unsuccessful immigrants at low inoculation densities and the existence of a small portion of very successful colonizers among a majority of less successful immigrants at high inoculation densities. The best fitting distribution assumed roughly similar numbers of sites with low or medium local carrying capacity, whereas the remaining very small portion of sites was characterized by high local carrying capacity. In the model, a high inoculation density meant that medium-J sites received immigrants in numbers that for the most part approximated or exceeded J, such that these cells did not differ much in RS from cells that landed in low-J sites. In contrast, the few high-J sites were occupied by cells in numbers well below J, allowing for a substantial number of offspring per cell. At low inoculation density, the low frequency of high-J sites prevented most, if not all, cells from landing in one of these sites, but as medium-J sites received cells in numbers well below J, there was still an opportunity for these cells to proliferate much more than cells that landed in low-J sites, where no or few doublings occurred.
Figure 5.
Variation in RS of virtual bacterial immigrants to a simulated leaf environment. The model is described in the text. It assumes a leaf with 104 discrete sites that vary in their carrying capacity J. This leaf was inoculated with 103 (a), 104 (b) or 105 (c) bacteria per leaf. For this simulation, we assumed that J=2 for 48.5% of the sites, J=48 for another 48.5% of the sites and J=1152 for the remaining 3% of the sites.
Discussion
We have determined the bacterial carrying capacity of bean leaves under controlled conditions in the laboratory. By bioreporter analysis, we were able to demonstrate that under these conditions, realized carrying capacity at different inoculation densities is a function of variation in the local carrying capacity of different sites on the leaf surface. Our findings are consistent with a model of phyllosphere colonization that features different fates for bacterial immigrants, depending on where and with how many others they land on the leaf surface. Our study offers a quantitative estimate for these fates, which is a significant step toward a more predictive understanding of bacterial colonization of leaf surfaces.
It is evident from our study that individual- and population-based data sets each offer an incomplete understanding of how bacteria colonize a leaf. CUSPER-based measurements have the limitation that information is lost beyond a RS of 5 (Remus-Emsermann and Leveau, 2010), so that the contribution of some immigrants to the population will be underestimated, whereas CFU-based measurements provide only the average contribution of cells to the population, thus ignoring the variability that clearly exists among immigrants. In combination, however, GFP- and CFU-based measurements offered significant novel insights. For example, the substantial number of cells that divide at most once upon arrival to the leaf surface at low inoculation density, and the corresponding high increase in population size as derived from CFU counts, suggests that the RS of cells that actually did divide was underestimated and that some cells could produce offspring in numbers that exceed 500 cells. Bacterial aggregates of this size and much larger have been found on bean leaves under appropriate conditions (Monier and Lindow, 2004), although it was not established whether they were derived from single cells. On the other hand, to explain high RS values for individual bacterial immigrants at high inoculation densities in the context of a constant or decreasing CFU count under the same conditions, one needs to assume that a substantial number of cells lose their ability to form a colony on agar plates. Indeed, such nonculturability has been demonstrated experimentally for P. syringae, another model colonizer of the phyllosphere, even under favorable conditions of relative high humidity (Wilson and Lindow, 1992). There is good evidence in the literature (for example, Unge et al., 1999; Lowder et al., 2000) that the GFP variant that we used in our study is extremely stable and continues to fluoresce in cells that enter this state of nonculturability.
In our current model of bacterial colonization of the phyllosphere, each leaf colonization event can be thought of as a bottleneck occurrence, where some percentage of the incoming population is prevented from reproducing, or even surviving, whereas other immigrants in that same population are numerically enriched because they settle in sites conducive to growth. On the basis of our derived estimates on the quantity and quality of sites, landing in a high-J site is unlikely for any individual bacterium, and at low inoculation densities, chance will determine whether such sites will be filled or not. Thus, the outcome of an inoculation event would be less predictable for low inoculation densities than for high ones.
Ecological theory holds that if a nutrient resource is limited and the number of organisms competing for this resource is high, the average success of the population goes down (Galliard et al., 2005; Rankin and Kokko, 2006). This is precisely what we observed in our experiments and simulations: high inoculation densities forced more bacterial individuals to share a site and resulted in overall reduced RS. This seems to support the notion that local carrying capacity on leaves is determined by local nutrient availability. Being perfectly isogenic, CUSPER cells can be considered each other's ultimate competitors, with a niche overlap index of 1 (Wilson and Lindow, 1994a, 1994b). Thus, unless a site offered very high levels of nutrients, bacterial immigrants would be relatively unable to reproduce, representing a convincing case of the ‘tragedy of the commons' (Hardin, 1968). However, a one-time immigration event of up to 107 bacteria per leaf is not a likely event in nature (Lindemann and Upper, 1985). Instead, immigration is a gradual process, with cells arriving to the leaf from the surrounding air at different times and typically at low rates (Upper and Hirano, 2002). Another aspect of our experimental setup that can be considered artefactual is that all immigrants were of clonal descent. In reality, the leaf surface is colonized by very diverse bacteria and other microorganisms with different niche overlap indexes, which allows for various degrees of coexistence (Knief et al., 2010a). However, it is important to note that the ability to set up such artificial colonization events allowed us to extract data on the quality of the leaf surface as a habitable environment. This high level of experimental amenability is quite unique to microbial ecology and is one of the advantages microbial ecologists have over ‘macro'-bial ecologists (Fierer, 2007). Future applications of CUSPER will address more realistic scenarios by assessing variation in RS of CUSPER cells in competition with other bacterial species and after immigration onto precolonized leaves.
Our observation that a small fraction (0.7–2.4%) of the cells was very successful in reproducing even at the highest inoculation density suggests the existence of sites on the leaf surface that offer high levels of nutrients, allowing these cells to contribute disproportionally (11–31%) to the final population size. Interestingly, this finding agrees with reports showing that relatively rare features on the leaf surface tend to harbor large numbers of bacteria on colonized leaves. Specifically, it is worth noting that only 0.6% of the bean leaf surface is covered by hooked and glandular trichomes (Monier and Lindow, 2005a, 2005b), yet 44.1% of Eh299R bacteria in aggregates were found to be associated with these structures after incubation under moist conditions for 5 days (Monier and Lindow, 2005a, 2005b). In at least one study (Yadav et al., 2005), the population size of epiphytic bacteria was positively correlated with the densities of glandular and nonglandular trichomes. This link between bacterial fate, population size and leaf topography calls for further investigation into the predictive value of trichome density for bacterial colonization of leaf surfaces.
As was pointed out before (Remus-Emsermann and Leveau, 2010), application of the CUSPER concept is not limited to leaf surfaces, but can be extended widely to many other bacterial habitats. Several bioreporter-based studies have revealed variation in the bacterial experience of such habitats (Moller et al., 1998; Jaspers et al., 2001; Herron et al., 2010), and all point to microscale heterogeneity in terms of the physical, chemical and biological conditions that an individual bacterium can be exposed to in a given environment. The CUSPER approach offers an insight in bacterial fate, which does not follow from knowing exactly what these conditions were; instead, it settles for knowing that these conditions were (or were not) permissive to the production of offspring. This interpretation of environmental heterogeneity matches quite well with the concept of local carrying capacity, where the microbial landscape, be it natural or man-made (for example, Keymer et al., 2006; Battin et al., 2007) features local variation in the ability of patches to sustain bacterial growth.
Acknowledgments
The study was funded by the Netherlands Organisation of Scientific Research (NWO) in the form of a personal VIDI grant to JHJL. This is NIOO-KNAW publication number 5156.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Battin TJ, Sloan WT, Kjelleberg S, Daims H, Head IM, Curtis TP, et al. Microbial landscapes: new paths to biofilm research. Nat Rev Microbiol. 2007;5:76–81. doi: 10.1038/nrmicro1556. [DOI] [PubMed] [Google Scholar]
- Elad Y, Kirshner B. Survival in the phylloplane of an introduced biocontrol agent Trichoderma harzianum and populations of the plant pathogen Botrytis cinerea; as modified by abiotic conditions. Phytoparasitica. 1993;21:303–313. [Google Scholar]
- Fierer N. Finding a place for microorganisms in the field of ecology. Ecology. 2007;88:1336–1337. [Google Scholar]
- Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- Herron PM, Gage DJ, Cardon ZG. Micro-scale water potential gradients visualized in soil around plant root tips using microbiosensors. Plant Cell Environ. 2010;33:199–210. doi: 10.1111/j.1365-3040.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- Jacobs JL, Carroll TL, Sundin GW. The role of pigmentation, ultraviolet radiation tolerance, and leaf colonization strategies in the epiphytic survival of phyllosphere bacteria. Microb Ecol. 2005;49:104–113. doi: 10.1007/s00248-003-1061-4. [DOI] [PubMed] [Google Scholar]
- Jacques MA, Kinkel LL, Morris CE. Population sizes, immigration, and growth of epiphytic bacteria on leaves of different ages and positions of field-grown endive (Cichorium endivia var. latifolia) Appl Environ Microbiol. 1995;61:899–906. doi: 10.1128/aem.61.3.899-906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers MCM, Meier C, Zehnder AJ, Harms H, van der Meer JR. Measuring mass transfer processes of octane with the help of an alkS-alkB∷gfp-tagged Escherichia coli. Environ Microbiol. 2001;3:512–524. doi: 10.1046/j.1462-2920.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- Keymer JE, Galajda P, Muldoon C, Park S, Austin RH. Bacterial metapopulations in nanofabricated landscapes. Proc Natl Acad Sci USA. 2006;103:17290–17295. doi: 10.1073/pnas.0607971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkel LL, Newton M, Leonard KJ.2002Resource aggregation in the phyllosphere: implications for microbial dynamics across spatial scalesIn: Lindow S, Hecht-Poinar E, Elliott V (eds).Phyllosphere Microbiology APS Press: Saint Paul, MN, USA; 317–340. [Google Scholar]
- Kinkel LL, Wilson M, Lindow SE. Plant species and plant incubation conditions influence variability in epiphytic bacterial population size. Microb Ecol. 2000;39:1–11. doi: 10.1007/s002489900182. [DOI] [PubMed] [Google Scholar]
- Knief C, Frances L, Vorholt JA. Competitiveness of diverse Methylobacterium strains in the phyllosphere of Arabidopsis thaliana and identification of representative models, including M. extorquens PA1. Microb Ecol. 2010a;60:440–452. doi: 10.1007/s00248-010-9725-3. [DOI] [PubMed] [Google Scholar]
- Knief C, Ramette A, Frances L, Alonso-Blanco C, Vorholt JA. Site and plant species are important determinants of the Methylobacterium community composition in the plant phyllosphere. ISME J. 2010b;4:719–728. doi: 10.1038/ismej.2010.9. [DOI] [PubMed] [Google Scholar]
- Leben C. Influence of humidity on migration of bacteria on cucumber seedlings. Can J Microbiol. 1965;11:671, 676. doi: 10.1139/m65-090. [DOI] [PubMed] [Google Scholar]
- Le Galliard JF, Fitze PS, Ferrière R, Clobert J. Sex ratio bias, male aggression, and population collapse in lizards. Proc Natl Acad Sci USA. 2005;102:18231–18236. doi: 10.1073/pnas.0505172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveau JHJ. Microbial Communities in the Phyllosphere. Biology of the Plant Cuticle. Riederer M and Müller C, Blackwell Publishing Ltd: Oxford, UK; 2006. pp. 334–367. [Google Scholar]
- Leveau JHJ, Lindow SE. Appetite of an epiphyte: quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc Natl Acad Sci USA. 2001a;98:3446–3453. doi: 10.1073/pnas.061629598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveau JHJ, Lindow SE. Predictive and interpretive simulation of green fluorescent protein expression in reporter bacteria. J Bacteriol. 2001b;183:6752–6762. doi: 10.1128/JB.183.23.6752-6762.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann J, Upper CD. Aerial dispersal of epiphytic bacteria over bean plants. Appl Environ Microbiol. 1985;50:1229–1232. doi: 10.1128/aem.50.5.1229-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindow SE, Brandl MT. Microbiology of the phyllosphere. Appl Environ Microbiol. 2003;69:1875–1883. doi: 10.1128/AEM.69.4.1875-1883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowder M, Unge A, Maraha N, Jansson JK, Swiggett J, Oliver JD. Effect of starvation and the viable-but-nonculturable state on green fluorescent protein (GFP) fluorescence in GFP-tagged Pseudomonas fluorescens A506. Appl Environ Microbiol. 2000;66:3160–3165. doi: 10.1128/aem.66.8.3160-3165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JV. Microbial Ecology: An Evolutionary Approach. Academic Press: Oxford, UK; 2006. [Google Scholar]
- Mercier J, Lindow SE. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl Environ Microbiol. 2000;66:369–374. doi: 10.1128/aem.66.1.369-374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller S, Sternberg C, Andersen JB, Christensen BB, Ramos JL, Givskov M, et al. In situ gene expression in mixed-culture biofilms: evidence of metabolic interactions between community members. Appl Environ Microbiol. 1998;64:721–732. doi: 10.1128/aem.64.2.721-732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier JM, Lindow SE. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl Environ Microbiol. 2004;70:346–355. doi: 10.1128/AEM.70.1.346-355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier JM, Lindow SE. Aggregates of resident bacteria facilitate survival of immigrant bacteria on leaf surfaces. Microb Ecol. 2005a;49:343–352. doi: 10.1007/s00248-004-0007-9. [DOI] [PubMed] [Google Scholar]
- Monier JM, Lindow SE. Spatial organization of dual-species bacterial aggregates on leaf surfaces. Appl Environ Microbiol. 2005b;71:5484–5493. doi: 10.1128/AEM.71.9.5484-5493.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix S, Burpee LL, Buck JW. Responses of 2 epiphytic yeasts to foliar infection by Rhizoctonia solani or mechanical wounding on the phylloplane of tall fescue. Can J Microbiol. 2009;55:1160–1165. doi: 10.1139/w09-072. [DOI] [PubMed] [Google Scholar]
- Rankin DJ, Kokko H. Sex, death and tragedy. Trend Ecol Evol. 2006;21:225–226. doi: 10.1016/j.tree.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Remus-Emsermann MNP, Leveau JHJ. Linking environmental heterogeneity and reproductive success at single-cell resolution. Isme J. 2010;4:215–222. doi: 10.1038/ismej.2009.110. [DOI] [PubMed] [Google Scholar]
- Tukey HB., Jr Leaching of metabolites from above-ground plant parts and its implications. Bulletin Torrey Bot Club. 1966;93:385–401. [Google Scholar]
- Unge A, Tombolini R, Molbak L, Jansson JK. Simultaneous monitoring of cell number and metabolic activity of specific bacterial populations with a dual gfp-luxAB marker system. Appl Environ Microbiol. 1999;65:813–821. doi: 10.1128/aem.65.2.813-821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upper C, Hirano S.2002Revisiting the roles of immigration and growth in the development of populations of Pseudomonas syringae in the phyllosphereIn: Lindow S, Hecht-Poinar E, Elliott V (eds).Phyllosphere Microbiology APS Press: Saint Paul, MN, USA; 69–79. [Google Scholar]
- Van Der Wal A, Leveau JHJ. Modelling sugar diffusion across plant leaf cuticles: the effect of free water on substrate availability to phyllosphere bacteria. Environ Microbiol. 2010;13:792–797. doi: 10.1111/j.1462-2920.2010.02382.x. [DOI] [PubMed] [Google Scholar]
- Wilensky U. NetLogo. Center for Connected Learning and Computer-Based Modeling, Northwestern University: Evanston, IL; 1999. [Google Scholar]
- Wilson M, Lindow S. Relationship of total viable and culturable cells in epiphytic populations of Pseudomonas syringae. Appl Environ Microbiol. 1992;58:3908–3913. doi: 10.1128/aem.58.12.3908-3913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Lindow S. Enhanced epiphytic coexistence of near-isogenic salicylate-catabolizing and non-salicylate-catabolizing Pseudomonas putida strains after exogenous salicylate application. Appl Environ Microbiol. 1995;61:1073–1076. doi: 10.1128/aem.61.3.1073-1076.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Lindow SE. Ecological similarity and coexistence of epiphytic ice-nucleating (Ice+) Pseudomonas syringae strains and a non-ice-nucleating (Ice−) biological control agent. Appl Environ Microbiol. 1994a;60:3128. doi: 10.1128/aem.60.9.3128-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Lindow SE. Inoculum density-dependent mortality and colonization of the phyllosphere by Pseudomonas syringae. Appl Environ Microbiol. 1994b;60:2232–2237. doi: 10.1128/aem.60.7.2232-2237.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Savka MA, et al. Altered epiphytic colonization of mannityl opine-producing transgenic tobacco plants by a mannityl opine-catabolizing strain of Pseudomonas syringae. Appl Environ Microbiol. 1995;61:2151–2158. doi: 10.1128/aem.61.6.2151-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ST, Ives AR, Nordheim EV, Andrews JH. Dispersal, density dependence, and population dynamics of a fungal microbe on leaf surfaces. Ecology. 2007;88:1513–1524. doi: 10.1890/05-2026. [DOI] [PubMed] [Google Scholar]
- Yadav R, Karamanoli K, Vokou D. Bacterial colonization of the phyllosphere of Mediterranean perennial species as influenced by leaf structural and chemical features. Microb Ecol. 2005;50:185–196. doi: 10.1007/s00248-004-0171-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.