Abstract
Lance-Adams syndrome (LAS) is a rare complication of successful cardiopulmonary resuscitation and is often accompanied by action myoclonus. LAS is seen in patients who have undergone a cardiorespiratory arrest, later regained consciousness, and then developed myoclonus days or weeks after the event. Less than 150 cases of LAS have been reported in the worldwide medical literature. Here, we present a 32-year-old man who suffered from myoclonus after hypoxic brain damage due to hanging himself. This case was diagnosed as Lance-Adams syndrome according to a history of hypoxic brain damage, the clinical features, and the neuroimages such as brain SPECT. Making an early diagnosis and properly managing LAS is positively related to improving the patient's functional outcome. If patients have posthypoxic myoclonus after successful cardiopulmonary resuscitation, we should consider the diagnosis of LAS and initiate a proper rehabilitation program.
Keywords: Lance-Adams syndrome, Posthypoxic myoclonus
INTRODUCTION
Posthypoxic myoclonus (PHM) is a neurological complication characterized by uncontrolled myoclonic jerks following cardiac arrest. PHM is divided into 2 types. The acute type of PHM, which is called myoclonic status epilepticus, occurs within 12 hours in most cases after hypoxic brain damage in patients who are deeply comatose. The chronic type of PHM, which is known as Lance-Adams syndrome (LAS), is characterized by action myoclonus beginning within days to weeks after cardiopulmonary resuscitation (CPR) and persists in patients who have recovered consciousness after CPR.1,2
Lance-Adams syndrome is a rare complication and less than 150 cases have been reported in the literature.2 We present here a patient who was diagnosed as having Lance-Adams syndrome after CPR due to hanging himself.
CASE REPORT
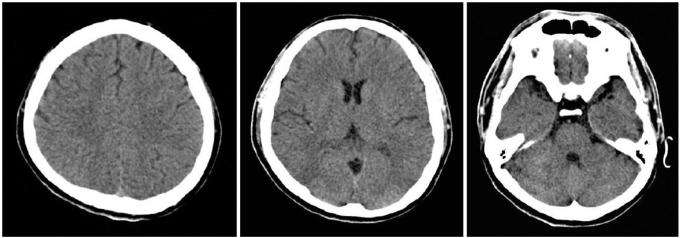
A 32-year-old man was admitted to our emergency department in a coma. He was found in cardiac arrest following a suicide attempt by hanging himself. After 6 minutes of CPR, his vital signs returned within the normal ranges. On arrival at the emergency department, he was still in a coma and brain computed tomography (CT) showed no abnormalities (Fig. 1).
Fig. 1.
The brain computed tomography scan shows normal findings.
On day 3 of hospitalization, he developed a generalized seizure in the intensive care unit after discontinuing an intravenous infusion of midazolam (7 mg per hour) and fentanyl (100 µg). Then, myoclonic movements were continuously observed over the entire body, including the face. At that time, the myoclonic movements were considered as generalized myoclonus secondary to hypoxic brain insult and these were empirically treated with sodium valproate. However, the myoclonic jerks were not controlled after increasing the dose of sodium valproate to 1,500 mg and phenytoin to 1,000 mg daily. The myoclonic jerks ceased with a bolus injection of midazolam or Norcuron, but the effect was transient. On day 6 of hospitalization, an EEG was performed and it showed complex spike waves in both frontal lobes lesions, which was consistent with cerebral dysfunction and status epilepticus. On day 12 of hospitalization, although the myoclonic jerks continued, the patient's mental status improved so that he was able to intermittently obey simple commands. On day 13 of hospitalization we started phenobarbital at a dose of 300 mg daily. The frequency of convulsion movements was reduced, yet the patient was overly sedated.
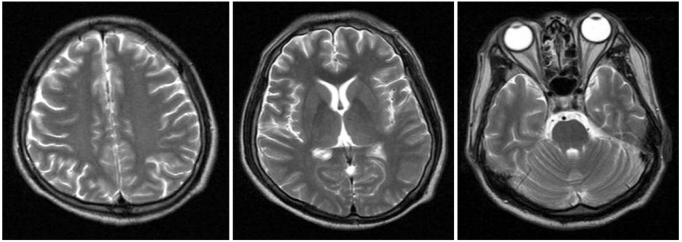
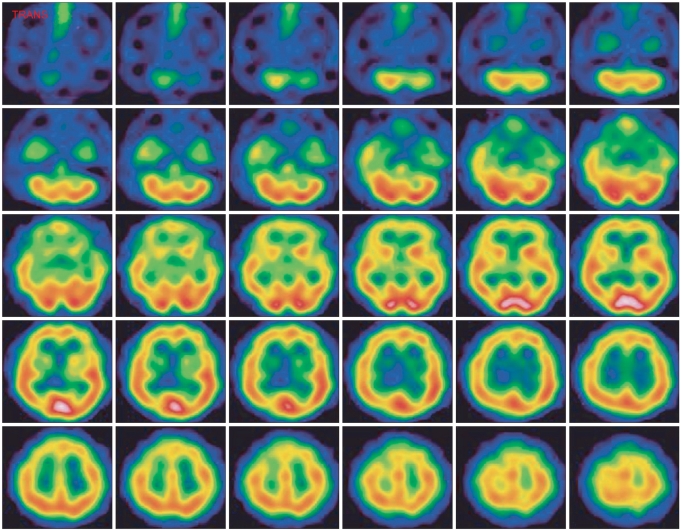
On day 20 of hospitalization, he was transferred to the rehabilitation department. His level of consciousness was 15 on the GCS. Manual muscle tests showed the muscles of all 4 extremities had a Medical Research Council grade of 4. The findings of the other neurological exams were not specific. The cerebellar function could not be evaluated due to intentional myoclonic jerks and he had dysarthria. The myoclonic jerks were aggravated by voluntary, coordinated action and disappeared at rest or during sleep. His Mini-Mental State Examination (MMSE) score was 19 of 30. He had short-term memory impairment, dyscalculia, and attention deficits. He was totally dependent for his activities of daily living, and his Modified Barthel Index (MBI) score was 0 of 100. Brain magnetic resonance imaging (MRI) showed no focal lesion except for mild diffuse brain atrophy, and the brain single photon emission computed tomography (SPECT) demonstrated a mild decrease of perfusion in the right basal ganglia and the left temporal lobe lesion (Fig. 2, 3). He was diagnosed as having Lance-Adams syndrome due to a history of hypoxic brain damage, the clinical features, and the brain SPECT.
Fig. 2.
The T2-weighted images of brain magnetic resonance imaging show mild diffuse brain atrophy.
Fig. 3.
The brain single photon emission computed tomography scan shows mild diffuse hypoperfusion in the brain and especially in the right basal ganglia and left temporal region 1 month after cardiorespiratory resuscitation
Based on his diagnosis, we started a rehabilitation program with a modified medication regimen. On day 27 of hospitalization, phenobarbital was stopped and clonazepam was initiated at 0.5 mg PO, tid and diazepam at 2 mg PO, tid was also administered. Changing the medications led to an improvement in the severity and frequency of the myoclonic jerks. During 3 weeks, the clonazepam and diazepam were gradually increased to 4 mg daily and 5 mg PO, tid, respectively, and we added levetiracetam at the dose of 250 mg PO, bid.
Initially, the rehabilitation program was focused on improving his postural balance and reducing the myoclonic jerks. To better control the myoclonus, he repeatedly performed a particular action in slow motion. Thirty days after transfer, he could perform some actions after 4 to 9 myoclonic jerks, but when trying to force himself to do something within a time limit, escalation of the myoclonus persisted. On discharge, he was able to walk 20 meters using a rolling walker with maximal assistance of relatives. His MMSE score was 22 of 30 and mild cognitive deficits remained. His dysarthria persisted to some extent.
DISCUSSION
Lance-Adams syndrome (LAS) was first described in the 1960s by Lance and Adams, who described 4 patients who developed myoclonic jerks within a few days following an episode of anoxia. After recovery of consciousness, the patients continued their abnormal clonic movements, which were triggered by intentional action or external stimuli, and they were relieved at resting or during sleep.1,2 Although the pathophysiology of LAS has not been clearly defined, the prognosis is known to be quite good.3 It is important to distinguish LAS from posthypoxic seizures so a correct prognosis can be provided. One of the important clinical features is consciousness. In the acute type of posthypoxic seizures, the patient's mental status persists as comatose, but in LAS, the patient later regains consciousness. Intentional myoclonus develops several days after the hypoxic brain insult and persists thereafter, but in posthypoxic seizures, generalized myoclonus usually occurs within 48 hours after CPR.2 The myoclonus in LAS has no consistent correlation with EEG abnormalities. The patient in this case had remarkable features that are consistent with LAS.
Diagnostic imaging tests such as CT or MRI of the brain are not helpful to make a diagnosis of LAS. Neuroimaging, such as brain SPECT or brain positron emission tomography (PET), has recently showed the anatomical and pathophysiological basis of LAS. Frucht et al. reported that compared with control groups, 7 patients with LAS had significantly increased glucose metabolism in the pontine tegmentum, mesencephalon, and ventrolateral thalamus.4 Zhang et al. presented 2 patients with LAS, and brain SPECT showed mild hypoperfusion in the left temporal lobe in one patient, and brain PET showed a mild bilateral decrease of glucose metabolism in the frontal lobes in the other patient.5 In our case patient, brain CT and brain MRI demonstrated no abnormalities, while brain SPECT revealed a mild hypoperfusion in the left temporal lobe.
The neurotransmitters related to LAS are known to be serotonin and gamma-aminobutyric acid (GABA). The loss of serotonin within the inferior olive nucleus has been thought to play a certain role6 and GABA may interact with the serotonin system to suppress posthypoxic myoclonus.7 The treatment of LAS has not been established and a combination of medications based on the neurotransmitters has been reported. Frucht and Fahn reviewed more than 100 patients with LAS and they found that clonazepam, valproate, and piracetam were effective in treating approximately 50% of the cases.8 Polesin and Stern recommended levetiracetam, zonisamide, clonazepam, and valproate as the first treatments of choice.9 In this case, the patient was treated with clonazepam and levetiracetam, which were effective in controlling the posthypoxic myoclonus.
Failure to recognize LAS may result in inappropriate anticonvulsant therapy and delayed treatment. Therefore, when a patient develops uncontrolled myoclonus after CPR and regaining consciousness, and the myoclonus is unsuccessfully treated with traditional anticonvulsants for a certain period, the possibility of LAS should be considered. This can lead to minimizing the patient's disabilities and improving the prognosis.
References
- 1.Lance JW, Adams RD. The syndrome of intention or action myoclonus as a sequel to hypoxic encephalopathy. Brain. 1963;86:111–136. doi: 10.1093/brain/86.1.111. [DOI] [PubMed] [Google Scholar]
- 2.English WA, Giffin NJ, Nolan JP. Myoclonus after cardiac arrest: pitfalls in diagnosis and prognosis. Anaesthesia. 2009;64:908–911. doi: 10.1111/j.1365-2044.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- 3.Werhahn KJ, Brown P, Thompson PD, Marsden CD. The clinical features and prognosis of chronic posthypoxic myoclonus. Mov Disord. 1997;12:216–220. doi: 10.1002/mds.870120212. [DOI] [PubMed] [Google Scholar]
- 4.Frucht SJ, Trost M, Ma Y, Eidelberg D. The metabolic topography of posthypoxic myoclonus. Neurology. 2004;62:1879–1881. doi: 10.1212/01.wnl.0000125336.05001.23. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YX, Liu JR, Jiang B, Liu HQ, Ding MP, Song SJ, Zhang BR, Zhang H, Xu B, Chen HH, et al. Lance-Adams syndrome : a report of two cases. J Zhejiang Univ Sci B. 2007;8:715–720. doi: 10.1631/jzus.2007.B0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh JP, Placantonakis DG, Warsetsky SI, Marquez RG, Bernstein L, Aicher SA. The serotonin hypothesis of myoclonus from the perspective of neuronal rhythmicity. Adv Neurol. 2002;89:307–329. [PubMed] [Google Scholar]
- 7.Matsumoto RR, Truong DD, Nguyen KD, Dang AT, Hoang TT, Vo PQ, Sandroni P. Involvement of GABA(A) receptors in myoclonus. Mov Disord. 2000;15(Suppl 1):47–52. doi: 10.1002/mds.870150709. [DOI] [PubMed] [Google Scholar]
- 8.Frucht S, Fahn S. The clinical spectrum of posthypoxic myoclonus. Mov Disord. 2000;15(Suppl 1):2–7. doi: 10.1002/mds.870150702. [DOI] [PubMed] [Google Scholar]
- 9.Polesin A, Stern M. Post-anoxic myoclonus: a case presentation and review of management in the rehabilitation setting. Brain Inj. 2006;20:213–217. doi: 10.1080/02699050500442972. [DOI] [PubMed] [Google Scholar]