Abstract
Extracorporeal filter cartridges, filled with activated carbon bead (ACB) adsorbent, have been used for removal of overdosed cancer drugs from the blood. Coatings on adsorbent matrices, poly (methyl methacrylate) (PMMA)/activated carbon bead and PMMA/chitosan/heparin/ACB composites, were tested to improve their biocompatibility and blood compatibility. PMMA coating on ACBs was accomplished in a straightforward manner using a PMMA solution in ethyl acetate. One-step hybrid coating of ACBs with PMMA-anticoagulant heparin required the use of acetone and water co-solvents. Multi-layer coatings with three components, PMMA, chitosan, and heparin involved three steps: PMMA was first coated on ACBs; chitosan was then coated on the PMMA coated surface; and finally heparin was covalently attached to the chitosan coating. Surface morphologies were studied by scanning electron microscopy. X-ray photoelectron spectroscopy confirmed −SO3− group. Adsorption, of a chemotherapy drug (doxorubicin) from both water and PBS, by the coated ACBs was examined. The adsorption isotherm curves were fitted using the Freundlich model. The current adsorption system might find potential applications in the removal of high dose regional chemotherapy drugs while maintaining high efficiency, biocompatibility, and blood compatibility.
Keywords: doxorubicin, drug removal, carbon beads, adsorption, heparin coating, blood compatibility
Introduction
Systemic drug delivery of potent anticancer agents exposes the entire body to toxic levels of these drugs. Localized and targeted chemotherapeutic drug delivery could potentially deliver significantly higher doses of toxic anti-cancer drugs, directly to a tumor where it is required, decreasing systemic toxicity, and improving drug efficacy and safety. Intra-arterial chemotherapy has recently been shown to result in remarkable clinical outcomes with minimal adverse effects as compared to systemic administration because of higher intratumoral concentrations of oncostatics.1–5 Increased antitumor effects are generally believed to correlate with higher dose intensity, but are also associated with severe toxicity.6 Doxorubicin (DOX) is a chemotherapy drug used in the treatment of a wide range of cancers including liver cancer. However, doxorubicin has significant side effects including, but not limited to, severe cardiac toxicity, damage to the immune system, acute nausea, severe vomiting, dermatological problems and hair loss. The most serious side effect of DOX, however, is irreversible heart tissue damage.7, 8 Activated-carbon filter cartridges have been used to adsorb toxic drugs removing them from the body, particularly in cases of overdose. Similarly, we envision activated-carbon filter cartridges might be used to efficiently remove the excess DOX after delivering it to a tumor site, such as the liver. This could be accomplished by isolating the liver from the systemic circulation, infusing DOX into the artery serving the liver, removing excess drug from the portal vein draining the liver using an extracorporeal filter cartridge, and returning blood free of DOX to the body. The major challenge of using such an approach is that currently available activated charcoal filters have a low first-pass adsorption capability, produce carbon debris, and activate the blood-clotting cascade and damage blood cells. Thus, there is an urgent need for filters with high adsorption efficiency, biocompatibility and blood compatibility.
We have previously reported that cellulose-heparin-coated charcoal is capable of adsorbing hydrophobic protein-bound drugs without causing large losses of blood proteins.9, 10 This cellulose-heparin-coated charcoal relied on a coating process utilizing a heparin imidazolium salt dissolved in a cellulose-containing room temperature ionic liquid and required extensive washing steps to remove the ionic liquid and convert the heparin imidazolium salt into the heparin sodium salt.
In the current study, PMMA, a biocompatible polymer,11 has been coated on the surface of ACBs as a potential adsorbent for extracorporeal filter cartridges. In addition, heparin was also incorporated in these coatings to improve blood compatibility by chemical conjugation (multi-layer coating containing PMMA, chitosan12 and heparin sodium) or physical blending (one-step hybrid coating of PMMA-heparin sodium). The heparin content, on the surface of coated ACBs, was determined by electron dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy. The chemical conjugation of heparin on the surface or ACBs also has potential advantage that heparin does not release into the medium, which avoids the formation of heparin-DOX complexes in solution. Adsorption of DOX by coated and uncoated ACBs was tested in both water and phosphate buffered saline (PBS, 10 mM sodium phosphate and 0.138 M sodium chloride at pH 7.4). The Freundlich model was used to study the adsorption isotherm.
Materials and Methods
Materials
Poly (methyl methacrylate) (PMMA, molecular weight of 120 kDa), doxorubicin hydrochloride, and high molecular weight chitosan (800 cps, Aldrich # 419419)) were purchased from Sigma-Aldrich (St. Louis, MO). All solvents were obtained from Fisher Scientific (Pittsburgh, PA). Heparin sodium salt (180 U/mg) from porcine intestinal was from Celsus Laboratories (Cincinnati, OH, USA). ACBs were obtained from Dr. Vladimir G. Nikolaev of the R. E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology NAS of Ukraine.
Characterization of ACBs
The bulk density and surface characteristics of the ACBs have been previously reported.4 The size distribution of the current batch of spherical ACBs was determined using optical microscopy to measure the diameter of 800 individual beads. Cross-sectional morphology was observed by imaging hemispherical beads prepared by cutting ACBs with a razor blade.
Coating of ACBs with PMMA
Dry ACBs (100 g) were added into a solution of PMMA in ethyl acetate (1200 mL of 2.5% w/v) and shaken in 2 L beaker for 6 h at 145 rpm. After coating the solution was decanted and the beads were dried under airflow in the hood for overnight after which they were further dried in a vacuum oven at 80°C.
One step coating of ACBs with PMMA-heparin blend layer
Heparin sodium (50 mg) was dissolved in water (2.5 mL) and then acetone (7.5 mL) was added to obtain a clear 0.5% (w/v) solution. PMMA was prepared in acetone as 5% (w/v) for 4 mL and then was added to 4 mL of the heparin solution slowly to avoid precipitation and final solution was stable and off-white. Two batches of beads (#1 and #2), weighing 2 g and 1 g, respectively, were immersed in 16 and 8 mL of the above PMMA-heparin solution respectively, and shaken at 180 rpm for 5 h. A total of six batches of ACBs were coated under a variety of different conditions.
Coating of ACBs with PMMA-chitosan
Chitosan was dissolved 0.25% (w/w) aqueous acetic acid solution at concentration of 120 mg/16 mL. Dry PMMA-coated ACBs (1.1 g) were added to chitosan solution (8 mL), and the resulting mixture was shaken in three batches for 1 h, 3 h and 5 h. After coating, the supernatant was decanted and the beads were filtered under vacuum through Millipore filter paper using Bucher funnel and rinsed with 0.1 N sodium hydroxide solution and further with water to remove residual sodium hydroxide. The PMMA-chitosan-coated beads were dried overnight in a vacuum oven at 80°C.
Immobilization of heparin on PMMA-chitosan-coated ACBs
The immobilization of heparin followed the literature with slight modification.13 Briefly, PMMA-chitosan-coated ACBs (0.3 g) were equilibrated in 2-(N-morpholino) ethanesulfonic acid (MES)-buffer (0.05 M, pH 5.6) for 1 h. Heparin solution (5 mL of 2% (w/w) in 0.05 M MES-buffer at pH 5.60) was activated by adding 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydoxysuccinimide (NHS) at a molar ratio of EDC:NHS:Heparin of 0.4:0.24:1.0. The pre-activation proceeded for 10 min after which 0.3 g of the buffer moistened PMMA-chitosan ACBs were added and shaken overnight at 180 rpm at room temperature. The beads were recovered by decanting and then washed for 24 h with three changes of water. The heparin-coated beads were dried overnight in vacuum oven at 40 °C.
Adsorption kinetics of doxorubicin
A 110 mg portion of either PMMA coated ACBs or PMMA-heparin one-step-coated ACBs were tested for their ability to adsorb 0.6 mg/mL of DOX in a 3 mL volume of water. A 0.1 mL aliquot was removed from the adsorption vial every 3 min, and added to 2.5 mL polypropylene centrifuge tube and brought to 0.9 mL with water. Water (0.1 mL) was added to the adsorption vial to maintain its volume. The corresponding concentrations were calculated using the appropriate dilution factor. The relative concentration was calculated as the ratio between concentration at any given time and the initial concentration. The relative concentration was then plotted as a function of time.
In the multi-layer coating a 110 mg portion of uncoated, PMMA-coated, PMMA-chitosan-coated and PMMA-chitosan-heparin-coated ACBs were tested using the similar procedure as used for the one-step coating. Two different solvent systems were examined, water and PBS.
Calibration curve for doxorubicin solution
A UV-Vis calibration curve was obtained by measuring absorption maximum at 480 nm and the baseline at 600 nm for five concentrations, 0.05, 0.025, 0.0125, 0.00625 and 0.003125 mg/mL in water. These five points were fit into a linear curve with R2 = 0.996. Solutions of concentration higher than 0.05 mg/mL were diluted into the range of the calibration curve to make determinations.
Adsorption isotherm
The determination of the adsorption isotherm was carried out in 2 mL doxorubicin solution in 2.2 mL polypropylene centrifuge vial, with 10 mg ACBs for each sample. The determination was performed for uncoated ACBs, PMMA-coated ACBs and PMMA-chitosan-heparin-coated ACBs. The initial concentration was in the range between 0.0126 – 4 mg/mL in both water and PBS. The solution was shaken (220 rpm) at room temperature (22°C) for 12 h. The adsorption capacity (q) versus equilibrium concentration (Ce) was plotted and fitted in to Freundilich isotherm equation.
Activated Partial Thromboplastin Time (aPTT)
ACBs or coated ACBs (250 mg) were gently mixed with 5 mL of human blood (citrated blood) on a rotator for 30 min. Plasma was separated by centrifugation at 1000 × g for 10 minutes, and aPTT was determined on an ACL8000 automated coagulation analyzer (Instrumentation Laboratory, Bedford, MA) using the SynthASil kit (Instrumentation Laboratory, Bedford, MA). Results were the average of three replicates for each type of beads. The aPTT activity of the heparin used in this study (at a concentration of 0.1 mg/ml) was 107 ± 3.0 s (s.d.).
Surface morphology characterization
Field emission-scanning electron microscopy (FE-SEM) was performed to study the surface morphology of fibers with a JEOL JSM-6335 FE-SEM (Tachikawa, Tokyo, Japan) equipped with a secondary electron detector at an accelerating voltage of 10 kV and at a working distance of 15 mm. Prior to performing the FE-SEM analysis, fibers were coated with gold by sputtering to form a conductive layer.
X-ray photoelectron spectroscopy (XPS)
XPS experiments were performed in an ultra high vacuum chamber using a hemispherical electron energy analyzer (HA100, VSW Ltd., UK) and a monochromatized Al X-ray source. The spectra were collected at a take-off angle of 45°. Survey spectra were acquired with pass energy of 117.4 eV. The binding energy scale was calibrated using the aliphatic component of the C 1s peak as 285.0 eV.
Results and discussions
Physical properties characterization of ACBs with and without coatings
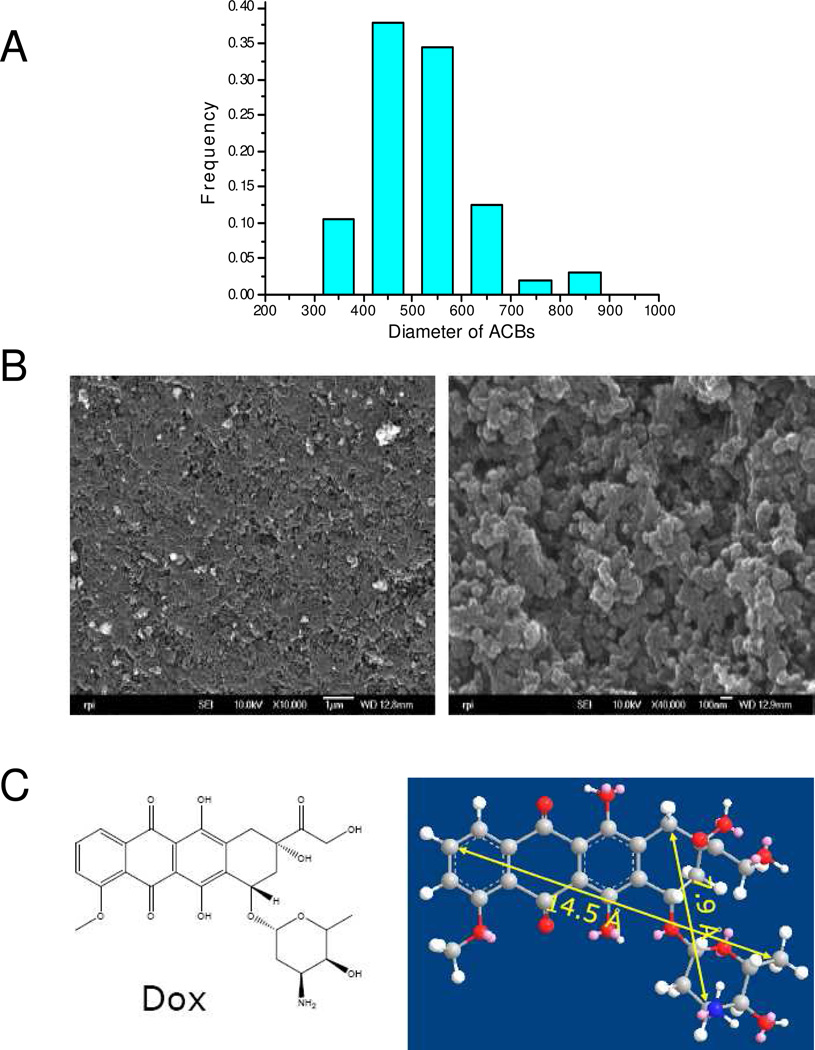
The ACBs used in this study were prepared by the pyrolysis spherical resin.4 The size distribution of the ACBs is shown in Figure 1A. The ACBs have and average diameter of 520 ± 102 µm (s.d.). The uncoated ACBs have a rough and porous surface, and cross-sectional images show matrices connected by nanoparticles (Figure 1B). The high porosity of both the surface and cross-section suggest the potential for a high adsorption rate.
Figure 1.
(A) Size distribution of uncoated ACBs based on 800 individual beads, (B) SEM images of uncoated ACBs, left image: surface, right image: cross-section, and (C) Chemical structure of DOX and its molecular dimensions determined by ChemDraw 3D.
In this study we hypothesized that ACBs having a porous coating would adsorb DOX with minimal activation of the coagulation cascade, if the coating did not change the pore size significantly. DOX has a molecular dimension of 0.79 × 1.45 nm, as measured by ChemDraw 3D (Figure 1C). The pore size, distribution, surface area and total pore volume of uncoated and coated ACBs were listed in Table 1 determined by BET isotherm. As we see, the most frequent pore size for all types of beads is large enough (>1.6 nm) to adsorb DOX molecules. The PMMA surface area did not change after coating, and following weight adjustment, compared to uncoated beads (2325 m2/g); however it decreased significantly after PMMA-chitosan and PMMA-chitosan-heparin coating following weight adjustment (2040 and 1462 m2/g respectively), and total pore volume also decreased accordingly, which could impact the adsorption capacity.
Table 1.
Physical properties of ACBs with and without coating by BET isotherm.
| Type of ACBs | Diameter (mm) | Surface area (m2/g) | Pore volume (mL/g) |
Most frequent Pore size (nm) |
||
|---|---|---|---|---|---|---|
| Non. | Ad. | Non. | Ad. | |||
| Uncoated beads | 520 ± 102 | 2325 ± 31 | 2325 ± 31 | 2.72 ± 0.13 | 2.72 ± 0.13 | 1.6 and 2.5–2.6 |
| PMMA coated beads | 520 ± 101 | 2070 ± 26 | 2325 ± 29 | 2.37 ± 0.14 | 2.72 ± 0.16 | 1.6 and 2.5–2.6 |
| PMMA-Chitosan coated beads | 520 ± 102 | 1632 ± 21 | 2040 ± 27 | 1.88 ± 0.11 | 2.35 ± 0.14 | 1.8 and 2.5–2.6 |
| PMMA-Chitosan-heparin coated beads | 520 ± 102 | 1140 ± 15 | 1462 ± 19 | 1.47 ± 0.11 | 1.88 ± 0.14 | 1.6 and 2.5–2.6 |
Avg.: Average; Non.: None adjustment; Ad.: Adjustment
The adsorption of DOX on one-step coated ACBs
Our initial attempts to coat PMMA/heparin blend layer on the ACBs surface was inspired based on our previous work,10 and a combination of two solvents, water and acetone, were selected to dissolve both PMMA and heparin sodium at certain ratios without precipitation. The surface distribution of heparin on coated beads was acquired by SEM-EDX; however, the loading of heparin on unit mass of beads was obtained by other way. First the heparin coated on the ACBs was extracted with water for overnight and then the amount of heparin in supernatant was determined in triplicate by carbazole assay.14 As shown in Table 2, batch #1 had much higher heparin loading compared to batch #2. This high heparin loading was also confirmed in triplicate by SEM-EDX analysis (converting the obtained sulfo content to heparin content).
Table 2.
Heparin content on the surface of coated ACBs as analyzed by carbazole and EDX.
| Coated batch | mg heparin/g beads by carbazole | % heparin on beads surface by EDX |
|---|---|---|
| #1 | 1.9+/−0.2 | 7.0%+/−0.2% |
| #2 | 0.34+/−0.03 | 1.2%+/−0.1% |
| PMMA only | 0 | 0 |
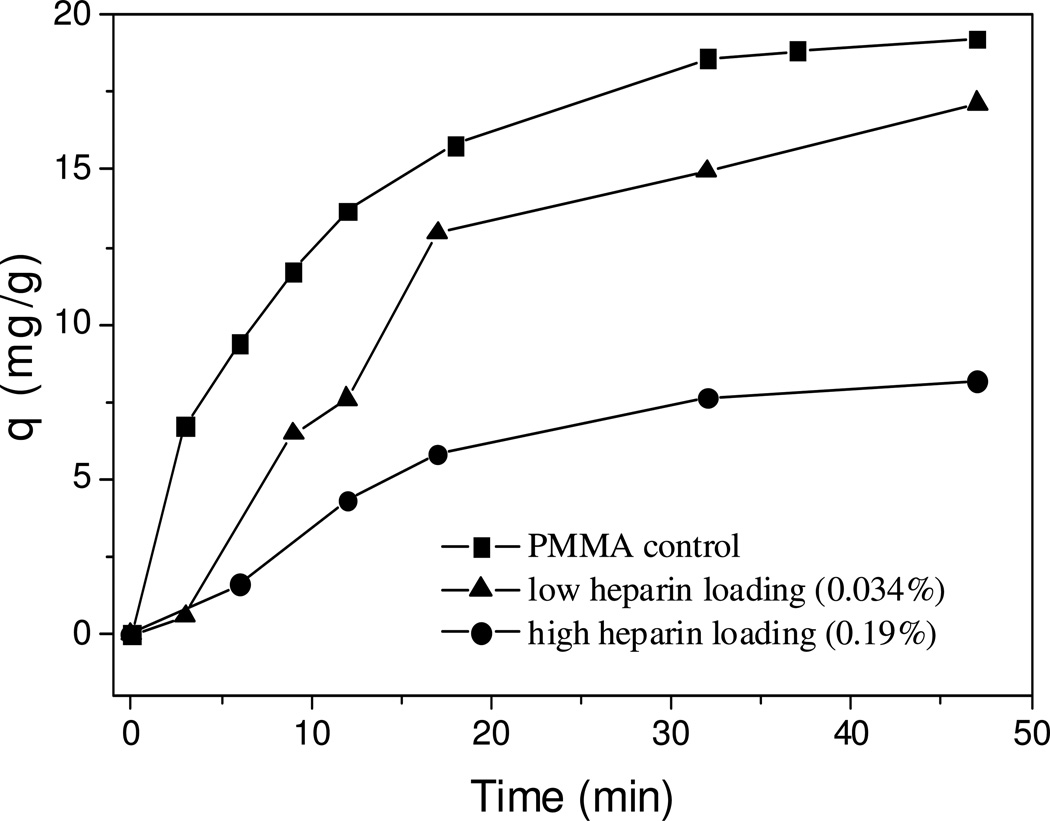
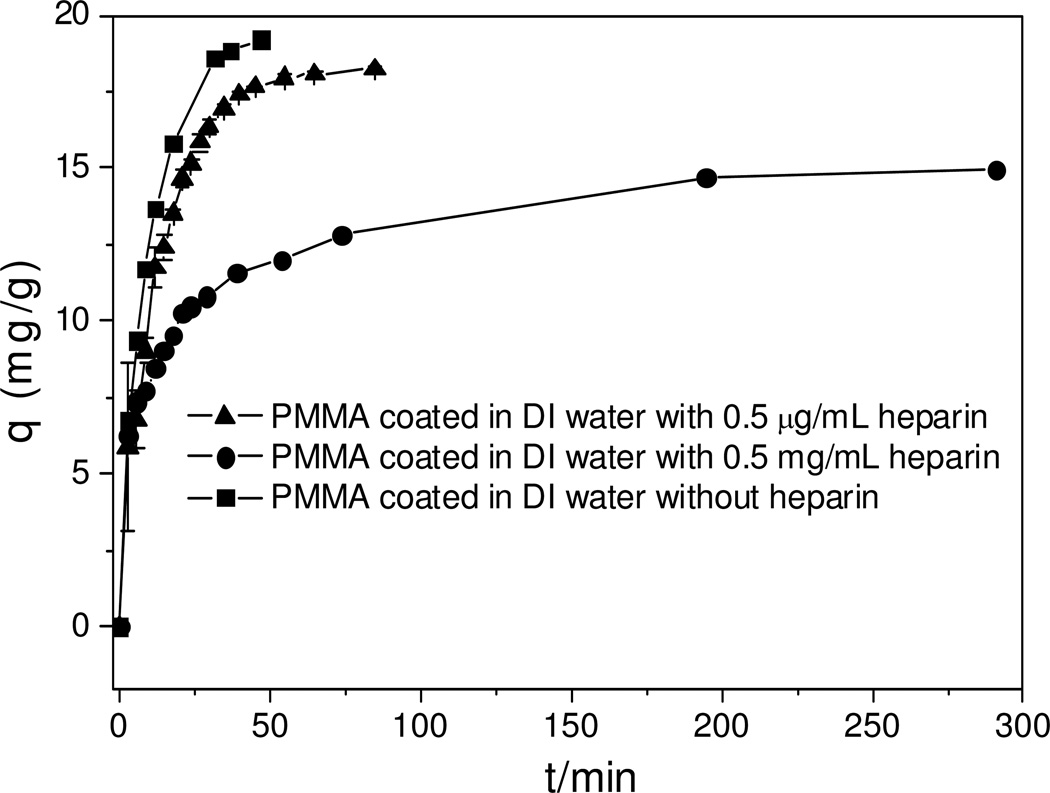
The adsorption kinetics curves for one-step blend-coating are shown in Figure 2. Surprisingly, compared to PMMA only coated ACBs, at higher loadings of heparin on ACBs (batch #1, 0.19%), much lower adsorption rate (0.25 mg•g−1 •min−1) and equilibrium adsorption capacity (8.2 mg•g−1) were observed. These reductions were also found in lower heparin loading (batch #2, 0.034%) with less significance. The coating of heparin may have adverse effect on the pore size and surface area of ACBs, however from our previous work;10 it does not have such a significant effect on the adsorption of drug. Considering the slow releasing of blend-coated heparin in aqueous solution (PMMA does not release in aqueous solution), the free heparin might have some interaction with DOX in the adsorption solution. Control experiments were designed and performed to address this issue. If batch #1 releases all heparin content in 3 mL adsorption solution, the concentration of heparin will be ~0.07 mg•mL−1, so two control adsorption experiments were tested at heparin concentrations of 0.5 µg•mL−1 and 0.5 mg•mL−1 in water. The adsorption kinetics curves obtained are shown in Figure 3.
Figure 2.
Kinetic curves for adsorption of DOX on beads with different coatings (adsorption performed in water, initial concentration 0.6 mg/mL).
Figure 3.
Heparin concentration effects on adsorption kinetics (initial concentration of DOX 0.6 mg/mL).
Initially, the adsorption rate for three conditions was similar, but equilibrium adsorption capacity was totally different. With 0.5 mg•mL−1 heparin in solution, the equilibrium adsorption capacity is around 15 mg•g−1 and it took much longer time to reach equilibrium compared to both solution with 0.5 µg•mL−1 heparin and without heparin. Lower heparin content had little effect on the equilibrium adsorption capacity, 18.2 versus 19.2 mg•g−1 (without heparin).
It has been reported that one molecule of heparin can potentially complex with up to 16 molecules of DOX in an electrostatic interaction and such complex reduces the cytotoxicity of DOX.15 The effect of heparin concentration on free DOX in aqueous solution was also reported.16 Based on an average molecular weight of heparin (12000 Da), the maximum molecular weight of heparin-DOX complex can be calculated to be 20696 Da. Such a large macromolecular complex would undoubtedly reduce the adsorption rate and the adsorption capacity for one-step hybrid coated ACBs.
Covalent attachment, instead of blending of heparin, on the PMMA coating is essential to reduce or eliminate the release of free heparin from the coating, improving adsorption efficiency. Unfortunately, PMMA has no available functional groups that can react directly with heparin. Chitosan, an established biocompatible matrix,17 was coated as a second layer over of PMMA, as its available amino groups could then be reacted with heparin.
Adsorption kinetics of multi-layer-coated ACBs and solvent effects
PBS solution was used to measure adsorption of DOX to mimic the pH conditions in human blood.18 PBS was also expected to reduce the electrostatic interaction between DOX and free heparin.
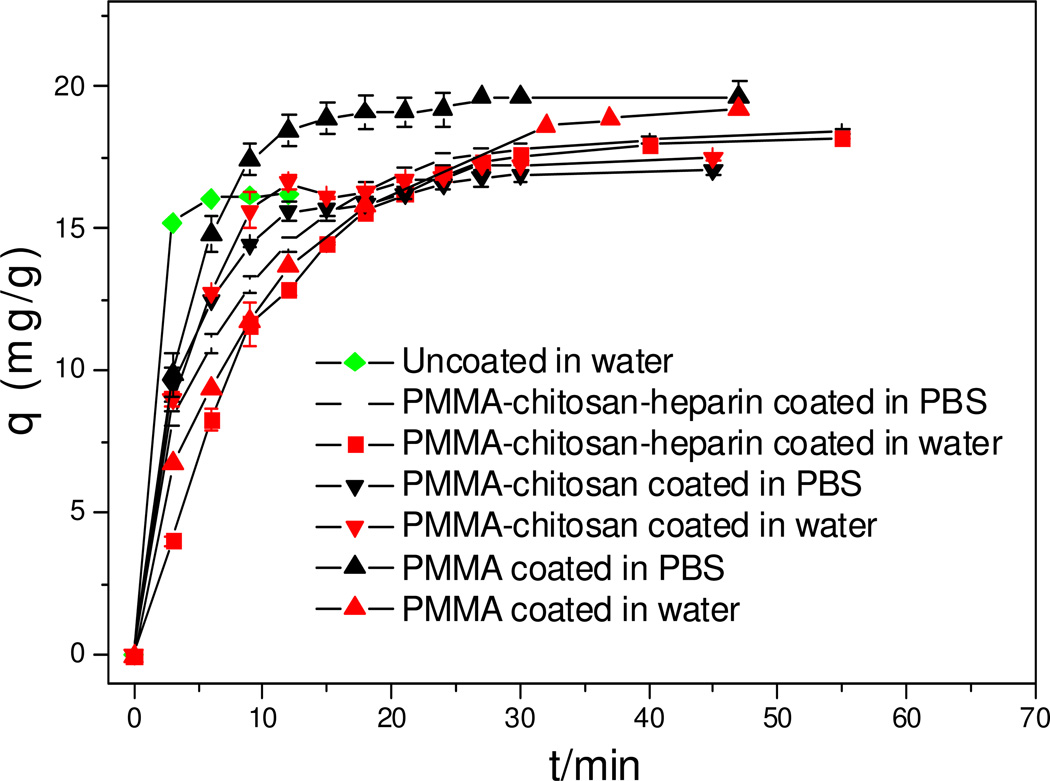
Doxorubicin adsorption was next measured in both water and PBS for all three types of coatings and in water only for the uncoated beads (Figure 4). The adsorption capacity has been adjusted according to the weight fraction of carbon beads. All beads with or without coating showed an initial fast adsorption and then equilibrium. The time to reach equilibrium and adsorption capacities at equilibrium are listed in Table 3.
Figure 4.
DOX adsorption kinetics for multi components coated beads (initial concentration of DOX 0.6 mg/mL).
Table 3.
The effects of coating types and solvent types on adsorption rate and adsorption capacity.
| Type of coatings |
Uncoated beads |
PMMA- coated beads |
PMMA- chitosan- coated beads |
PMMA- chitosan- heparin-coated beads |
|
|---|---|---|---|---|---|
| Type of solvents | |||||
| In PBS |
Time to reach equilibrium (min) |
N/A | 18 | 30 | 30 |
| Equilibrium capacity (mg/g) |
N/A | 19.6 | 17.1 | 18.2 | |
| In water |
Time to reach equilibrium (min) |
6 | 47 | 30 | 30 |
| Equilibrium capacity (mg/g) |
16.1 | 19.1 | 17.5 | 18.1 | |
The uncoated beads reached equilibrium most quickly in water compared to all three types of coatings; however, its equilibrium adsorption capacity was lowest. This can be explained as it is still too far away to reach saturation on uncoated beads and a lower fraction of total available adsorption sites are used compared to coated beads. PMMA-chitosan coated and PMMA-chitosan-heparin coated beads show similar adsorption behavior both in water and in PBS, and it took almost same amount of time for both to reach equilibrium both in water and in PBS. The adsorption capacity at equilibrium for PMMA-chitosan-heparin coated beads was slightly higher than that of PMMA-chitosan coated beads, which might be attributed to formation of heparin-DOX complex on the surface of PMMA-chitosan-heparin coated beads. In terms of PMMA coated beads, the types of solvent used do have a significant effect on the adsorption rate but not on equilibrium adsorption capacity. As we can see, these beads quickly reach equilibrium in PBS (18 min); however they took 47 min to reach equilibrium in water. Since PMMA coated beads have a hydrophobic surface, which might repel a hydrophilic drug, DOX may be prevented from quickly passing through the coating and reaching the adsorption sites deep within the beads. Negatively charged phosphate ion may bind 2–3 DOX hydrochloride molecules and allow the hydrophilic portion of the drug to stay together inside and hydrophobic portion of drug to stay outside. Thus, DOX would quickly pass through hydrophobic coating layer and reach the adsorption sites of beads and be adsorbed.
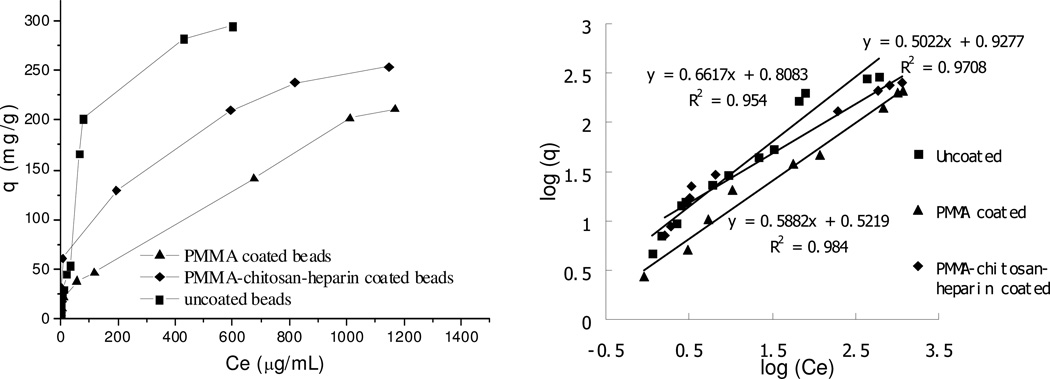
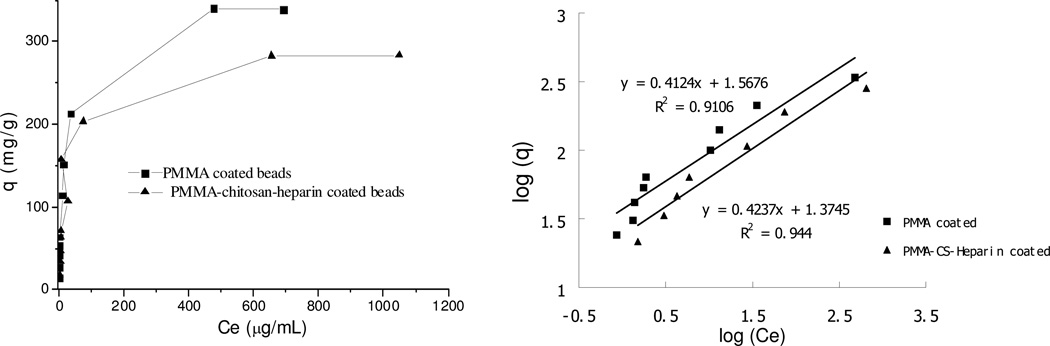
The adsorption isotherm of DOX on multi-layer-coated ACBs
The adsorption isotherm studies the relationship between equilibrium adsorption capacity and equilibrium concentration at same temperature. Both water and PBS was used to obtain the maximum equilibrium adsorption capacity, up to 4 mg/mL DOX. However, it was found that at very high DOX concentration in PBS, uncoated beads promoted the precipitation of DOX and resulted in incorrect concentration of DOX in supernatant. So no adsorption isotherm data for DOX in PBS are presented in current study. Two models are typically used for studies of adsorption, the Langmuir isotherm and Freundlich isotherm. The Langmuir isotherm is valid for monolayer adsorption on a surface with a finite number of identical sites, so it is not suitable for the current study since the R2 values are less than 90%. The Freundlich isotherm may be better to describe the current adsorption based on the heterogeneous surface of ACBs having different affinities. Its equation is as follows:19
| (1) |
where q is the amount of doxorubicin (mg) adsorbed per unit mass of the beads (g), Ce is the equilibrium concentration (µg/mL), KF and n are Freundlich constants related to adsorption capacity at equilibrium concentration of 1 µg/mL and adsorption intensity of the adsorbent.
The fitted curves are shown in Figure 5 and 6, and the Freundlich constants and R2 are listed in Table 4 and 5 for water and PBS, respectively. It was found that both PMMA coated and PMMA-chitosan-heparin coated beads have significantly higher KF value and n value in PBS compared to those in water. Although the KF value in PBS for PMMA coated beads was much higher than that of PMMA-chitosan-heparin coated beads due to a great difference in surface area, in water the opposite effects on the KF value were observed due to a higher hydrophobicity of PMMA coating. These observations confirmed the effects of hydrophobicity of coating and solvent types on adsorption capacity.
Figure 5.
Adsorption isotherm (left) and linear fitting (right) on beads with different coatings in water. (~10 mg in 2 mL solution for all types of beads, equilibrium time: 12 h)
Figure 6.
Adsorption isotherm (left) and linear fitting (right) on beads with different coatings in PBS. (~10 mg in 2 mL solution for all types of beads, equilibrium time: 12 h)
Table 4.
Freundilich isotherm model constants and correlation coefficients for adsorption of DOX on beads with and without coating at 25° C in water.
| Beads | KF | n | R2 |
|---|---|---|---|
| Uncoated | 6.4 | 1.5 | 0.95 |
| PMMA coated | 3.3 | 1.7 | 0.98 |
| PMMA-chitosan-heparin coated | 8.5 | 2 | 0.97 |
Table 5.
Freundilich isotherm model constants and correlation coefficients for adsorption of DOX on beads with and without coating at 25° C in PBS.
| Beads | KF | n | R2 |
|---|---|---|---|
| PMMA coated | 37 | 2.4 | 0.91 |
| PMMA-chitosan-heparin coated | 24 | 2.4 | 0.94 |
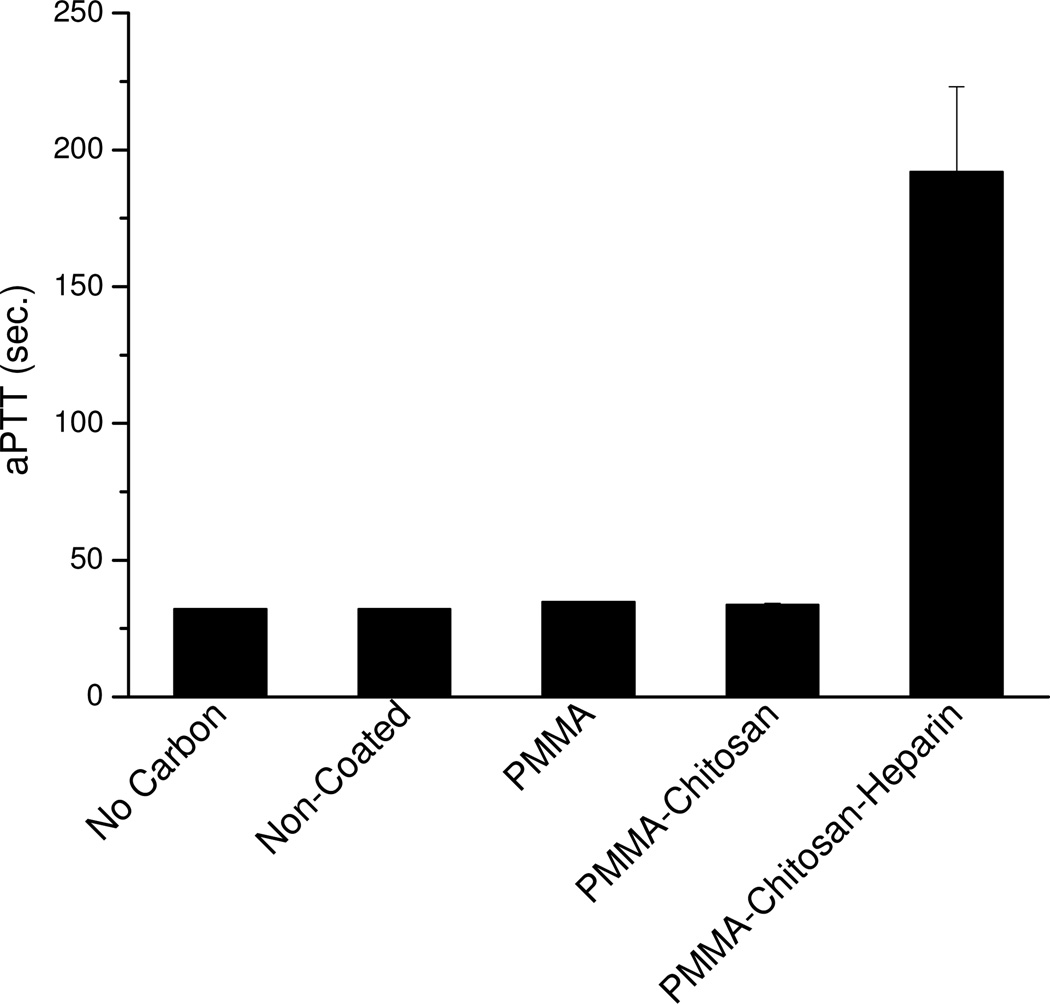
Blood compatibility of ACBs with different coatings
Activated partial thromboplastin time (aPTT), an indication of blood compatibility, was performed to measure anticoagulant activity of PMMA-chitosan-heparin coated beads as well as other coated beads in human plasma (Figure 7). It was found that both PMMA and PMMA-chitosan coated beads showed similar aPTT value to uncoated beads (32–35 s); however, PMMA-chitosan-heparin coated beads showed significantly higher aPTT value (192 ± 31 s) than did other coated beads, which is almost double of control heparin at 0.1 mg/mL and further confirmed that the covalently bonded heparin on the surface of PMMA-chitosan-heparin coated beads did not lose anticoagulation activity.
Figure 7.
aPTT results for four different types of beads and a control without beads.
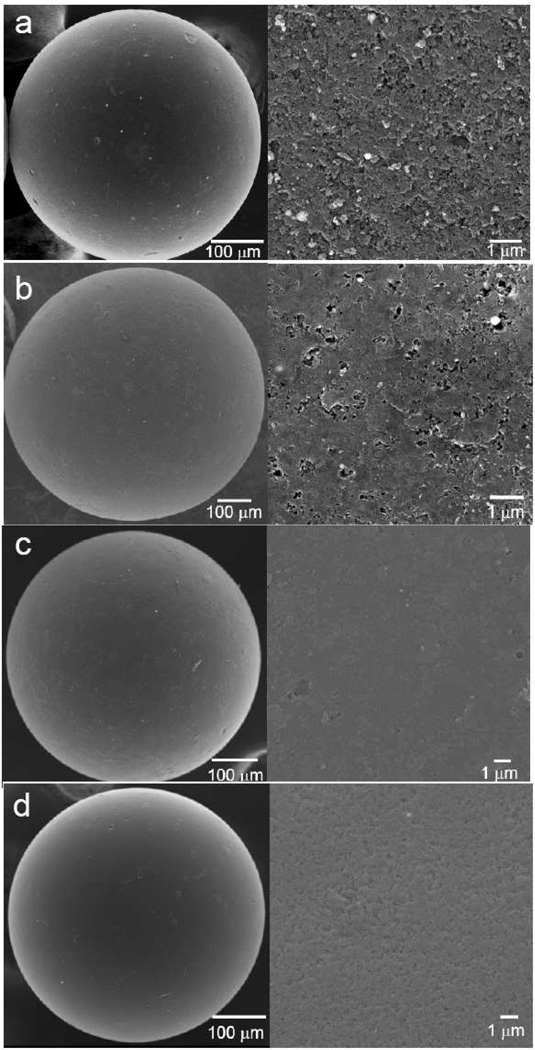
The surface morphologies of ACBs with different coatings
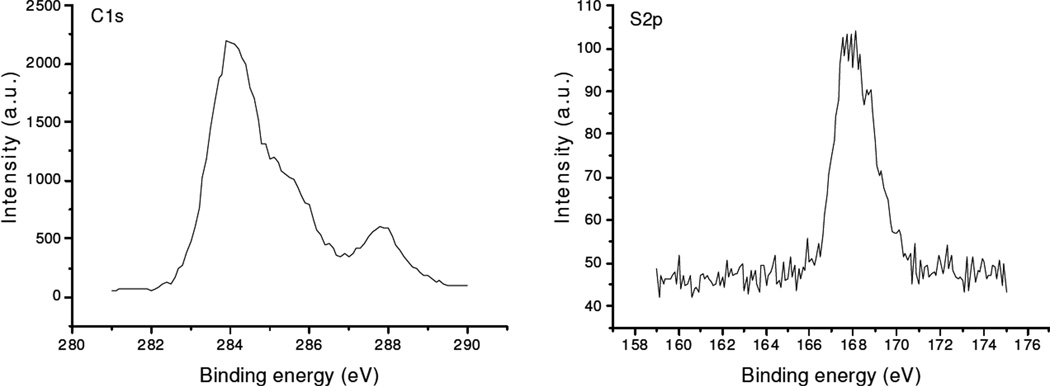
The surface morphologies of beads with and without coating(s) were obtained by FESEM (Figure 8). The surface of uncoated beads has a rough and highly porous structure with micron and submicron pore size. The internal bead structure is composed by aggregation of submicron particles seen in a cross-section of a bead (Figure 2, right). The surface showed more smoothness than that of uncoated beads with large amount of available submicron-sized pore on the beads surface after coating with PMMA. PMMA-chitosan beads were much smoother than either uncoated or PMMA coated beads. PMMA-chitosan-heparin coated beads have a relatively coarse surface compared to PMMA-chitosan coated beads and showed a large number of available nano-sized pores for adsorption of DOX. Surface elemental analysis by XPS (Figure 9) showed signal from sulfo groups and ester groups (carbon signal), confirming the presence of the immobilized heparin on the PMMA-chitosan layer.
Figure 8.
FESEM images of (a) uncoated beads, (b) PMMA coated beads, (c) PMMA-chitosan coated beads, (d) PMMA-chitosan-heparin coated beads. Left panel with low magnification, right panel with high magnification.
Figure 9.
XPS on the PMMA-chitosan-heparin coated beads, left C1s signal right S2p signal.
Conclusions
PMMA coated ACBs and PMMA-chitosan-heparin coated ACBs were prepared to improve biocompatibility and blood compatibility of these supports. One-step hybrid coating of PMMA-heparin was also performed in an acetone-water co-solvent. Increasing heparin loading on the PMMA-heparin coated beads significantly decreased the adsorption rate and equilibrium adsorption capacity. A three-layered coating consisting of PMMA, chitosan and covalently conjugated heparin was used to reduce the release of heparin from the coating. The adsorption kinetics indicated that solvents (water and PBS) had little effect on the adsorption rate and equilibrium adsorption capacity for both PMMA-chitosan and PMMA-chitosan-heparin coated beads, but had significant effect on the adsorption rate for PMMA coated beads. The Freundlich model was used to study the adsorption isotherm and indicated the significant solvent effects on maximum adsorption capacity for both PMMA and PMMA-chitosan-heparin coated beads.
A great concern of physicians using systematic drug removal systems is the sight of carbon debris leaving the filter during the procedure since these carbon fragments can lodge in small arterioles and capillaries of the lung and mimic a pulmonary embolism. Filters previously used in clinical trials exhibited low first-pass adsorption capability, produced carbon debris, and activated the blood-clotting cascade. The current adsorption system should alleviate these problems and might find potential applications in the removal of high dose regional chemotherapy drugs while maintaining high efficiency, biocompatibility, and blood compatibility.
Acknowledgement
The authors thank the financial support from JNC Corporation and the National Institutes of Health in the form of Grant # 1R41CA153973-01 entitled “Enabling high dose regional chemotherapy while minimizing systemic toxicity”
References and Notes
- 1.Eckman WW, Patlak CS, Fensterm Jd. Critical Evaluation of Principles Governing Advantages of Intra-Arterial Infusions. J. Pharmacokinet. Biopharm. 1974;2(3):257–285. doi: 10.1007/BF01059765. [DOI] [PubMed] [Google Scholar]
- 2.Vermorken JB. The Role of Chemotherapy in Squamous-Cell Carcinoma of the Uterine Cervix - a Review. Int. J. Gynecol. Cancer. 1993;3(3):129–142. doi: 10.1046/j.1525-1438.1993.03030129.x. [DOI] [PubMed] [Google Scholar]
- 3.Kusunoki N, Ku Y, Tominaga M, Iwasaki T, Fukumoto T, Muramatsu S, Sugimoto T, Tsuchida S, Takamatsu M, Suzuki Y, Kuroda Y. Effect of sodium thiosulfate on cisplatin removal with complete hepatic venous isolation and extracorporeal charcoal hemoperfusion: A pharmacokinetic evaluation. Ann. Surg. Oncol. 2001;8(5):449–457. doi: 10.1007/s10434-001-0449-y. [DOI] [PubMed] [Google Scholar]
- 4.Tominaga M, Ku Y, Iwasaki T, Suzuki Y, Kuroda Y, Saitoh Y. Pharmacological evaluation of portal venous isolation and charcoal haemoperfusion for high-dose intra-arterial chemotherapy of the pancreas. Br. J. Sur. 1997;84(8):1072–1076. [PubMed] [Google Scholar]
- 5.Jones A, Alexander HR. Development of isolated hepatic perfusion for patients who have unresectable hepatic malignancies. Surg. Oncol. Clin. N. Am. 2008;17(4):857–876. doi: 10.1016/j.soc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Maruo T, Motoyama S, Hamana S, Yoshida S, Ohara N, Yamasaki M, Ku Y. Percutaneous pelvic perfusion with extracorporeal chemofiltration for advanced uterine cervical carcinoma. Surg. Oncol. Clin. N. Am. 2008;17(4):843–856. doi: 10.1016/j.soc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009;16(25):3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 8.Samuel M, Chow PKH, Shih-Yen EC, Machin D, Soo KC. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst. Rev. 2009;(1) doi: 10.1002/14651858.CD001199.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park TJ, Govindaiah P, Hwang T, Kim E, Choi SW, Kim JH. Biocompatible charcoal composites prepared by ionic liquids for drug detoxification. Macromol. Res. 2011;19(7):734–738. [Google Scholar]
- 10.Park TJ, Lee SH, Simmons TJ, Martin JG, Mousa SA, Snezhkova EA, Sarnatskaya VV, Nikolaev VG, Linhardt RJ. Heparin-cellulose-charcoal composites for drug detoxification prepared using room temperature ionic liquids. Chem. Commun. 2008;(40):5022–5024. doi: 10.1039/b809791g. [DOI] [PubMed] [Google Scholar]
- 11.Jager M, Wilke A. Comprehensive biocompatibility testing of a new PMMA-HA bone cement versus conventional PMMA cement in vitro. J. Biomater. Sci. Polym. Ed. 2003;14(11):1283–1298. doi: 10.1163/156856203322553491. [DOI] [PubMed] [Google Scholar]
- 12.Lim CK, Yaacob NS, Ismail Z, Halim AS. In vitro biocompatibility of chitosan porous skin regenerating templates (PSRTs) using primary human skin keratinocytes. Toxicol. in Vitro. 2010;24(3):721–727. doi: 10.1016/j.tiv.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Wissink MJB, Beernink R, Pieper JS, Poot AA, Engbers GHM, Beugeling T, van Aken WG, Feijen J. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials. 2001;22(2):151–163. doi: 10.1016/s0142-9612(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 14.Han JY, Zhang FM, Xie J, Linhardt RJ, Hiebert LM. Changes in cultured endothelial cell glycosaminoglycans under hyperglycemic conditions and the effect of insulin and heparin. Cardiovasc. Diabetol. 2009;8:46. doi: 10.1186/1475-2840-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuno Y, Hara T, Tachibana S, Uragoh K, Akazawa K, Ueda K. Doxorubicin-Heparin Complex - Reduction of Cardiotoxicity of Doxorubicin. J. Cancer Res. Clin. Oncol. 1995;121(8):469–473. doi: 10.1007/BF01218363. [DOI] [PubMed] [Google Scholar]
- 16.Kummerle A, Krueger T, Dusmet M, Vallet C, Pan Y, Ris HB, Decosterd LA. A validated assay for measuring doxorubicin in biological fluids and tissues in an isolated lung perfusion model: matrix effect and heparin interference strongly influence doxorubicin measurements. J. Pharm. Biomed. Anal. 2003;33(3):475–494. doi: 10.1016/s0731-7085(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 17.VandeVord PJ, Matthew HWT, DeSilva SP, Mayton L, Wu B, Wooley PH. Evaluation of the biocompatibility of a chitosan scaffold in mice. J. Biomed. Mater. Res. 2002;59(3):585–590. doi: 10.1002/jbm.1270. [DOI] [PubMed] [Google Scholar]
- 18.Waugh A, Grant A. Anatomy ans Physiology in Health and Illness (Tenth ed.) Churchill Livingstone Elsevier; 2007. p. 22. [Google Scholar]
- 19.Chen Z, Pierre D, He H, Tan SH, Chuong PH, Hong H, Huang JL. Adsorption behavior of epirubicin hydrochloride on carboxylated carbon nanotubes. Int. J. Pharm. 2011;405(1–2):153–161. doi: 10.1016/j.ijpharm.2010.11.034. [DOI] [PubMed] [Google Scholar]