Abstract
Processing speed deficits affect reading efficiency, even among individuals who recognize and decode words accurately. Children with ADHD who decode words accurately can still have inefficient reading fluency, leading to a bottleneck in other cognitive processes. This “slowing” in ADHD is associated with deficits in fundamental components of executive function underlying processing speed, including response selection. The purpose of the present study was to deconstruct processing speed in order to determine which components of executive control best explain the “processing” speed deficits related to reading fluency in ADHD. Participants (41 ADHD, 21 controls), ages 9-14, screened for language disorders, word reading deficits, and psychiatric disorders, were administered measures of copying speed, processing speed, reading fluency, working memory, reaction time, inhibition, and auditory attention span. Compared to controls, children with ADHD showed reduced oral and silent reading fluency, and reduced processing speed—driven primarily by deficits on WISC-IV Coding. In contrast, groups did not differ on copying speed. After controlling for copying speed, sex, severity of ADHD-related symptomatology, and GAI, slowed “processing” speed (i.e., Coding) was significantly associated with verbal span and measures of working memory, but not with measures of response control/inhibition, lexical retrieval speed, reaction time, or intra-subject variability. Further, “processing” speed (i.e., Coding, residualized for copying speed) and working memory were significant predictors of oral reading fluency. Abnormalities in working memory and response selection (which are frontally-mediated and enter into the output side of processing speed) may play an important role in deficits in reading fluency in ADHD, potentially more than posteriorally-mediated problems with orienting of attention or perceiving the stimulus.
Keywords: Reading, Attention, Child, Dyslexia, Fluency, Working Memory, Executive Function
Processing Speed and ADHD
Slowed processing speed (PS) has been described as a sensitive but not specific characteristic of a broad range of disorders common to childhood (Willcutt, Sonuga-Barke, Nigg, & Sergeant, 2008). Processing speed is typically defined as speed of completion of a task with reasonable accuracy; PS measures include such disparate tasks as quickly associating numbers with symbols (e.g., Coding from the WISC), searching for and responding to specific targets, and rapid naming of visual stimuli (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005). Children with Attention-Deficit/Hyperactivity Disorder (ADHD) have been shown to demonstrate slowed PS, relative to typically developing peers, across a wide variety of such tasks including: 1) graphomotor speed, as measured by WISC-IV Processing Speed subtests (Chhabildas, 2001; Hinshaw, 2002; Rucklidge & Tannock, 2002; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005) or by Trailmaking tests (Shanahan, 2006); 2) naming speed, as measured by rapid automatized naming (RAN) tasks (Rucklidge & Tannock, 2002) or tasks such as Stroop color naming or word reading (Shanahan, 2006; Willcutt, 2010) and, 3) reaction time on continuous performance or go-no go tasks (Rucklidge & Tannock, 2002; Shanahan, 2006; Wodka, Mahone, et al., 2007).
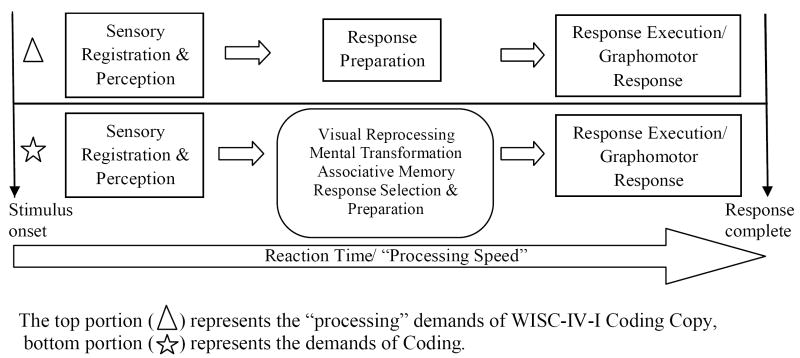
Interpretation of ADHD-related deficits on these measures of processing speed is complicated by the observation that children with ADHD are also slower than controls on measures of skelemotor (Cole, Mostofsky, Larson, Denckla, & Mahone, 2008) and oculomotor speed (Mahone, Mostofsky, Lasker, Zee, & Denckla, 2009). These more “basic” deficits in motor speed among children with ADHD likely contribute to the overall slowing observed on a variety of timed tasks, although perhaps more toward the output (as opposed to perceptual) side of processing speed for ADHD (Sergeant, 2005). Thus, since nearly all processing speed measures rely on some form of motor output, it remains unclear to what extent the slowed performance in children with ADHD is a function of inefficient (slow) motor control, rather than slowed “processing” of information at the level of response selection and preparation, prior to execution of the motor response. The PS deficits associated with ADHD have been described as occurring not at the level of “orienting to” or “perceiving” a stimulus, which is related to posterior brain systems, but rather between sensation/perception and action, and involve a state of preparedness to respond, including selection of an appropriate response, which is related to premotor and prefrontal circuits (Denckla, 1996; Kelly, Margulies, & Castellanos, 2007; Mostofsky & Simmonds, 2008; Simmonds, et al., 2007). Thus, when studying ADHD-related PS deficits, it is important to clarify the contributions of both motor and non-motor components. Figure 1 illustrates the potential components of PS—defined as the time between stimulus onset and response completion (e.g., a motor response), including sensory registration and perception of the stimulus, response selection and preparation, and response execution. Reaction time from stimulus onset to completed response thus reflects a chain of events that can be deconstructed, allowing better identification of the executive control components of processing speed that are separable from sensory registration/perception and motor output. The most common measure of PS in children is the WISC-IV Processing Speed Index, which is comprised of tests examining all of the components of this process from sensory registration to the motor response.
Figure 1.
Components of “processing speed” or reaction time between stimulus onset and response completion.
Processing speed is thus a promising candidate for a neuropsychological deficit in ADHD that can contribute uniquely to reading difficulties (Denckla & Cutting, 1999; Rucklidge & Tannock, 2002; Willcutt, 2005), particularly as it may influence efficiency of reading fluency among those who can read single words accurately. The effects of PS on reading fluency can subsequently affect further development of more complex academic skills such as reading comprehension. Indeed, Wolf and colleagues (Wolf & Katzir-Cohen, 2001) proposed a definition of reading fluency that incorporates comprehension: “reading fluency refers to a level of accuracy and rate at which decoding is relatively effortless; at which oral reading is smooth and accurate, with correct prosody; and at which attention can be allocated to comprehension” (p.219). In this definition, comprehension is dependent on automaticity. As reading becomes more automatized, less mental effort and attentional resources are required for ongoing decoding and accurate word reading; thus, these resources can be allocated to the task of translating text into meaning. Children with ADHD, even those without comorbid reading or language disorders, often show weaker reading fluency compared to controls (Ghelani, Sidhu, Jain, & Tannock, 2004; Willcutt, Pennington, Olson, & DeFries, 2007). Further, children with ADHD and children with globally-defined reading difficulties both demonstrate a common pattern of deficits in both PS and working memory—although perhaps for different reasons (Willcutt, 2005). Recently, Willcutt et al. (Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005) recommended additional research to clarify the taxonomy of processing speed tasks, by examining relationships among measures and by contrasting performance on these measures between clinical groups. At this point, however, much remains unknown about the specific influence of ADHD on reading fluency and comprehension among children without deficits in single word reading or decoding, since most studies of ADHD do not control for the high degree of overlap between ADHD and word reading deficits. There is emerging evidence, though, that higher level reading difficulties among children with adequate word recognition are linked to executive dysfunction (Cutting, 2009; Locascio, in press; Sesma, Mahone, Levine, Eason, & Cutting, 2009). To this end, the present study addresses these issues by dissociating the response preparation components of PS from the graphomotor output components, and examining the contribution of each to reading fluency.
Summary
The present study examined the performance of children with ADHD and typically developing controls across the tasks of the WISC-IV Processing Speed Index (PSI), dissociating the perceptual, response preparation, and graphomotor output components of PS, and exploring the contribution of executive control components to prediction of reading fluency. We hypothesized that children with ADHD without comorbid reading disorders would show deficits in PS, as measured by the WISC-IV PSI, and would demonstrate difficulty with the both the executive and graphomotor output components of PS. Further, we hypothesized that the cognitive (executive) control component of PS would be associated with ADHD-related decrements in reading fluency.
Method
Participants
Following approval from the Johns Hopkins Medicine Institutional Review Board, participants were recruited from outpatient clinics at the Kennedy Krieger Institute and from local area pediatricians, local chapters of Children and Adults with ADHD (CHADD), schools, social/service organizations (e.g., Boy/Girl Scouts), and advertisements in the community (e.g., postings at libraries). The sample included 62 children (61.3% male), of whom 41 met criteria for a diagnosis of ADHD. This study is part of a larger project examining brain-behavior relationships in children; therefore, participants were screened for comorbidities commonly associated in ADHD (details below). All participants and their parents signed a consent form that met Institutional Review Board standards. Participants ranged in age from 9 to 14 years (M = 11.13, SD = 1.49). The majority (71.0%) were Caucasian, 21.0% were African-American, 3.2% were multiracial, 3.2% Asian, and 1.6% Pacific Islander. Two participants (3.2%) were of Hispanic ethnicity.
Children included in the study had intellectual ability scores of 80 or higher on the General Ability Index (GAI) of the Wechsler Intelligence Scale for Children-IV-Integrated (Wechsler, 2004). Screening criteria were similar for both groups. Children were excluded if they were identified with a history of speech/language disorder or word reading difficulties, either through telephone screening before the initial visit, or based on prior school assessment (completed within one year). Further exclusion criteria included evidence of visual or hearing impairment, history of other neurological disorder, psychotropic medication use other than stimulants in the ADHD group, or comorbid diagnoses in children with ADHD other than Oppositional Defiant Disorder or Specific Phobia. Demographic information, school, and developmental histories were obtained through telephone screenings with parents of participants. Children with ADHD who were taking stimulant medication were removed from the medication on the day of and day prior to testing.
Screening Measures
Following initial telephone screening, participants were screened for psychiatric diagnoses using a structured parent interview (Diagnostic Interview for Children and Adolescents, Fourth Edition—DICA-IV; Reich, Welner, & Herjanic, 1997). Additionally, parents and teachers of both children with ADHD and controls completed ADHD-specific and broad behavior rating scales. The Conners’ Parent/Teacher Rating Scale-Revised (CPRS-R/CTRS-R, Conners, 1997) and ADHD Rating Scale-IV (DuPaul, Power, Anastopoulos, & Reid, 1998) were used to confirm ADHD diagnosis using the following criteria: 1) positive DSM-IV ADHD diagnosis on DICA-IV; and, 2) T-scores greater than 65 on the DSM-IV Hyperactive/Impulsive or Inattentive scales of the CPRS-R or CTRS-R; and, 3) 6 of 9 DSM-IV symptoms met (item rating of 2 or 3) on the Hyperactive/Impulsive or Inattention scales of the ADHD Rating Scale-IV, home or school version. Children with DSM-IV diagnoses other than Oppositional Defiant Disorder and Specific Phobias were excluded from both groups. Additional exclusionary criteria for both groups included any history of mental health services for behavior or emotional problems (other than for ADHD-related behaviors in the ADHD group), history of academic problems requiring school based intervention services, or history of defined primary reading or language-based learning disability.
Parents of controls also completed the DICA-IV, CPRS-R, and ADHD Rating Scale-IV, and teachers completed the CTRS-R and teacher form of the ADHD Rating Scale-IV. Controls with T-scores greater than 60 on either the DSM-IV Inattentive or Hyperactive/Impulsive scales of the CPRS-R or CTRS-R, or item ratings of 2 or greater for 4 or more symptoms of inattention or hyperactivity/impulsivity from the ADHD Rating Scale-IV (Home or School), were also excluded.
All participants were screened to rule out evidence of word reading difficulties, which were operationally defined as a score less than 25th percentile on the Basic Reading Composite of the Woodcock Johnson-III Tests of Achievement (WJ-III, Woodcock, McGrew, & Mather, 2001). Participants with Basic Reading scores below the 25th percentile were excluded from the study.
Data were collected over two days of testing, less than one month apart. Assessments included measures of intellectual functioning, language, reading, and executive function. On the first day, children were administered the WJ-III and Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-IV, Semel, Wiig, & Secord, 2004), Gray Oral Reading Test, Fourth Edition (GORT-IV, Wiederholt & Bryant, 2000), Test of Word Reading Efficiency (TOWRE, Torgeson, 1999), Rapid Automatized Naming Test (RAN, Wolf & Denckla, 2005), and Sentence Span (Swanson, 1989). Measures completed on the second day included the WISC-IV (Wechsler, 2004), Spatial Span and Coding Copy subtests of the WISC-IV Integrated (WISC-IV-I, Wechsler, et al., 2004), Cambridge Neuropsychological Test Automated Battery (CANTAB, Cognition, 1996) and Go/No-Go (GNG) measures (Wodka et al., 2007).
Study Measures
Intellectual Ability
WISC-IV-I GAI
The WISC-IV-I General Ability Index score (GAI) served as a measure of participants’ broad intellectual ability. As noted above, children included in the sample had GAI scores above 80.
Processing Speed
WISC-IV-I Coding and Symbol Search
Participants were administered the WISC-IV-I, with measures of interest in the current study including scaled scores on subtests comprising the Processing Speed Index (Coding and Symbol Search).
WISC-IV-I Coding Copy
Participants were also administered the Coding Copy subtest of the WISC-IV-I. The Coding Copy subtest requires the child to copy the same symbols used on the WISC-IV Coding subtest; however, the items are not paired with numbers in a key at the top of the page, but instead are immediately above each of the boxes in which the child copies the symbols —thus reducing the divided attention demands of the task. The child copies the symbols as quickly as possible for 2 minutes. The scaled score represents the number of correctly copied symbols. The Coding Copy subtest also provides process scores for performance across each quarter (e.g., number of items completed during each 30 second interval) of the test.
Reading Fluency
GORT-IV Fluency
The child is asked to read aloud contextual text passages of increasing difficulty, with the instruction to read for comprehension. The Fluency score represents both the child’s speed (Rate) of reading and Accuracy (# of deviations from print) for each passage. Scaled scores are calculated for both Rate and Accuracy and are combined to produce the overall Fluency score.
WJ-III Reading Fluency
The WJ-III Reading Fluency subtest is a timed measure of silent text reading fluency in which the child is asked to read simple sentences silently, determine whether the sentence is true or false, and circle the appropriate corresponding letter (e.g., T or F). The total standard score represents the number of correct responses within a three-minute time limit.
Test of Word Reading Efficiency
The TOWRE is an assessment of the child’s single word reading and single pseudoword decoding isolated (non-contextual) word fluency under timed conditions. The child is asked to read as many individual words (Sight Word Efficiency) or non-words (Phonetic Decoding Efficiency) of increasing length and phonetic difficulty as possible in 45 seconds. Scaled scores for Sight Word Efficiency and Phonetic Decoding Efficiency represent the number of correctly read words within the time limit.
Verbal Working Memory
WISC-IV-I Digit Span Backward
The Digit Span test is a subtest of the WISC-IV. Children are asked to repeat auditorily presented digit strings, both forward and backward. Norms are provided for both forward and backward span. The backward span was used to measure the manipulate component of working memory and the forward span was used to measure the maintain component.
WISC-IV-I Letter-Number Sequencing
The Letter-Number Sequencing subtest requires the child to read a sequence of numbers and letters and then recall the numbers in ascending order and the letters in alphabetical order. It consists of ten items with three trials each, with the total score consisting of the number of correct item trials.
Spatial Working Memory
CANTAB Spatial Working Memory (SWM)
The CANTAB SWM test is a computerized self-ordered pointing test consisting of a series of trials in which sets of visually presented stimulus items (ranging from two to eight) are spatially arranged on a display, varied across trials. On each trial the participant is required to point to an item not pointed to on a previous trial. The measure of interest was the spatial working memory between error score, which represents the number of “rule break” errors in which the child failed to hold in mind previously searched locations.
WISC-IV-I Spatial Span Backward
The Spatial Span subtest is divided into two tasks (Spatial Span Forward and Spatial Span Backward) with seven items each. Each item is composed of two trials of the same span length, on which the examiner taps a series of blocks. On Spatial Span Backward, the child is asked to repeat the sequence of blocks tapped by the examiner, in reverse order.
Auditory Attention Span
CELF-4 Recalling Sentences
This measure is a simple verbal span task, on which the child is read sentences of increasing length and is required to repeat each sentence verbatim. The measure of interest was the total number of sentences recalled correctly.
WISC-IV-I Digit Span Forward
On the Digit Span subtest, children are asked to repeat orally presented digit strings, in the same sequence (forward) as they were presented, as well as in reverse sequence (backward). The measure of interest was the scaled score for digits recalled correctly in the same sequence as presented (forward condition).
Reaction Time, Intra-subject Variability, and Inhibition
Simple Reaction Time and Go/No-go Tests
Three versions of computerized tests were administered. The simple reaction time test measure flashed green spaceships and participants were required to push a button for each spaceship (all “go” condition). The two subsequent measures had both go and no-go conditions; the computer flashed red and green spaceships. Participants were instructed to push a button as quickly as possible in response to green spaceships only. Use of familiar color elements (green for “go”; red for “no-go”) minimized the working memory load of the test. In the fixed interstimulus interval (ISI) condition, cues appeared on the screen for 300 msec and were presented once every 1500 msec (1500 msec interstimulus interval). Cues were weighted towards green spaceships at a ratio of 3:1 (162 go cues; 54 no-go cues), intensifying the need to inhibit a rapid, habitual skeletomotor response. In the jittered ISI condition, stimuli were presented with a variable ISI, using a moderate (33.3%) level of jitter in which five ISIs were presented randomly, ranging from 1000 to 2000 msec (i.e., 1000, 1250, 1500, 1750, 2000). The go/no-go cue ratio for the jittered ISI condition was the same as in the fixed ISI condition. The total time for each of the three tasks was 6 minutes 30 seconds. For all three measures, the variable of interest was mean reaction time (MRT, for correct hits). The simple reaction time test was administered first, with the two go/no-go tests administered subsequently in a counterbalanced order. For the go/no-go tests only, additional variables of interest included inhibition, measured by commission errors, and intra-subject variability (ISV), calculated as (standard deviation of response time) / (mean response time).
Naming Speed
Rapid Automatized Naming
On the RAN subtests, the child is asked to name each stimulus item (a series of 5 colors, numbers, or letters repeated randomly in 5 rows of 10) as quickly as possible without making mistakes. Time to completion is recorded separately for each condition (e.g., colors, letters, and numbers) and converted to a standard score.
Data Analyses
Initial between group (ADHD vs. control) comparisons on the study measures were made using independent t-tests or chi-square analyses, as appropriate (Table 1). Girls with ADHD were rated by their parents as exhibiting more severe ADHD-related symptomatology (CPRS-R Scale N: DSM-IV Total) than were boys (F1,38 = 7.882, p = .008). There was not a significant difference in ADHD-related symptomatology between control girls and boys (F1,14) = .231, p = .639). Additionally, there were slightly more boys in the ADHD group, potentially contributing to sex differences in scores on the WISC-IV-I Coding subtest. Therefore, sex and the CPRS-R Scale N score were used as covariates in regression analyses examining group differences on measures of processing speed and reading fluency. Additional analyses investigating those skills potentially contributing to group differences in Coding scores used a consistent hierarchical regression model in which variables were entered in the following order: sex, Coding Copy, CPRS-R Scale N score, and GAI in the preliminary blocks, followed by the cognitive variable of interest (i.e., verbal span, verbal working memory, spatial working memory, reaction time, etc.) added in the final block. Thus, in each analysis, the unique variance contributed by the cognitive variable of interest was examined for significance, after controlling for sex, Coding Copy, symptom severity, and intellectual ability.
Table 1.
Demographic, IQ and Reading Information
| ADHD n = 41 |
Control n = 21 |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | η2p | |
| Sex (% male) | 68.3 | 47.6 | .117 | .040 | ||
| Age | 10.97 | 1.41 | 11.46 | 1.63 | .222 | .025 |
| WISC-IV-I | ||||||
| GAI | 105.66 | 11.88 | 116.10 | 12.42 | .002 | .148 |
| PSI | 91.49 | 14.96 | 106.43 | 14.89 | .000 | .188 |
| Coding | 7.49 | 3.23 | 10.95 | 3.06 | .000 | .217 |
| Coding Copy | 8.38 | 3.15 | 8.78 | 3.19 | .662 | .004 |
| Symbol Search | 9.46 | 2.93 | 11.24 | 3.55 | .040 | .068 |
| Reading Fluency | ||||||
| GORT-IV Fluency | 10.83 | 3.27 | 13.86 | 3.12 | .001 | .171 |
| WJ-III RF | 100.22 | 13.85 | 110.10 | 18.63 | .023 | .084 |
| TOWRE PDE | 103.24 | 12.64 | 113.05 | 11.03 | .004 | .129 |
| TOWRE SWE | 103.83 | 11.30 | 109.60 | 11.04 | .064 | .057 |
Note. WISC-IV-I = WISC-IV Integrated; GAI = General Ability Index; PSI = Processing Speed Index; GORT-IV = Gray Oral Reading Test-IV; WJ-III RF = Woodcock Johnson-III Reading Fluency; TOWRE = Test of Word Reading Efficiency; PDE = Phonemic Decoding Efficiency; SWE = Sight Word Efficiency
Results
Sample Characteristics
Sample characteristics are listed in Table 1. There were no significant differences between ADHD and control groups in age [t(60) = 1.235, p = .222], SES [t(58) = 0.902, p = .371], handedness [χ2(1) = 0.109, p = .741], or racial distribution [χ2(4) = 4.452, p = .348]. There was a slightly but not significantly greater proportion of boys in the ADHD group than the control group [χ2(1) = 2.502, p = .114]. Children in the sample were of average to above average ability (GAI M = 109.19, SD = 12.96), and the control group had a higher mean GAI than the ADHD group [t(60) = 3.224, p = .002].
Group Performance on Behavioral Variables
Processing Speed
Participant performance on the processing speed measures is listed in Table 1. After controlling for group differences in sex distribution, children with ADHD performed significantly worse than controls on the WISC-IV-I PSI (ΔR2 = .152, p = .001) (see Table 1). Examining the subtests comprising the PSI, there were significant group differences (ADHD < control) on the Coding subtest (ΔR2 = .177, p < .001), but not on the Symbol Search subtest (ΔR2 = .054, p = .068). There were also no significant differences between the ADHD and control groups on WISC-IV-I Coding Copy test (ΔR2 = .000, p = .909). The group differences on Coding remained after controlling for both sex and Coding Copy (ΔR2 = .126, p = .001), as well as after controlling for sex, Coding Copy, and GAI (ΔR2 = .078, p = .009) suggesting that the differences seen between children with ADHD and controls on this type of “processing speed” measure (i.e., WISC-IV-I Coding) reflect differences beyond basic graphomotor speed or basic stimulus evaluation speed (which were common to both Coding and Coding Copy), such as differences in “executive” processes critical to selecting an accurate motor response.
Reading Fluency
Performance on oral and silent reading fluency measures is listed in Table 2. After controlling for sex and GAI, children with ADHD had significantly lower scores on both oral contextual reading fluency (GORT-IV Fluency, ΔR2 = .064, p = .032) and oral non-contextual fluency (TOWRE Phonetic Decoding; ΔR2 = .075, p = .030).
Table 2.
Group Differences in Reading Fluency Scores (Controlling for Sex and GAI)
| Reading Fluency Measure | ΔR2 | p |
|---|---|---|
| GORT-IV Fluency | .064 | .032 |
| WJ-III RF | .008 | .450 |
| TOWRE PDE | .075 | .030 |
| TOWRE SWE | .011 | .400 |
Note. GAI = WISC-IV General Ability Index, GORT-IV = Gray Oral Reading Test-IV; WJ-III RF = Woodcock Johnson-III Reading Fluency; TOWRE = Test of Word Reading Efficiency; PDE = Phonemic Decoding Efficiency; SWE = Sight Word Efficiency
To further investigate the role of response preparation/processing speed in reading fluency, we created a residualized Coding score by regressing sex and Coding Copy performance on the Coding standard score, in order to control for these two variables. This variable (i.e., residualized Coding—CodingR) was considered to reflect processes necessary to response selection and preparation that are separable from graphomotor output speed (see Figure 1). CodingR and the CodingR –by-group interaction were examined as predictors of reading fluency in subsequent regression analyses (Table 3). CodingR accounted for significant proportion of variance when predicting two of three measures of oral reading fluency: GORT-IV Fluency (R2 = .092, p = .027) and TOWRE Phonetic Decoding Efficiency (R2 = .121, p = .011). The interaction between CodingR and group was not significant when predicting measures of oral reading fluency, indicating that the relationship between this (non-graphomotor) element of response selection and oral reading fluency is similar among children with and without ADHD.
Table 3.
Variance in Reading Fluency Accounted for by CodingR
| Reading Fluency Measure | ΔR2 | p |
|---|---|---|
| GORT-IV Fluency | .092 | .027 |
| WJ-III RF | .013 | .417 |
| TOWRE PDE | .121 | .011 |
| TOWRE SWE | .003 | .693 |
Note. CodingR = WISC-IV Coding (residualized for sex, group, and Coding Copy), GORT-IV = Gray Oral Reading Test-IV; WJ-III RF = Woodcock Johnson-III Reading Fluency; TOWRE = Test of Word Reading Efficiency; PDE = Phonemic Decoding Efficiency; SWE = Sight Word Efficiency.
Processing Speed: Supplemental Analyses
Additional analyses were used to investigate which elements of attention and executive control might best explain the persisting group differences on measures of processing speed (i.e., Coding). Seven constructs were identified that were hypothesized to play a role in processing speed, including verbal attention span, verbal working memory, spatial working memory, reaction time, intra-subject variability of responding, inhibition, and automaticity of retrieval for overlearned information (rapid naming). Variables representing each of these constructs were entered on the second step of separate hierarchical regression analyses, after controlling for sex, Coding Copy, severity of ADHD symptomatology (CPRS-R Scale N score), and GAI on the first step. Of these hypothesized constructs, several accounted for a significant proportion of additional unique variance in Coding, over and above that accounted for by sex, Coding Copy, ADHD-related symptom severity, and GAI (Table 4). Significant constructs included verbal attention/span (as measured by Digit Span Forward and CELF-IV Recalling Sentences), verbal working memory (as measured by Sentence Span, Letter-Number Sequencing and Digit Span Backward, after controlling for simple verbal attention –i.e., Digits Forward), and spatial working memory (as measured by WISC-IV-I Spatial Span Backward, CANTAB Spatial Working Memory Between errors, after controlling for spatial attention –i.e., Spatial Span Forward). The total R2 for the models including these three constructs ranged from .594 to .633, indicating that these models accounted for up to 63.3 percent of the variance in Coding scores. Measures of reaction time, intra-subject variability, inhibition, and naming speed did not account for a significant proportion of additional variance in Coding scores, over and above the measures already in the model (e.g., sex, Coding Copy, symptom severity, and GAI).
Table 4.
Hierarchical Regression Analysis Predicting Coding Scores
| Predictor | β | ΔR2 | p | Model R2 | |
|---|---|---|---|---|---|
| Base Model | .486 | ||||
| Sex | .226 | .051 | .097 | ||
| Coding Copy | .572 | .295 | .000 | ||
| CPRS-R Scale N | -.387 | .146 | .001 | ||
| GAI | -.010 | .000 | .937 | ||
|
| |||||
| Verbal Attention/Span | .094 | .016 | .594 | ||
| Digit Span Forward | .320 | .011 | |||
| Recalling Sentences | -.228 | .061 | |||
| Verbal Working Memory, controlling for verbal span | .076 | .025 | .633 | ||
| Letter Number Seq. | .384 | .007 | |||
| Digit Span Backward | -.121 | .338 | |||
| Spatial Working Memory, controlling for spatial span | .115 | .012 | .599 | ||
| CANTAB SWM | .086 | .477 | |||
| Spatial Span Backward | .434 | .003 | |||
| Reaction Time | .016 | .819 | .450 | ||
| Simple Reaction Time | .067 | .734 | |||
| GNG Fixed MRT | -.162 | .484 | |||
| GNG Jittered MRT | .169 | .468 | |||
| Naming Automaticity | .022 | .603 | .508 | ||
| RAN Colors | -.085 | .584 | |||
| RAN Letters | -.121 | .546 | |||
| RAN Numbers | .253 | .201 | |||
| Intra-subject Variability | .009 | .722 | .467 | ||
| GNG Fixed Variability | -.027 | .853 | |||
| GNG Jittered Variability | -.085 | .532 | |||
| Inhibition | .005 | .852 | .463 | ||
| GNG Fixed Commissions | -.021 | .936 | |||
| GNG Jittered Commissions | -.052 | .839 | |||
Note. In each regression, Sex, Coding Copy, CPRS N Score, and GAI were entered first, followed by the predictors of interest in the final block.
Further analyses suggest that verbal working memory also contributes significantly to children’s performance on measures of oral reading fluency when tested directly. After controlling for sex and GAI, the verbal working memory measures predicted a significant proportion of variance in oral contextual and non-contextual reading fluency performance (GORT Fluency, ΔR2 = .137, p = .028; TOWRE PDE, ΔR2 = .221, p = .005).
Discussion
The current study sought to examine performance of children with ADHD versus typically developing controls across the tasks of the WISC-IV-I Processing Speed Index, dissociating the executive “processing” components necessary to response selection and preparation from the initial perceptual and terminal graphomotor output demand of PS. We also sought to explore the contribution of these elements to reading fluency. In this well screened sample of children without basic word reading or language difficulties, there were no differences between ADHD and control groups in basic graphomotor copying speed (i.e., Coding Copy); whereas, there were significant differences in the aspects involving response selection (i.e., CodingR), with the ADHD group slower than controls. As a group, children with ADHD were also significantly less efficient on measures of oral contextual and non-contextual reading fluency and silent non-contextual reading fluency. Further, this non-graphomotor element “processing speed” (i.e., that involving response selection) was significantly associated with oral reading fluency performance. Importantly, these measures of fluency required not just rapid performance, but both efficient and accurate responding, thus including a substantial executive demand in response selection and preparation. Among constructs hypothesized to play a role in this efficient responding, verbal attention span as well as the executive control elements of verbal and spatial working memory accounted for a significant proportion of additional unique variance in children’s performance on Coding, over and above that accounted for by sex, Coding Copy, ADHD-related symptom severity, and GAI. In contrast, measures of reaction time, rapid naming speed, inhibition, and intra-individual response variability did not contribute. Notably, measures of verbal and spatial working memory accounted for unique variance in Coding, even after controlling for the respective maintain demand (e.g., verbal span/spatial span). Working memory (in these analyses, assessed primarily by the WISC-IV-I Letter-Number Sequencing and Spatial Span Backwards tasks) may play an important role in performance of this task due to the effortful nature of cognitive control required to complete the tasks, the additional “step” of controlled processing required in re-ordering or transforming information mentally, or its more novel presentation. Each of these characteristics highlights the executive control involved, which may help to explain the contribution to the response selection component of PS.
Taken together, these results suggest that the “cognitive” (executive) and graphomotor output components of PS can be dissociated, with these cognitivecomponents contributing uniquely to slowed output speed in ADHD (Chhabildas, 2001; Hinshaw, 2002; Rucklidge & Tannock, 2002; Willcutt, 2005). Given previous findings of skeletomotor and oculomotor slowing in children with ADHD (Cole, et al., 2008; Mahone, et al., 2009), one might expect that ADHD-related deficits on the WISC-IV-I PSI are due to largely to the motor control demands of the tasks. Our findings, suggest, however, that difficulty with response selection may be uniquely responsible for at least part of these observed findings. The Coding task does present a greater vertical scanning demand than the Coding Copy task so there may also be differences in perceptual demand which contribute to different group performance on these tasks; however, the reading fluency measures demand horizontal scanning rather than vertical scanning/tracking, so it is unlikely that this perceptual difference alone contributes to group differences on reading fluency measures. The observed differences between groups on a residualized measure of Coding, controlling for sex and Coding Copy performance, suggest that differences may exist between groups at the level of response selection (i.e., CodingR), with this difference in response selection potentially contributing to differences in apparent response efficiency. To our knowledge, this is the first study to examine this dissociation between components of the WISC-IV-I Coding in children with ADHD.
The observed differences between children with ADHD and controls in more frontally-mediated executive components of processing speed also contributed to differences in performance on measures of reading fluency, even in this highly screened sample. Given recent findings that children with ADHD, even without comorbid reading or language disorders, still show weaker reading fluency than controls (Ghelani, et al., 2004; Willcutt, et al., 2007), and our findings that verbal span and working memory predict reading fluency for both children with and without ADHD, these results support the idea that executive control skills, especially working memory, play an important role in development of reading fluency, beyond word reading accuracy (e.g., Willcutt, et al., 2005) for all children, and thus are of particular importance in later elementary school years and beyond. These data are consistent with recent work suggesting that impairments in specific components of executive control contribute to deficits in higher-level aspects of reading skills, even for children with intact decoding skills (Locasio et al., in press; Sesma et al., 2009).
Results implicating executive aspects of processing speed, including working memory, provide new evidence for involvement of skills dependent on more anterior (premotor, prefrontal) circuits, as well as on long-range frontal-posterior connections involving these areas, in children with ADHD. These behavioral findings are consistent with recent anatomic MRI evidence (Shaw, 2009; Shaw, 2008; Shaw, 2007) implicating anomalous frontal lobe development in children with ADHD, including decreased volumes of supplementary motor complex (SMC) and prefrontal (dorsolateral, medial) cortex volumes, especially among boys with ADHD (Mahone, Richardson, et al., 2009). The SMC, in particular the rostral portion or “pre-SMA”, is thought to be critical to response control and selection (Mostofsky & Simmonds, 2008), and may contribute to reduced efficiency of response speed, whereas anomalous prefrontal development may drive the working memory contributions to ADHD-related behavioral inefficiency.
Limitations and Future Directions
As a function of the high level of screening in the present study, participants were of average to above average intellectual ability, with control participants tending to show higher than average GAI (with less variability than the normative sample). This may reflect a potential sampling bias with regard to parental willingness to involve their children in research, either due to an interest in potentially identifying areas of concern in children with ADHD or in identifying superior cognitive ability in control participants. On a related note, given the attempt to statistically adjust for this difference in mean GAI score between groups, our results may actually represent a low estimate of the effects of executive aspects of “processing” on children’s reading fluency. Due to the overlap in essential elements between conventional measures of IQ and executive function, especially involving working memory and response preparation/processing speed, which were of particular interest in this paper, statistical “control” of IQ has been suggested to produce anomalous results when examining group differences in executive functioning (Dennis et al., 2009). Furthermore, a recent meta-analysis suggests that reduced IQ scores in the ADHD population relative to typically developing controls may be specifically related to attentional dysregulation and poor test-taking behavior rather than deficits in “intelligence,” per se (Jepsen, Fegerlund & Mortensen, 2009). It is important to note that children with ADHD in this study were tested off medication, which Jepsen and colleagues (2009) also suggest is likely to result in lower IQ scores. Furthermore, as some data suggest the relation of children’s executive function to intellectual ability may differ at the ends of the IQ distribution (Delis, 2007; McGee, 2009), the observed relation of working memory to PS and reading fluency measures may be different in children with lower levels of verbal ability. The current sample was not large enough to test for differences in this association across levels of ability.
In addition, the recent evidence from structural neuroimaging (Mahone et al., 2009) suggests that boys and girls with ADHD may share a common anomaly in premotor circuit (especially SMC) development; whereas in boys (but not girls) with ADHD (especially in this age range), the anomalous development of prefrontal circuits may persist throughout childhood (Qiu et al., 2009). Thus, the observed association between “processing”, verbal working memory, and reading fluency may differ across sexes. Future research with larger and more equally distributed samples of boys and girls will be required to address that issue.
The current study used PS measures from the WISC-IV-I, which was designed to allow for a more careful examination (e.g., “process analysis”) of the component skills involved in completion of the more traditional WISC tasks. Development of additional measures permitting the dissociation of response components will be helpful in further examining differences in aspects of PS, including motor or verbal output and the executive components of response selection and preparation. Furthermore, it will be important to investigate the association between the slowed “processing” typical of children with ADHD and reading comprehension skills, as reading comprehension is believed to rely even more explicitly on efficient “processing” of material and verbal working memory skills.
Acknowledgments
Supported by P50 HD 52121, HD-24061 (Intellectual and Developmental Disabilities Research Center), and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1-RR025005.
Footnotes
Portions of this manuscript were presented at the 38th annual North American meeting of the International Neuropsychological Society in Acapulco, Mexico on February 5, 2010.
References
- Cambridge Cognition. CANTAB. Cambridge, England: Cambridge Cognition Limited; 1996. [Google Scholar]
- Chhabildas N, Pennington BF, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29(6):529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Gidley Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners’ Rating Scales - Revised. North Tonawanda, New York: Multi-Health Systems, Inc; 1997. [Google Scholar]
- Cutting LE, Materek A, Cole CA, Levine TM, Mahone EM. Effects of fluency, oral language, and executive function on reading comprehension performance. Annals of Dyslexia. 2009;59:34–54. doi: 10.1007/s11881-009-0022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Houston WS, Wetter S, Han SD, Jacobson M, Holdnack J, et al. The importance of testing higher-level executive functions in school-age children and adolescents. Journal of Psychoeducational Assessment. 2007;25(1):29–40. [Google Scholar]
- Denckla MB. Biological correlates of learning and attention: What is relevant to learning disability and Attention-Deficit/Hyperactivity Disorder? Developmental and Behavioral Pediatrics. 1996;17(2):114–119. [PubMed] [Google Scholar]
- Denckla MB, Cutting LE. History and significance of rapid automatized naming. Annals of Dyslexia. 1999;49(1):29–42. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV. New York: Guilford Press; 1998. [Google Scholar]
- Ghelani K, Sidhu R, Jain U, Tannock R. Reading comprehension and related reading abilities in adolescents with reading disabilities and Attention-Deficit/Hyperactivity Disorder. Dyslexia. 2004;10:364–384. doi: 10.1002/dys.285. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP, Carte ET, Sami N, Treuting JJ, Zupan BA. Preadolescent girls with attention-deficit/hyperactivity disorder: II. Neuropsychological performance in relation to subtypes and individual classification. Journal of Consulting and Clinical Psychology. 2002;70(5):1099–1111. doi: 10.1037//0022-006x.70.5.1099. [DOI] [PubMed] [Google Scholar]
- Jepsen JRM, Fagerlund B, Mortensen EL. Do attention deficits influence IQ in children and adolescents with ADHD? Journal of Attention Disorders. 2009;12:551–562. doi: 10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Margulies DS, Castellanos FX. Recent advances in structural and functional brain imaging studies of attention-deficit/hyperactivity disorder. Current Psychiatry Reports. 2007;9(5):401–407. doi: 10.1007/s11920-007-0052-4. [DOI] [PubMed] [Google Scholar]
- Locascio G, Mahone EM, Eason S, Cutting LE. Executive function among children with reading comprehension deficits. Journal of Learning Disabilities. doi: 10.1177/0022219409355476. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Mostofsky SH, Lasker AG, Zee D, Denckla MB. Oculomotor anomalies in Attention-Deficit/Hyperactivity Disorder: Evidence for deficits in response preparation and inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(7):749–756. doi: 10.1097/CHI.0b013e3181a565f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone EM, Richardson ME, Crocetti D, Clauss JA, Denckla MB, Mostofsky SH. Regional frontal lobe anomalies in girls with ADHD. Journal of the International Neuropsychological Society. 2009;15(S1):197. [Google Scholar]
- McGee CL, Delis DC, Holdnack AL. Cognitive discrepancies in children at the ends of the bell curve: A note of caution for clinical interpretation. The Clinical Neuropsychologist. 2009;23:1160–1172. doi: 10.1080/13854040902794995. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20(5):751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller M, et al. Basal ganglia volume and shape in children with ADHD. American Journal of Psychiatry. 2009;166(1):74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- Rucklidge JJ, Tannock R. Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry. 2002;43(8):988–1003. doi: 10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Semel E, Wiig E, Secord W. Clinical evaluation of language fundamentals, Fourth Edition (CELF-4) Toronto, Canada: The Psychological Corporation/A Harcourt Assessment Company; 2004. [Google Scholar]
- Sergeant JA. Modeling Attention-Deficit/Hyperactivity Disorder: A critical appraisal of the cognitive–energetic model. Biological Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Sesma HW, Mahone EM, Levine T, Eason SH, Cutting LE. The contribution of executive skills to reading comprehension. Child Neuropsychology. 2009;15(3):232–246. doi: 10.1080/09297040802220029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Yerys BE, Scott A, Boada R, Willcutt EG, et al. Processing speed deficits in Attention Deficit/Hyperactivity Disorder and reading disability. Journal of Abnormal Child Psychology. 2006;34:585–602. doi: 10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, et al. Psychostimulant treatment and the developing cortex in Attention Deficit Hyperactivity Disorder. American Journal of Psychiatry. 2008;166(1):58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, et al. Development of cortical asymmetry in typically developing children and its disruption in Attention-Deficit/Hyperactivity Disorder. Archives of General Psychiatry. 2009;66(8):888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Swanson HL, Cockran KF, Ewers CA. Working memory in skilled and less skilled readers. Journal of Abnormal Child Psychology. 1989;17(2):145–156. doi: 10.1007/BF00913790. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Wechsler DL. Wechsler Intelligence Scale for Children. Fourth Edition. San Antonio, TX: The Psychological Corporation; 2004. [Google Scholar]
- Wechsler DL, Kaplan E, Fein D, Kramer JH, Morris R, Delis DC. Wechsler Intelligence Scale for Children - Fourth Edition: Integrated, technical and interpretive manual. San Antonio, Tx: Harcourt Assessment, Inc; 2004. [Google Scholar]
- Welsh MC, Pennington BF, Grossier DB. A normative study of executive functions: a window on prefrontal function in children. Developmental Neuropsychology. 1991;7:131–149. [Google Scholar]
- Wiederholt L, Bryant B. Examiner’s Manual: Gray Oral Reading Test-Fourth Edition. Austin, Tx: Pro-Ed; 2000. [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, Hulslander J. Neuropsychological analyses of comorbidity between reading disability and Attention Deficit Hyperactivity Disorder: In search of the common deficit. Developmental Neuropsychology. 2005;27(1):35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, DeFries JC. Understanding comorbidity: A twin study of reading disability and Attention-Deficit/Hyperactivity Disorder. American Journal of Medical Genetics Part B. 2007;144:709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Sonuga-Barke E, Nigg J, Sargeant J. Recent developments in neuropsychological models of childhood psychiatric disorders. Biological Child Psychiatry. 2008;24:195–226. [Google Scholar]
- Willcutt EG. Attention-Deficit/Hyperactivity Disorder. In: Yeates KO, Ris MD, Taylor MG, Pennington BF, editors. Pediatric Neuropsychology. 2. NY: Guilford; 2010. pp. 393–417. [Google Scholar]
- Wodka EL, Mahone EM, Blankner JG, Gidley Larson JC, Fotedar S, Denckla MB, et al. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinincal and Experimental Neuropsychology. 2007;29(4):345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Wodka EL, Mostofsky SH, Prahme C, Gidley Larson JC, Loftis C, Denckla MB, et al. Process examination of executive function in ADHD: Sex and subtype effects. Clinical Neuropsychology. 2008;22(5):826–841. doi: 10.1080/13854040701563583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Denckla MB. Rapid Automatized Naming and Rapid Alternating Stimulus tests. Austin, TX: Pro-Ed; 2005. [Google Scholar]
- Wolf M, Katzir-Cohen T. Reading fluency and its intervention. Scientific Studies of Reading. 2001;5:211–238. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson – III. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]