Abstract
Particle-associated periprosthetic osteolysis remains a major issue in joint replacement. Ongoing bone loss resulting from wear particle-induced inflammation is accompanied by continued attempts at bone repair. Previously we showed that mesenchymal stem cells (MSCs) are recruited systemically to bone exposed to continuous infusion of ultra high molecular weight polyethylene (UHMWPE) particles. The chemokine-receptor axis that mediates this process is unknown. We tested two hypotheses: (1) the CCR1 receptor mediates the systemic recruitment of MSCs to UHMWPE particles and (2) recruited MSCs are able to differentiate into functional mature osteoblasts and decrease particle-associated bone loss. Nude mice were allocated randomly to four groups. UHMWPE particles were continuously infused into the femoral shaft using a micro-pump. Genetically modified murine wild type reporter MSCs were injected systemically via the left ventricle. Non-invasive imaging was used to assay MSC migration and bone mineral density. Bioluminescence and immunohistochemistry confirmed the chemotaxis of reporter cells and their differentiation into mature osteoblasts in the presence of infused particles. Injection of a CCR1 antagonist decreased reporter cell recruitment to the UHMWPE particle infusion site and increased osteolysis. CCR1 appears to be a critical receptor for chemotaxis of MSCs in the presence of UHMWPE particles. Interference with CCR1 exacerbates particle-induced bone loss.
Keywords: Arthroplasty, Wear debris, CCR1 receptor, Mesenchymal Stem Cells chemotaxis, Osteolysis
Introduction
Total hip arthroplasties (THA) with metal-on-conventional polyethylene bearings have shown excellent survivorship rates up to 80.9% free of revision or removal of the implant, at over twenty-five years follow-up [1]. The use of highly cross-linked polyethylene has further reduced the generation of wear debris, compared to conventional non-highly cross-linked polyethylene [2]. Nevertheless, aseptic loosening accounts for more than two-thirds of revisions of THA, and almost one-half of total knee arthroplasties (TKA) respectively [3, 4]. Although the mechanisms leading to aseptic loosening are multi-factorial, Sundeldt et al.[3] and others[5–8] conclude that a substantial role is played by wear particles. Ultra high molecular weight polyethylene (UHMWPE) particles stimulate biological reactions in the local microenvironment [9] as well as systemically [10–12]. Macrophages are the key cells driving the immunological reaction. Indeed after phagocytosis or cell membrane contact [8], activated macrophages release pro-inflammatory mediators such as cytokines (IL-1, IL-6, TNF-a), growth factors (macrophage colony-stimulating factor-1) and chemokines (MIP-1a, MCP-1) as shown by tissue retrieval studies [13–15]. Subsequently, locally and systemically recruited activated macrophages differentiate into multinucleated giant cells and osteoclasts leading to bone resorption around implants within a foreign body reaction [16]. Among the large number of chemokine receptors, CCR1 (C-C motif receptor 1) plays a major role in the recruitment of mesenchymal stem cells (MSCs) [17–19]. Huang et al. [20] have shown the ability of CCR1 to increase MSC chemotaxis, viability and engraftment using a murine model of injured myocardium. They established that once recruited, CCR1-MSCs have a lower percentage of apoptosis. Moreover, evidence of the role played by CCR1 in MSC chemotaxis has been established by Honczarenko et al. [21] CCR1 is a chemokine receptor which is able to bind three chemokines including MIP-1a (CCL3), MCP-3 (CCL7) and RANTES (Regulated upon Activation, Normal T-cell Expressed, and Secreted, CCL5) [22]. As a chemokine receptor, CCR1 belongs to the G-protein coupled receptor superfamily [23]; its gene identification (ID) is 1230 in humans and 12768 in mice [24]. In humans, MSCs belong to the somatic lineage and many studies have detected CCR1 on the cell surface of hMSCs [21, 25, 26]. CCR1 can be blocked by specific antagonists [27, 28].
In a previous in vitro study from our laboratory, Huang et al. [29] demonstrated a critical role for MIP-1a, a CCR1 ligand, to promote the chemotaxis of MSCs to polymethylmethacrylate (PMMA) particles. Whether CCR1 is involved in the systemic recruitment of MSCs to clinically relevant UHMWPE particles in vivo is unknown. We hypothesized that polyethylene wear particles, known to incite an inflammatory reaction, also induce the systemic recruitment of MSCs, which is mediated in part by CCR1. In this study, we test this hypothesis using a murine model of continuous intramedullary infusion of clinically relevant UHMWPE particles. Given the facts that periprosthetic osteolysis is due to systemic migration of macrophages to UHMWPE particles, subsequent bone destruction and inadequate bone repair [10, 12, 30, 31], and that CCR1 is one of the most expressed CC chemokine receptors on the cell surface of MSCs [21], modulation of pathways involving CCR1 may provide another strategy to mitigate osteolysis.
Materials and methods
Animals and experimental design
Eighteen, 8–10 weeks old nude mice nu/nu (Charles River Laboratory Inc, Wilmington, MA) were housed and fed in our Animal Facility. The experimental design was approved by the Institutional Administration Panel for Laboratory Animal Care. We strictly followed university guidelines for care and use of laboratory animals. Animals were divided into four groups (Table 1). Group 1 (n = 5) animals had UHMWPE particles infused into the distal femur via a subcutaneous minipump; the animals were also injected intraperitoneally with 0.1 mL of the carrier solution: 0.9% sodium chloride (Hospira Inc, Lake Forest, IL) every day. Group 2 (n = 5) also had infused UHMWPE particles; the animals were injected intraperitoneally with 0.1 mL of J-113863 (a soluble competitive CCR1 receptor inhibitor, Tocris Bioscience, Ellisville, MO) every day at the concentration of 10 mg/kg. For Groups 1 and 2, genetically modified wild type murine mesenchymal reporter stem cells (WTMSCs) were injected through an intra-cardiac route. Groups 3 (n = 5) had saline solution infused into the distal femur; WTMSCs were injected through an intra-cardiac route. Groups 4 (n = 3) had infused UHMWPE particles and was injected through an intra cardiac injection with CCR1 −/− mesenchymal stem cells.
Table 1.
Experimental design. UHMWPE = UHMWPE particles, ICI = intracardiac injection, WTMSCs = wild type mesenchymal stem cells, CCR1 −/− = mesenchymal stem cells deficient in the CCR1 receptor.
| n = | UHMWPE In pump |
CCR1 antagonist |
Carrier | Cells injected Via ICI |
|
|---|---|---|---|---|---|
| Group 1 | 5 | ✓ | ✓ | WTMSCs | |
| Group 2 | 5 | ✓ | ✓ | WTMSCs | |
| Group 3 | 5 | WTMSCs | |||
| Group 4 | 3 | ✓ | CCR1−/− MSCs |
For Groups 1 and 2, saline and J-113863 (a competitive antagonist of the CCR1 receptor) respectively were injected daily for five weeks (beginning one week before surgery and continuing for four weeks after surgery). Cells were injected one week after surgery (day 0) when wound healing was complete. Animals underwent microCT one day before surgery and at day 28 for bone mineral density analysis. Bioluminescence (BLI) was performed at day 0, 2, 4, 6, 8, 10, 14, 21, 28 for all groups. All animals were euthanized after imaging and their femurs were harvested for histomorphometry.
Surgery
The surgical procedure was performed at day -7, i.e. 7 days before imaging began. All animals received an injection of buprenorphine (0.1 mg/kg; Ben Venue Laboratories, Bedford, OH), subcutaneously before the procedure. Animals were anesthetized with 2–3% isoflurane in 100% oxygen at a flow rate of 1 L/min and were operated on a warm small animal surgery station. Using sterile technique, the left patellar tendon was exposed through a 5 mm skin incision. Then the intercondylar notch of the distal femur was exposed through a medial parapatellar arthrotomy. We used a series of needles from 25 to 21 gauge to manually drill through the intercondylar notch. In order to implant the osmotic pump subcutaneously, another incision was made posteriorly between the scapulae. We then filled the femoral shaft with UHWMPE particles and a 23 gauge hollow, 6-mm long rod, connected to the pump via polyvinyl tubing was press fit into the distal femur through the drill hole. After implant insertion, the quadriceps-patellar complex was closed by one 6.0 chromic-gut resorbable suture and the skin (knee and dorsal incisions) was repaired with 5.0 vicryl sutures and biocompatible glue. We checked the animals each day postoperatively for general health and activity.
Particles and pumps
We used conventional non-highly cross-linked UHMWPE particles (a gift from Dr Timothy Wright, Hospital for Special Surgery, New York) obtained from knee joint simulator tests and isolated according to an established protocol [32]: The particles were isolated by density gradient centrifugation and sterilized by incubating with 95% ethanol overnight. Then frozen aliquots of the particles containing serum were lyophilized for 4–7 days. The dried material was digested in 5M sodium hydroxide at 70°C for 2 hours. The digested particle suspension was centrifuged through a 5% sucrose gradient at 40 K rpm at 10°C for 3 hours. The collected particles at the surface of the sucrose solution were ultrasonicated and centrifuged again through an isopropanol gradient (0.96 and 0.90 g/cm3) at 40 K rpm at 10°C for 1 hour. The purified particles at the interface between the two layers of isopropanol were harvested and the isopropanol was evaporated from the particle mixture until dry. Particles were then resuspended in 95% ethanol which was evaporated completely. Ultimately, UHMWPE particles were washed in 70% ethanol and resuspended in phosphate buffered saline prior to filling the diffusion pumps. The concentration of UHMWPE was 15mg/mL. The particles tested negative for endotoxin using a Limulus Amebocyte Lysate Kit (BioWhittaker, Walkersville, MD). The mean diameter of the particles was 1.0 ± 0.1 mm (mean±SE) measured by electron microscopy. We used a Model 2006 Alzet osmotic pump (DURECT Corp, Cupertino, CA) loaded with the particles and connected it to silicon tubing overlying a 6 mm long hollow titanium rod at the opposite end. According to our previous study, 100 µL of the pump contents could be pumped out during four weeks, which represents 3.0 × 109 particles infused into the femoral medullary canal.
Reporter cells: wild type mesenchymal stem cells and CCR1 −/− MSCs
We used murine passage 6 wild type mesenchymal stem cells and murine passage 5 knockout CCR1 mesenchymal stem cells, both transfected with the lentiviral vector to express the bioluminescent optical reporter gene firefly luciferase (fluc), and a red fluorescent reporter gene (tomato). The cells were grown in Minimum Essential Medium Alpha + GlutaMAX™ 32571 (Invitrogen, Grand Island, NY) + 10% Fetal Bovin Serum (Invitrogen) + 1% antibiotic-antimycotic (Invitrogen). Each mouse received 2 × 106 cells suspended in 0.1 mL Hank’s Balanced Salt Solution (HBSS) 14170 (Invitrogen, Carlsbad, CA) with 30 units/mL heparin through an intracardiac injection into the left ventricle.
Imaging: microCT and bioluminescence (BLI)
The imaging was performed in the Small Animal Imaging Facility at Stanford University (Clark Center). A microCT scan was performed one day before surgery (day -8) and at day 28 for all groups in order to detect changes in bone mineral density (BMD). At day 28, we euthanized the animals, removed the implanted titanium rod from the distal femur in order to avoid metal artifact, and performed microCT. We used a phantom made of an epoxy-based resin which mimics hydroxyapatite and contains water and air inclusion for calibration. Anesthesia was maintained by mask inhalation of isoflurane and animals were placed in the ventral position in the device, MicroCAT™ microCT scanner (ImTek, Inc, Knoxville, TN) with 80 mm resolution during 9 minutes. Before the procedure, scout images were made to confirm that both femurs were entirely scanned. After scanning, we used the MicroCAT™ software (Imtek, Inc) for acquisition and COBRA Reconstruction interface software (Exxim computing corporation, Pleasanton, CA) for reconstruction. For BMD assessment we used the MicroView software (GE medical Systems) and a 3D region of interest (ROI) was created (4 mm × 3 mm × 3 mm) at the level of the distal femur for both femurs. The data was collected as to mg/cc.
For bioluminescence, we used an in vivo imaging system (IVIS) employing a cooled device camera (Caliper LifeScience, Hopkinton, MA). Animals were anesthetized with 1.5% isoflurane during the process. We performed the bioluminescence with 3mg/mouse of Luciferase substrate D-Luciferin (Biosynth International), administrated intraperitoneally. Five minutes later, images were taken of the whole mouse. With regard to the interpretation of the images, we drew uniformly sized region of interest (ROI) (1.2 cm × 0.5 cm) at the level of the distal femur, for each femur. The data was collected as to photon/second/cm2/steradian (p/s/cm2/sr).
Histology and immunohistochemistry
After imaging, all animals were euthanized using both CO2 inhalation and cervical dislocation. Then the femora were collected from the animals (26 femora). Femora were decalcified using paraformaldehyde (PFA) for three days followed by ethylenediaminetetraacetic acid (EDTA) twice for five days each. Frozen sections of 7 µm were cut using a cryostat (Cambridge Instruments, Buffalo, NY) to include the distal third of the femora, where particles were infused. The sections collected were used for immunostaining. Image-iT® FX Signal Enhancer was first used for 1 hour followed by PBS with 10% normal goat serum for 1 hour at room temperature, in order to decrease non-specific binding and background staining. Mouse anti-Luciferase (Santa Cruz Biotechnology, CA) was used to detect migrated reported cells and rabbit anti-osteocalcin (Santa Cruz) was use to detect osteoblasts. The secondary antibody used for immunofluorescence was goat anti-mouse/rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA). ProLong® Gold Antifade Reagent with DAPI (Invitrogen) was used for mounting. We decreased background autofluorescence using the light spectra for specific fluorophores.
We also performed staining with hematoxylin and eosin (H&E) (Sigma, Steinheim, Germany) on cut histological sections. Osteoclast-like cells were identified using a leukocyte acid phosphatase kit, TRAP, (Sigma).
Statistical analysis
Bioluminescence data (ratio of operated divided by non-operated femora within ROI) and microCT data (bone mineral density) were analyzed by an analysis of variance and post hoc unpaired t-tests between Group 1 versus Group 2 and Group 1 versus Group 3 (Prism Software, GraphPad Software Inc.). Results of Group 4 were used for descriptive purposes due to loss of several animals during the injection process. Intergroup comparisons of osteoclast numbers were made of counting data using the Mann-Whitney U test.
Results
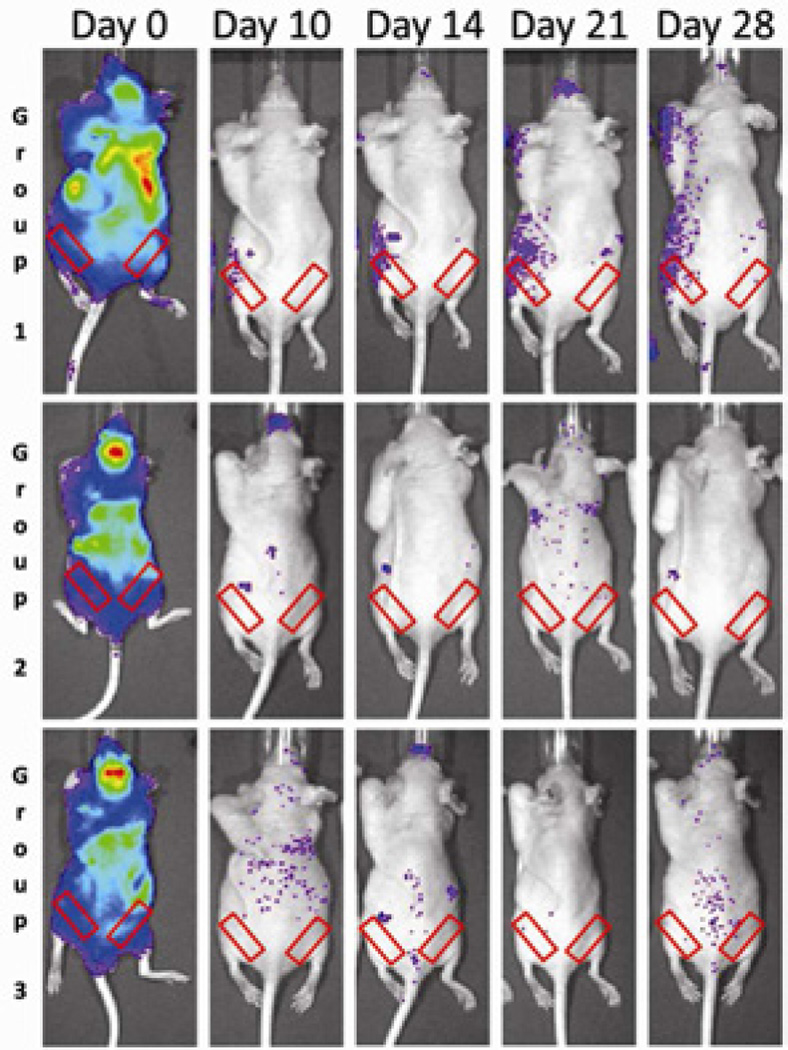
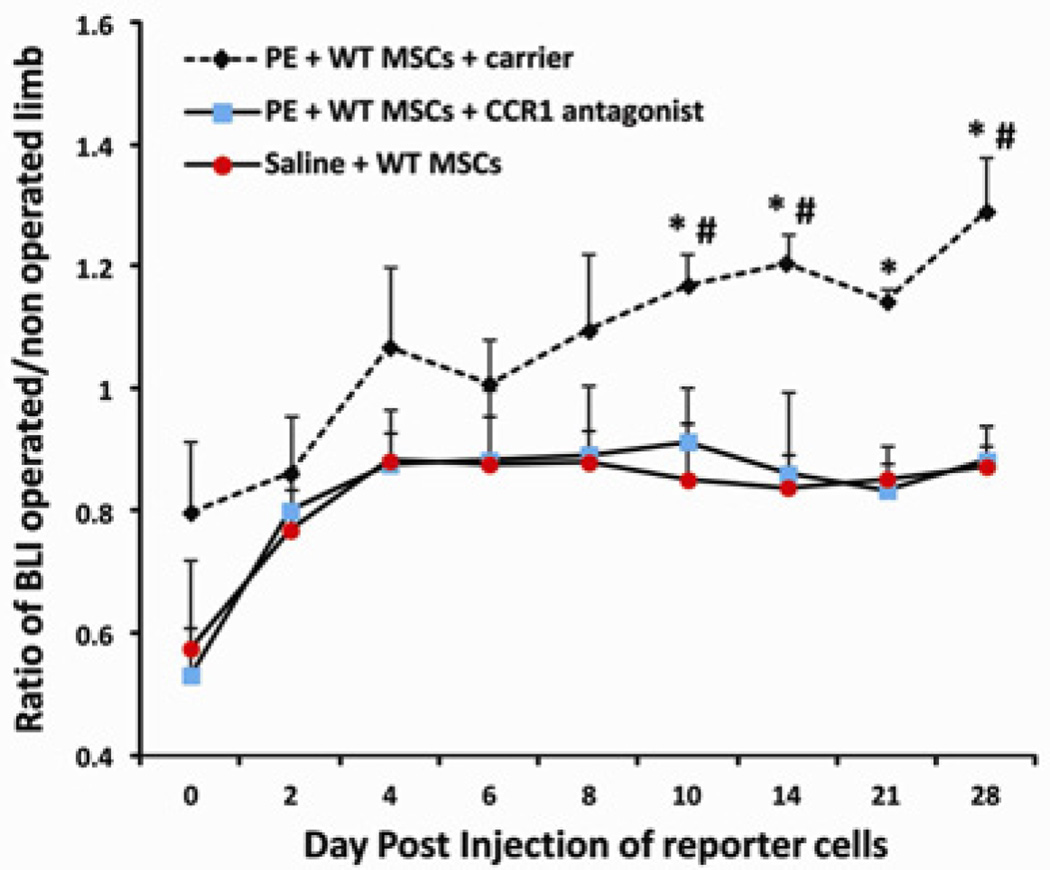
At day 0, immediately after cell injection, we confirmed the successful MSCs injection into the left heart using bioluminescence; cells were noted to be diffusely spread throughout the entire body or slightly concentrated in the lungs (Fig. 1). From day 10 to day 28, reporter WT MSCs were found to be systemically recruited toward the particle-infused femurs in Group 1. No increased bioluminescence was observed when the CCR1 antagonist was given (Group 2) or when the femur was infused with saline solution (Group 3). In Group 1, we observed a significant increase in systemic migration of WT MSCs at day 10, 14, 21, 28 compared to Group 2 (receiving the CCR1 antagonist) and at day 10, 21 and 28 compared to Group 3 (saline-infused group). At day 10, the ratio (operated limb divided by non-operated limb) of bioluminescence was 1.17 ± 0.05 for Group 1 whereas it was 0.91 ± 0.09 for Group 2 receiving the CCR1 antagonist (p = 0.04) and 0.85 ± 0.09 for Group 3 with saline-infused femurs (p = 0.01). At day 28, the ratio was 1.29 ± 0.09 for Group 1 whereas it was 0.88 ± 0.05 for Group 2 (p = 0.005) and 0.87 ± 0.03 for Group 3 (p = 0.002) (Fig. 2). No increased bioluminescence was observed for Group 4 (receiving CCR1−/− MSCs, data non shown).
Fig 1.
Bioluminescence (BLI) of each murine group at critical time points. At day 0, reporter cells were successfully injected into the left ventricle. In Group 1 (UHMWPE + MSCs + Carrier), reporter MSCs systemically migrated toward the infusion site (left femur) whereas no systemic migration was observed when the CCR1 antagonist was also given (Group 2). Red rectangles indicates the region of interest for BLI analysis.
Fig 2.
Graph showing the effect of the CCR1 antagonist. Reporter cells were injected through an intra-cardiac injection. At day 10, 14, 21 and 28, systemic migration of MSCs was significantly decreased when the CCR1 antagonist was used. * = p < 0.05; # = p < 0.05; *Group 1 vs. 2; #Group 1 vs. 3.
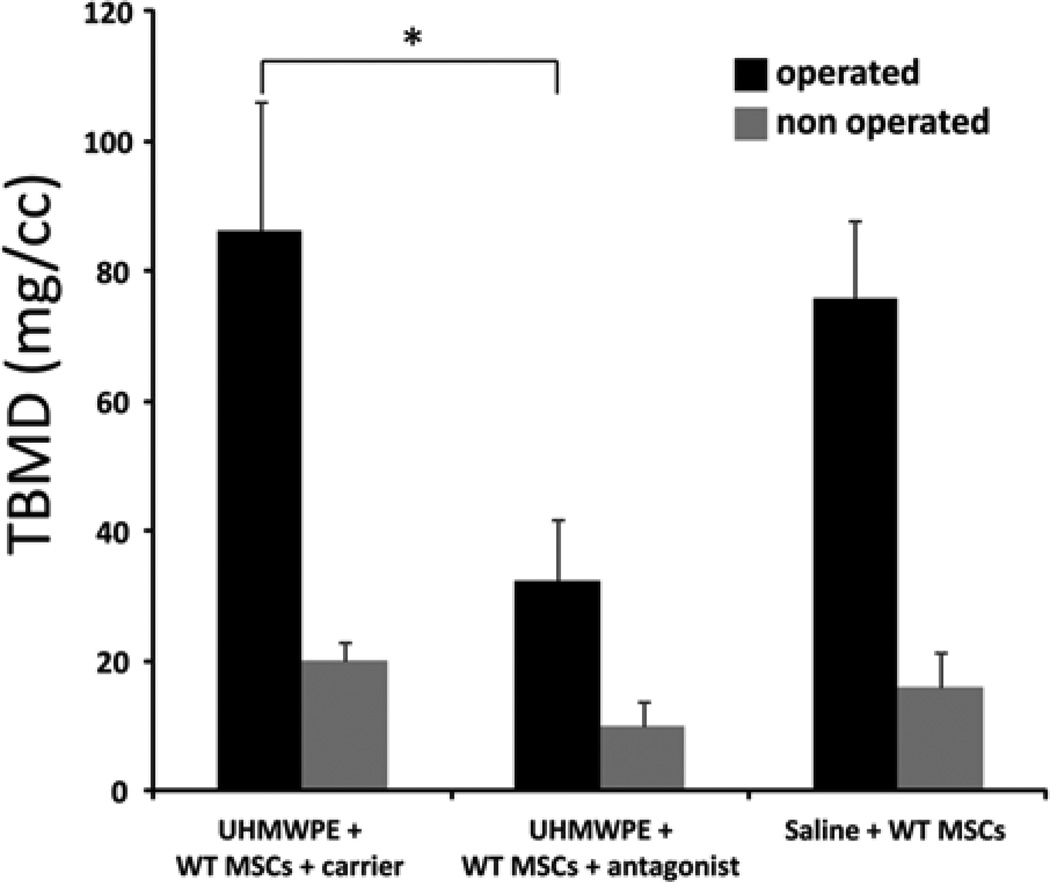
MicroCT analysis confirmed the critical role of CCR1 to recruit MSCs. Total bone mineral density (TBMD) was significantly decreased for Group 2 (receiving particles and the CCR1 antagonist) compared to Group 1 (receiving particles plus the carrier solution) at day 28. After normalization (post minus pre values for microCT), the TBMD was 86.06 ± 19.88 for Group 1 versus 32.29 ± 9.53 for Group 2 (p = 0.041) and 75.83 ± 11.87 for group 3 (Fig. 3). TBMD was similarly affected on the non-operated side for the different groups, indicating a so called “crossover effect” on the contra-lateral side that paralleled the systemic effects. This effect was seen in our previous studies using UHMWPE particles [10, 30].
Fig 3.
Bone loss (decreased BMD) was significantly higher when mice were treated with CCR1 antagonist. WTMSCs = wild type mesenchymal stem cells. TBMD = total bone mineral density.
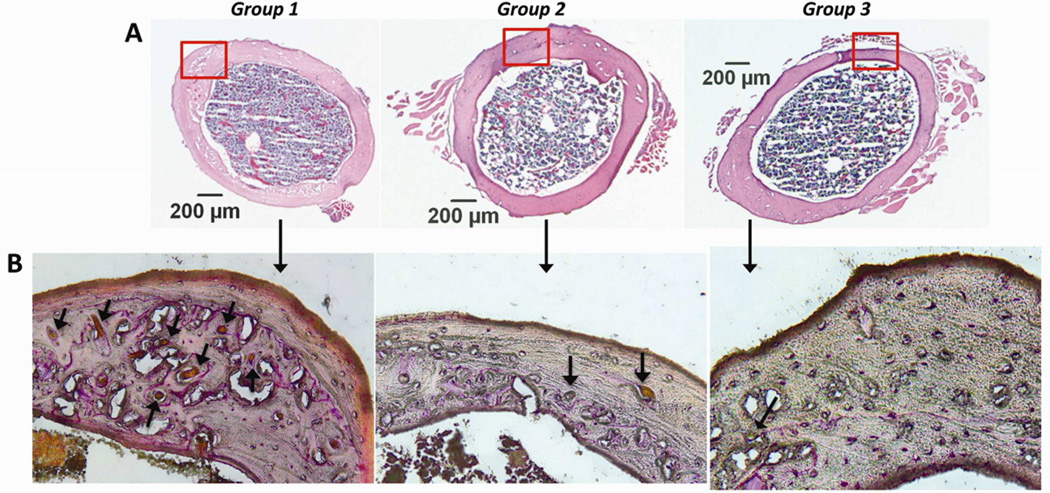
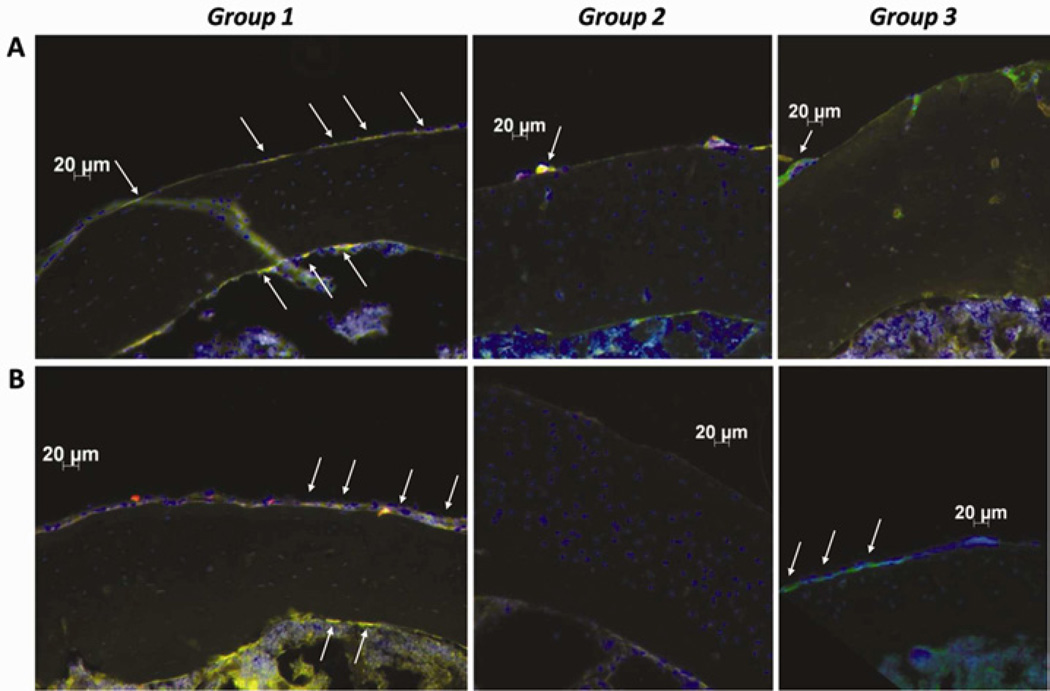
H&E-stained sections (Fig. 4A) and immunohistochemistry confirmed the migration of reporter cells to the femurs that were infused with UHWMPE particles. For Group 1, reporter MSCs migrated to the periosteum and the endosteum and differentiated into osteoblasts that were positive for osteocalcin. For Group 2 (UHMWPE plus CCR1 antagonist) reporter cells were rarely found in the periosteum and endosteum. For Group 3, only mature endogenous (reporter negative) osteoblasts were found within the periosteum (Fig. 5). Osteoclast-like cells were more common for group 1 compared to group 2 and 3 (p= 0.0012, Fig. 4B).
Fig 4.
Hematoxylin and eosin staining (A) and TRAP staining (B) for Groups 1, 2 and 3. Red rectangles indicate the area for immunohistochemistry. Few osteoclasts (arrows) were observed for group 2 and 3 compared to group 1. (H&E original magnification = ×50; TRAP original magnification =×200).
Fig 5.
Immunohistochemistry with mouse anti-luciferase (A) and rabbit anti-osteocalcin (B) antibodies. Exogenous reporter cells were transfected with tomato (red), the secondary antibody was conjugated with Alexa Fluor 488 (green), overlay of tomato and Alexa Fluor 488 shows migrated reporter cells (yellow). Few reporter MSCs (arrows) were observed when CCR1 antagonist was used (Group 2). Reporter MSCs differentiated into mature osteoblasts (Group 1, B), no exogenous but endogenous mature osteoblasts were found with infused saline solution (Group 3). (Original magnification = ×200)
Discussion
The aim of the current study was to demonstrate that CCR1 is a critical chemokine receptor for the systemic migration of MSCs in the presence of UHMWPE wear particles. WTMSCs were found to undergo systemic trafficking to the UHMWPE infusion site from the remote area of injection, subsequently enhancing bone formation and mitigating osteolysis. Our current study provides strong experimental evidence of a relationship between the CCR1 receptor and systemic trafficking of MSCs in the presence of UHMWPE wear particles. When the CCR1 antagonist was administered, MSC chemotaxis was decreased and the particle-associated adverse effects on bone mineral density were more profound. This finding may provide a strategic opportunity for the use of MSCs in treating osteolysis. This approach is already being considered for other orthopaedic conditions involving loss of articular cartilage, trauma with bone loss, injuries to tendons and ligaments, meniscus, and hip osteonecrosis [33–36]. Thus, local or systemic delivery of MSCs or biomolecules to enhance chemotaxis may be viable strategy to diminish osteolysis associated with wear debris.
Limitations of our study include the use of a short-term murine model that does not recapitulate all of the clinical, mechanical and biological variables associated with osteolysis in humans. However, the model does simulate continuous deliver of particles, similar to the clinical situation. In addition, CCR1 is not the only receptor involved in MSC chemotaxis in vivo [17, 21, 37]. The bioluminescence data show that when the CCR1 antagonist is given, systemic recruitment of MSCs still occurs. Others ligand/receptor axes involved in the biological reaction to orthopaedic wear debris may explain this remaining recruitment. Indeed, particle-induced macrophage activation results in the secretion of a number of different chemokines including Interleukin-8 (IL-8) [8]. Its receptor is CXCR2 [22] which is also highly represented on the cell surface of primary murine MSCs [38]. Thus the IL-8/CXCR2 ligand/receptor axis may further enhance MSC chemotaxis. Other ligand-receptor axes may also be involved [17, 21, 37].
Another interesting finding was the consistence between immunohistochemistry and microC results. Indeed, bone mineral density was similar for Group 1 (UHMWPE + WTMSCs + carrier) and for Group 3 (SALINE + WTMSCs). This finding was confirmed by immunohistochemistry where reporter cells systemically migrated to the periosteum and the endosteum and differentiated into mature osteoblasts in Group 1. However, in Group 3, few reporter cells migrated systemically (which is consistent with the BLI data), and only endogenous mature osteoblasts were found in increased numbers in the periosteum, explaining the high level of bone mineral density. The high level of bone mineral density for Group 3 (with saline infusion) may be explained in two ways. First, the mice were 10–12 weeks of age prior to euthanasia; this age is an intense period of bone growth in mice [39]. Second, the mechanical trauma due drilling of the femur though the intercondylar notch induces a regenerative response that facilitates bone repair. Therefore, even without polyethylene particle infusion, some level of systemic recruitment of MSCs, consistent with the BLI data, may occur through the MCP-3/CCR1 ligand receptor axis [22] as shown by Shinohara et al. [40] The dual effect of the CCR1 antagonist (which interrupts MSC recruitment), together with the infusion of polyethylene particles (which increases macrophage migration, osteoclast differentiation and proliferation) explains the decreased bone mineral density in Group 2. The same trend was observed when we used CCR1 −/− MSCs (Group 4) but due to issues related to animal survival, only 3 mice were included in this group. Indeed, because of the high mortality rate during the injection of the CCR1−/− MSCs (cell clumping after injection), only 3 animals survived. Due to ethical reasons no more animals were added to the group and the first three groups were only used for statistical purposes.
Conclusion
This work underscores the importance of CCR1 as a key receptor for systemic recruitment of MSCs in the presence of UHMWPE particles. Using a clinically relevant murine model of continuous local infusion of UHMWPE particles, our hypothesis was confirmed: MSCs were able to systemically migrate toward the particle infusion site and mitigate particle-associated bone loss increasing the bone mineral density. MSCs or biologics that facilitate MSC trafficking may provide a therapeutic strategy to improve biocompatibility of joint replacements.
Acknowledgements
This research was supported by the Ellenburg Chair in Surgery at Stanford University, 1R01AR055650-8 04 from the National Institute of Health and the French Granting Agency E.F.M.C. №1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84-A:171–177. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ries MD, Scott ML, Jani S. Relationship between gravimetric wear and particle generation in hip simulators: conventional compared with cross-linked polyethylene. J Bone Joint Surg Am. 2001;83-A(Pt 2) Suppl 2:116–122. doi: 10.2106/00004623-200100022-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop. 2009;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glant TT, Jacobs JJ, Molnar G, Shanbhag AS, Valyon M, Galante JO. Bone resorption activity of particulate-stimulated macrophages. J Bone Miner Res. 1993;8:1071–1079. doi: 10.1002/jbmr.5650080907. [DOI] [PubMed] [Google Scholar]
- 6.Archibeck MJ, Jacobs JJ, Roebuck KA, Glant TT. The basic science of periprosthetic osteolysis. Instr Course Lect. 2001;50:185–195. [PubMed] [Google Scholar]
- 7.Bauer TW. Particles and periimplant bone resorption. Clin Orthop. 2002;405:138–143. doi: 10.1097/00003086-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Tuan RS, Lee FY, Y TK, Wilkinson JM, Smith RL. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J Am Acad Orthop Surg. 2008;16 Suppl 1:S42–S48. doi: 10.5435/00124635-200800001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konttinen YT, Zhao D, Beklen A, Ma G, Takagi M, Kivela-Rajamaki M, et al. The microenvironment around total hip replacement prostheses. Clin Orthop. 2005;430:28–38. doi: 10.1097/01.blo.0000150451.50452.da. [DOI] [PubMed] [Google Scholar]
- 10.Gibon E, Ma T, Ren P-G, Fritton K, Biswal S, Yao Z, et al. Selective inhibition of the MCP- 1-CCR2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles. Jour Orthop Res. 2011 doi: 10.1002/jor.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren PG, Lee SW, Biswal S, Goodman SB. Systemic trafficking of macrophages induced by bone cement particles in nude mice. Biomaterials. 2008;29:4760–4765. doi: 10.1016/j.biomaterials.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren PG, Huang Z, Ma T, Biswal S, Smith RL, Goodman SB. Surveillance of systemic trafficking of macrophages induced by UHMWPE particles in nude mice by noninvasive imaging. J Biomed Mater Res A. 2010;94:706–711. doi: 10.1002/jbm.a.32744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman SB, Huie P, Song Y, Schurman D, Maloney W, Woolson S, et al. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br. 1998;80:531–539. doi: 10.1302/0301-620x.80b3.8158. [DOI] [PubMed] [Google Scholar]
- 14.Al-Saffar N, Revell PA. Differential expression of transforming growth factor-alpha and macrophage colony-stimulating factor/colony-stimulating factor-1R (c-fins) by multinucleated giant cells involved in pathological bone resorption at the site of orthopaedic implants. J Orthop Res. 2000;18:800–807. doi: 10.1002/jor.1100180518. [DOI] [PubMed] [Google Scholar]
- 15.Xu JW, Konttinen YT, Waris V, Patiala H, Sorsa T, Santavirta S. Macrophage-colony stimulating factor (M-CSF) is increased in the synovial-like membrane of the periprosthetic tissues in the aseptic loosening of total hip replacement (THR) Clin Rheumatol. 1997;16:243–248. doi: 10.1007/BF02238958. [DOI] [PubMed] [Google Scholar]
- 16.Goodman SB, Chin RC, Chiou SS, Schurman DJ, Woolson ST, Masada MP. A clinical-pathologic- biochemical study of the membrane surrounding loosened and nonloosened total hip arthroplasties. Clin Orthop. 1989;244:182–187. [PubMed] [Google Scholar]
- 17.Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43:596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooke G, Tong H, Levesque J-P, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17:929–940. doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- 19.Song CH, Honmou O, Furuoka H, Horiuchi M. Identification of chemoattractive factors involved in the migration of bone marrow-derived mesenchymal stem cells to brain lesions caused by prions. J Virol. 2011;85:11069–11078. doi: 10.1128/JVI.05318-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, et al. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010;106:1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 22.Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahlaei M, Madadkar-Sobhani A, Fassihi A, Saghaie L. Exploring a Model of a Chemokine Receptor/Ligand Complex in an Explicit Membrane Environment by Molecular Dynamics Simulation: The Human CCR1 Receptor. J Chem Inf Model. 2011;51:2717–2730. doi: 10.1021/ci200261f. [DOI] [PubMed] [Google Scholar]
- 24.Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin J-P, Davenport AP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 25.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 26.Von Luttichau I, Notohamiprodjo M, Wechselberger A, Peters C, Henger A, Seliger C, et al. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329–336. doi: 10.1089/scd.2005.14.329. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Botran R. A small-molecule antagonist of human and murine CCR1 receptors. Expert Opin Investig Drugs. 2001;10:1387–1389. doi: 10.1517/13543784.10.7.1387. [DOI] [PubMed] [Google Scholar]
- 28.Naya A, Sagara Y, Ohwaki K, Saeki T, Ichikawa D, Iwasawa Y, et al. Design, synthesis, and discovery of a novel CCR1 antagonist. J Med Chem. 2001;44:1429–1435. doi: 10.1021/jm0004244. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Ma T, Ren P-G, Smith RL, Goodman SB. Effects of orthopedic polymer particles on chemotaxis of macrophages and mesenchymal stem cells. J Biomed Mater Res A. 2010;94:1264–1269. doi: 10.1002/jbm.a.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren PG, Irani A, Huang Z, Ma T, Biswal S, Goodman SB. Continuous Infusion of UHMWPE Particles Induces Increased Bone Macrophages and Osteolysis. Clin Orthop. 2011;469:113–122. doi: 10.1007/s11999-010-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman SB, Ma T. Cellular chemotaxis induced by wear particles from joint replacements. Biomaterials. 2010;31:5045–5050. doi: 10.1016/j.biomaterials.2010.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell P, Ma S, Yeom B, McKellop H, Schmalzried TP, Amstutz HC. Isolation of predominantly submicron-sized UHMWPE wear particles from periprosthetic tissues. J Biomed Mater Res. 1995;29:127–131. doi: 10.1002/jbm.820290118. [DOI] [PubMed] [Google Scholar]
- 33.Hernigou P, Poignard A, Zilber S, Rouard Hln. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43:40–45. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernigou P, Poignard A, Manicom O, Mathieu G, Rouard H. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87:896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 35.Hernigou P, Daltro G, Filippini P, Mukasa MM, Manicom O. Percutaneous implantation of autologous bone marrow osteoprogenitor cells as treatment of bone avascular necrosis related to sickle cell disease. Open Orthop J. 2008;2:62–65. doi: 10.2174/1874325000802010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee EH, Hui JH. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg Br. 2006;88:841–851. doi: 10.1302/0301-620X.88B7.17305. [DOI] [PubMed] [Google Scholar]
- 37.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 38.Chamberlain G, Wright K, Rot A, Ashton B, Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PloS One. 2008;3:e2934–e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 40.Shinohara K, Greenfield S, Pan H, Vasanji A, Kumagai K, Midura RJ, et al. Stromal cellderived factor-1 and monocyte chemotactic protein-3 improve recruitment of osteogenic cells into sites of musculoskeletal repair. J Orthop Res. 2011;29:1064–1069. doi: 10.1002/jor.21374. [DOI] [PubMed] [Google Scholar]