Abstract
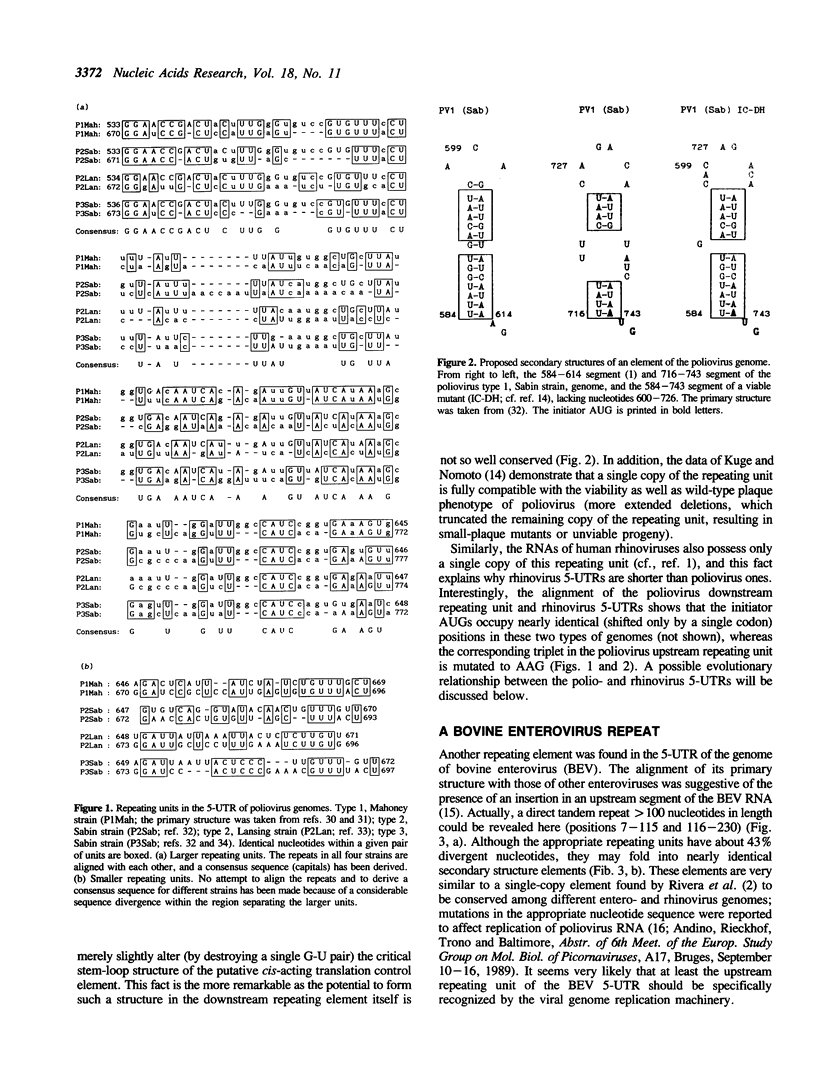
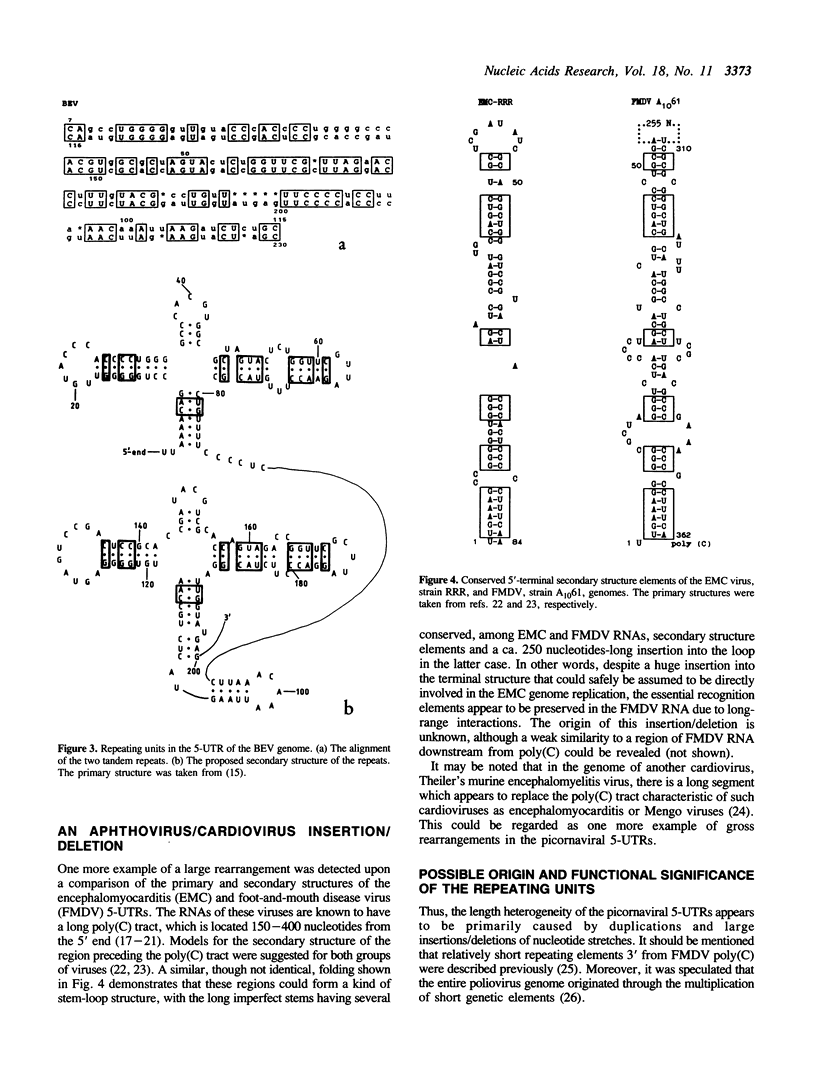
An analysis of reported nucleotide sequences revealed several cases of gross rearrangements in the 5'-untranslated region (5-UTR) of picornaviral genomes. A large (greater than 100 nt) duplication was discovered in a downstream region of poliovirus 5-UTR involved in the translational control. Properties of the poliovirus mutants with large deletions [Kuge and Nomoto (1987) J. Virol. 61, 1478-1487] show that a single copy of the appropriate repeating unit is compatible with a wild type phenotype of the virus. In contrast to poliovirus and another enterovirus genomes, human rhinovirus RNAs contain only a single copy of this repeating unit. Another similarly large repeat was found in an upstream segment of the bovine enterovirus 5-UTR. A comparison of the primary and secondary structures of cardio- and aphthovirus 5-UTRs demonstrated the existence of a large (ca. 250 nucleotides) insertion/deletion in a region preceding the poly(C) tract. The two latter rearrangements appear to involve elements of the viral genome replication machinery. Possible origin as well as evolutionary and functional implications of these structural peculiarities are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowska-Szewczyk K., Ehrenfeld E. An internal 5'-noncoding region required for translation of poliovirus RNA in vitro. J Virol. 1988 Aug;62(8):3068–3072. doi: 10.1128/jvi.62.8.3068-3072.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F., Newman J., Stott J., Porter A., Frisby D., Newton C., Carey N., Fellner P. Poly(C) in animal viral RNAs. Nature. 1974 Sep 27;251(5473):342–344. doi: 10.1038/251342a0. [DOI] [PubMed] [Google Scholar]
- Chumakov K. M., Agol V. I. Poly(C) sequence is located near the 5'-end of encephalomyocarditis virus RNA. Biochem Biophys Res Commun. 1976 Jul 26;71(2):551–557. doi: 10.1016/0006-291x(76)90822-6. [DOI] [PubMed] [Google Scholar]
- Chumakov K. M., Chichkova N. V., Agol V. I. 5'-kontsevaia posledovatel'nost' RNK virusa éntsefalomiokardita: lokalizatsiia poliTs-trakta i rol' v transliatsii. Dokl Akad Nauk SSSR. 1979;246(4):994–996. [PubMed] [Google Scholar]
- Clarke B. E., Brown A. L., Currey K. M., Newton S. E., Rowlands D. J., Carroll A. R. Potential secondary and tertiary structure in the genomic RNA of foot and mouth disease virus. Nucleic Acids Res. 1987 Sep 11;15(17):7067–7079. doi: 10.1093/nar/15.17.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Wright R. M. DNA sequence of the excision sites of a mitochondrial plasmid from senescent Podospora anserina. Nucleic Acids Res. 1983 Apr 11;11(7):2111–2119. doi: 10.1093/nar/11.7.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle J. A., Skuce R. A., Fleming C. S., Hoey E. M., Martin S. J. The complete nucleotide sequence of a bovine enterovirus. J Gen Virol. 1988 Feb;69(Pt 2):253–263. doi: 10.1099/0022-1317-69-2-253. [DOI] [PubMed] [Google Scholar]
- Gorbalenia A. E., Donchenko A. P., Blinov V. M. O vozmozhnoi obshchnosti proiskhozhdeniia belkov virusa poliomielita, vypolniaiushchikh raznye funktsii. Mol Gen Mikrobiol Virusol. 1986 Jan;(1):36–41. [PubMed] [Google Scholar]
- Harris T. J., Brown F. The location of the ploy(C) tract in the RNA of foot-and-mouth disease virus. J Gen Virol. 1976 Dec;33(3):493–501. doi: 10.1099/0022-1317-33-3-493. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Baltimore D. The mechanism of RNA recombination in poliovirus. Cell. 1986 Nov 7;47(3):433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kuge S., Nomoto A. Construction of viable deletion and insertion mutants of the Sabin strain of type 1 poliovirus: function of the 5' noncoding sequence in viral replication. J Virol. 1987 May;61(5):1478–1487. doi: 10.1128/jvi.61.5.1478-1487.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Saito I., Nomoto A. Primary structure of poliovirus defective-interfering particle genomes and possible generation mechanisms of the particles. J Mol Biol. 1986 Dec 5;192(3):473–487. doi: 10.1016/0022-2836(86)90270-6. [DOI] [PubMed] [Google Scholar]
- La Monica N., Meriam C., Racaniello V. R. Mapping of sequences required for mouse neurovirulence of poliovirus type 2 Lansing. J Virol. 1986 Feb;57(2):515–525. doi: 10.1128/jvi.57.2.515-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5'-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989 Jul;3(7):1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Newton S. E., Carroll A. R., Campbell R. O., Clarke B. E., Rowlands D. J. The sequence of foot-and-mouth disease virus RNA to the 5' side of the poly(C) tract. Gene. 1985;40(2-3):331–336. doi: 10.1016/0378-1119(85)90057-5. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Kaplan G., Racaniello V. R., Sonenberg N. Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5' noncoding region. Mol Cell Biol. 1988 Mar;8(3):1103–1112. doi: 10.1128/mcb.8.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Pestova T. V., Maslova S. V., Potapov V. K., Agol V. I. Distinct modes of poliovirus polyprotein initiation in vitro. Virus Res. 1989 Oct;14(2):107–118. doi: 10.1016/0168-1702(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Pevear D. C., Calenoff M., Rozhon E., Lipton H. L. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987 May;61(5):1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Chernov B. K., Dmitrieva T. M., Agol V. I. Conservation of the secondary structure elements of the 5'-untranslated region of cardio- and aphthovirus RNAs. Nucleic Acids Res. 1989 Jul 25;17(14):5701–5711. doi: 10.1093/nar/17.14.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko E. V., Blinov V. M., Romanova L. I., Sinyakov A. N., Maslova S. V., Agol V. I. Conserved structural domains in the 5'-untranslated region of picornaviral genomes: an analysis of the segment controlling translation and neurovirulence. Virology. 1989 Feb;168(2):201–209. doi: 10.1016/0042-6822(89)90259-6. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Meriam C. Poliovirus temperature-sensitive mutant containing a single nucleotide deletion in the 5'-noncoding region of the viral RNA. Virology. 1986 Dec;155(2):498–507. doi: 10.1016/0042-6822(86)90211-4. [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Welsh J. D., Maizel J. V., Jr Comparative sequence analysis of the 5' noncoding region of the enteroviruses and rhinoviruses. Virology. 1988 Jul;165(1):42–50. doi: 10.1016/0042-6822(88)90656-3. [DOI] [PubMed] [Google Scholar]
- Romanova L. I., Blinov V. M., Tolskaya E. A., Viktorova E. G., Kolesnikova M. S., Guseva E. A., Agol V. I. The primary structure of crossover regions of intertypic poliovirus recombinants: a model of recombination between RNA genomes. Virology. 1986 Nov;155(1):202–213. doi: 10.1016/0042-6822(86)90180-7. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J., Harris T. J., Brown F. More precise location of the polycytidylic acid tract in foot and mouth disease virus RNA. J Virol. 1978 May;26(2):335–343. doi: 10.1128/jvi.26.2.335-343.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. A., Racaniello V. R., Dunn G., Cooper J., Minor P. D., Almond J. W. New model for the secondary structure of the 5' non-coding RNA of poliovirus is supported by biochemical and genetic data that also show that RNA secondary structure is important in neurovirulence. J Mol Biol. 1989 May 20;207(2):379–392. doi: 10.1016/0022-2836(89)90261-1. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Maslova S. V., Agol V. I. The genomes of attenuated and virulent poliovirus strains differ in their in vitro translation efficiencies. Virology. 1985 Dec;147(2):243–252. doi: 10.1016/0042-6822(85)90127-8. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Pestova T. V., Maslova S. V., Agol V. I. Point mutations modify the response of poliovirus RNA to a translation initiation factor: a comparison of neurovirulent and attenuated strains. Virology. 1988 Oct;166(2):394–404. doi: 10.1016/0042-6822(88)90510-7. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Trono D., Andino R., Baltimore D. An RNA sequence of hundreds of nucleotides at the 5' end of poliovirus RNA is involved in allowing viral protein synthesis. J Virol. 1988 Jul;62(7):2291–2299. doi: 10.1128/jvi.62.7.2291-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartapetian A. B., Mankin A. S., Skripkin E. A., Chumakov K. M., Smirnov V. D., Bogdanov A. A. The primary and secondary structure of the 5'-end region of encephalomyocarditis virus RNA. A novel approach to sequencing long RNA molecules. Gene. 1983 Dec;26(2-3):189–195. doi: 10.1016/0378-1119(83)90189-0. [DOI] [PubMed] [Google Scholar]
- del Angel R. M., Papavassiliou A. G., Fernández-Tomás C., Silverstein S. J., Racaniello V. R. Cell proteins bind to multiple sites within the 5' untranslated region of poliovirus RNA. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8299–8303. doi: 10.1073/pnas.86.21.8299. [DOI] [PMC free article] [PubMed] [Google Scholar]


