Abstract
Omega-3 fatty acids have been proposed as an adjuvant treatment option in psychiatric disorders. Given their other health benefits and their relative lack of toxicity, teratogenicity and side effects, they may be particularly useful in children and in females of child-bearing age, especially during pregnancy and postpartum. A comprehensive mechanistic understanding of their effects is needed. Here we report translational studies demonstrating the phenotypic normalization and gene expression effects of dietary omega-3 fatty acids, specifically docosahexaenoic acid (DHA), in a stress-reactive knockout mouse model of bipolar disorder and co-morbid alcoholism, using a bioinformatic convergent functional genomics approach integrating animal model and human data to prioritize disease-relevant genes. Additionally, to validate at a behavioral level the novel observed effects on decreasing alcohol consumption, we also tested the effects of DHA in an independent animal model, alcohol-preferring (P) rats, a well-established animal model of alcoholism. Our studies uncover sex differences, brain region-specific effects and blood biomarkers that may underpin the effects of DHA. Of note, DHA modulates some of the same genes targeted by current psychotropic medications, as well as increases myelin-related gene expression. Myelin-related gene expression decrease is a common, if nonspecific, denominator of neuropsychiatric disorders. In conclusion, our work supports the potential utility of omega-3 fatty acids, specifically DHA, for a spectrum of psychiatric disorders such as stress disorders, bipolar disorder, alcoholism and beyond.
Keywords: alcoholism, bipolar, DHA, genomics, omega-3, stress
Introduction
‘First do no harm'
–Hippocratic Oath
There is a strong need for better treatments, with less side effects, for stress, mood and alcohol use disorders. Natural compounds may offer a source for such treatments, but have been in general insufficiently studied in preclinical models, and a molecular understanding is lacking. Omega-3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid (DHA)) are essential fatty acids, with DHA being the final metabolic pathway compound. They have been speculated to have had an evolutionary role in the development of the brain in higher organisms,1 and their relative depletion compared with proinflammatory omega-6 fatty acids in modern Western diets has been invoked as having a role in the pathophysiology of multiple diseases.2 Omega-3 fatty acids, particularly DHA, have been described to have mood- and psychosis-modulating properties, in both preclinical models and some clinical trials. For example, deficits in omega-3 fatty acids have been linked to increased depression and aggression in animal models3, 4 and humans.5, 6 Of note, deficits in DHA have been reported in erythrocytes7 and in the post-mortem orbitofrontal cortex of patients with bipolar disorder, and were greater in those who had high vs those who had low alcohol abuse.8 Omega-3 fatty acids have been reported to be clinically useful in the treatment of both mood9, 10, 11, 12 and psychotic disorders.13, 14, 15 To date, there is no clear understanding of how they work in terms of psychotropic effects, or indeed how well they actually work. Unlike most psychiatric drugs, these natural compounds have minimal side effects, and intriguing evidence for favorable health benefits.16, 17, 18 Particularly for children and female patients of child-bearing age, the potential developmental and teratogenic side effects of mood-stabilizing and antidepressant medications are a major issue. As such, if the action of omega-3 fatty acids in mood disorders and other related disorders could be substantiated by understanding their mechanistic effects and the identification of candidate molecular biomarkers for treatment response, they would become an important consideration as an addition to the therapeutic armamentarium of psychiatrists, pediatricians and primary care doctors.
We have previously identified the circadian clock gene D-box binding protein (DBP) as a potential candidate gene for bipolar disorder,19 as well as for alcoholism20 and schizophrenia,21 using a convergent functional genomics (CFG) approach. In follow-up work, we established mice with a homozygous deletion of DBP (DBP knockout (KO)) as a stress-reactive genetic animal model of bipolar disorder and co-morbid alcoholism.22 We reported that DBP KO mice have lower locomotor activity and blunted responses to stimulants and they gain less weight over time. In response to a chronic stress paradigm, the mice exhibit a diametric switch in these phenotypes. DBP KO mice are also activated by sleep deprivation, similar to bipolar patients, and that activation is prevented by treatment with the mood stabilizer drug valproate. Moreover, these mice show increased alcohol intake following exposure to stress. Microarray studies of brain and blood revealed a pattern of gene expression changes that may explain the observed phenotypes. CFG analysis of the gene expression changes identified a series of novel candidate genes and blood biomarkers for bipolar disorder, alcoholism and stress reactivity.
Based on the above, we decided to test omega-3 fatty acids, specifically DHA, at a phenotypic, gene expression and blood biomarker level, in this animal model (DBP KO mice subjected to a chronic stress paradigm), using a case–case design23 to increase signal detection and focus on the effects of DHA. We also studied the effects of DHA on modulating alcohol consumption in these mice and in an independent animal model, the alcohol-preferring (P) rats, a well-established model of alcoholism. Of note, there is a high degree of co-morbidity of alcoholism with depression24, 25 as well as with bipolar disorder.26 The work described has important translational implications for understanding and validating a new treatment approach, which follows the Hippocratic principle of ‘first do no harm' and may favorably impact multiple co-morbid medical and psychiatric conditions.
Materials and methods
Mouse colony
The generation of transgenic mice carrying DBP-KO has been previously described in detail.22 DBP (+/−) heterozygous (HET) mice were bred to obtain mixed littermate cohorts of DBP (+/+) wild-type (WT), HET and DBP (−/−) KO mice. Mouse pups were weaned at 21 days and housed by gender in groups of two to four in a temperature- and light-controlled colony on reverse cycle (lights on at 2200 h, lights off at 1000 h), with food and water available ad libitum. DNA for genotyping was extracted by tail digestion with a Qiagen DNeasy Tissue kit, following the protocol for animal tissue (Qiagen, Valencia, CA, USA). The following three primers were used for genotyping by PCR:
Dbp forward: 5′-TTCTTTGGGCTTGCTGTTTCCCTGCAGA-3′
Dbp reverse: 5′-GCAAAGCTCCTTTCTTTGCGAGAAGTGC-3′ (WT allele)
lacZ reverse: 5′-GTGCTGCAAGGCGATTAAGTTGGGTAAC-3′ (KO allele)
WT or KO mice, 8–12 weeks old, were used for experiments.
Animal housing, diets and treatment
All mice were housed for at least 1 week before each experiment in a room set to an alternating light cycle with 12 h of darkness from 1000 to 2200 h, and 12 h of light from 2200 to 1000 h. At the start of the experiment, male and female DBP (+/+) WT or DBP (−/−) KO mice were placed on one of the two diets: (1) low DHA custom research diet (TD 00522, Harlan Teklad, Madison, WI, USA), a DHA-depleting low n-3 PUFA test diet adequate in all other nutrients (n-6/n-3 ratio of 85:1 with 6% fat as safflower oil);27 or (2) high DHA custom research diet (TD 07708 low-DHA diet supplemented with 0.69% algal DHA; Martek Bioscience, Columbia, MD, USA).27 The DBP mice were fed the low-DHA diet (0% DHA) or high-DHA diet (0.69% DHA) for 28 days. Mice and food and water were weighed twice a week. Water was refilled once a week.
Mice were subjected to a chronic stress paradigm consisting of isolation (single housing) for 28 days, with an acute stressor (behavioral challenge tests) on day 21. The behavioral challenge tests consisted of sequential administration of the forced swim test (FST), tail flick test and tail suspension test.
At 4 weeks (day 28), the mice were injected with saline to keep handling consistent with previous work22 and their open field locomotor activity was assessed with SMART II video-tracking software (San Diego Instruments, San Diego, CA, USA). After video tracking, brain and blood were harvested as previously described22 for use in microarray studies.
Behavioral challenge tests
Forced Swim Test
FST experiments were performed on day 21 of treatment during the dark cycle. Mice were placed one at a time in a transparent plexiglas cylinder (64 cm height × 38 cm diameter), with water depth of 30 cm and temperature of 23±2 °C. Water was replaced after each mouse tested. Time spent immobile in a 10-min interval was scored live by two independent observers blinded to the genotype and treatment group of the animals.
Tail flick
Immediately following the FST, the mice were dried with paper towels and placed in the Plexiglas chamber of the Tail Flick Analgesia Meter System (San Diego Instruments). The mouse's tail was placed over a window located on the Tail Flick platform where a light beam shines to heat the tail at a reliable, reproducible rate for 20±1 s. This test was performed as an acute stressor, and not as a way to measure the mouse's response to pain, as it is confounded by the preceding test.
Tail suspension
For the third part of the acute stress paradigm, the mouse was suspended by its tail, ∼30 cm above the ground for 5 min. This test was performed as an acute stressor, and not as a way to measure the mouse's behavior, as it is confounded by the preceding tests.
Locomotion testing
A SMART II Video Tracker (VT) system (San Diego Instruments) under normal light was used to track the movement of mice. The mice were placed in the lower-right-hand corner of one of four adjacent, 41 × 41 × 34 cm3 enclosures. Mice were not allowed any physical contact with other mice during testing. Each enclosure had nine predefined areas, that is, center area, corner area and wall area. The movements of the mice were recorded for 30 min. The enclosures were cleaned with water after each tracking. Measures of total distance traveled, center entry, center time, fast movement, resting time, average velocity (V mean) and maximum velocity (V max) were obtained.
Clustering analysis of locomotion pattern using GeneSpring
GeneSpring GX (Agilent Technologies, Palo Alto, CA, USA), the most widely used, commercially available, microarray gene expression analysis software, was adapted for the novel use of analyzing and visualizing phenotypic data. We have inputted the scores on phenotypic items numbers in lieu of the usual use of gene expression intensity numbers. All the subsequent analyses were carried out using the same tools as for gene expression data sets, as per the manufacturer's instructions (www.chem.agilent.com). Unsupervised two-way hierarchical clustering of normalized (Z-scored) behavioral data from locomotor testing was carried out using methodology previously described.22, 28
Alcohol consumption experiments in mice
To create an alcohol free-choice drinking paradigm, male DBP (+/+) WT or DBP (−/−) KO mice were placed in individual cages with both a bottle of ∼250 ml cold tap water and a bottle of ∼250 ml 10% ethanol, the customary concentration used in mouse studies of alcohol consumption, and either a low- or high-DHA diet for 28 days, with an acute stressor (behavior challenge tests described above) on day 21. The amount of ethanol and water consumed was recorded twice a week, at which time the locations of the bottles were switched to prevent positional bias. The bottles were refilled with fresh solution once a week.
Alcohol consumption experiments in alcohol-preferring (P) rats
Experimentally naive, male P rats, 4–6 months of age at the start of the experiment, were used as subjects. They were placed on three diets (1) low DHA custom research diet (TD 00522, Harlan Teklad); (2) high omega-3 custom research diet (TD 07708, 0.69% DHA), similar to the DBP KO mice experiments; and (3) normal rat diet (7001, Harlan Teklad) for a duration of 28 days. Food and water were available ad libitum throughout the experiments. Rats were given continuous free-choice access in the home cage to 15% v/v ethanol and water, the customary concentration used in rat studies of alcohol consumption. Ethanol intake was measured daily throughout the experiment.
Behavioral statistical analysis
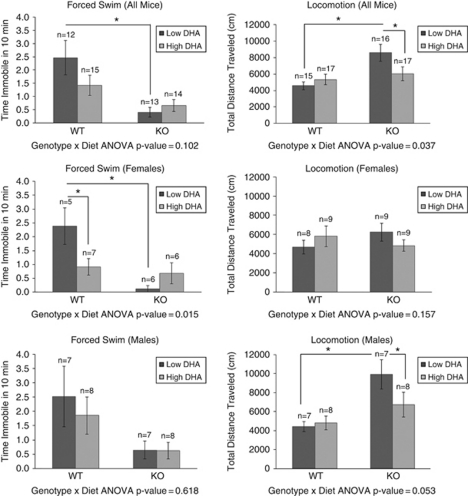
Behavioral data are expressed as the mean±s.e.m. Two-way analysis of variance was used to determine statistically significant differences for factors of gender, genotype and diet, using SPSS statistical software (SPSS, Chicago, IL, USA). We used a one-tailed, two-sample independent t-tests assuming unequal variance to determine significant differences between individual groups. Differences between groups were considered significant at a P<0.05 (Figure 1).
Figure 1.
Effects of docosahexaenoic acid (DHA) on stressed mice behavior: DBP (+/+) wild-type (WT) and DBP (−/−) knockout (KO) mice on a diet either high or low in DHA were subjected to a chronic stress paradigm consisting of isolation (single housing) for 28 days, with an acute stressor (behavioral challenge tests, including forced swim test) at day 21.On day 28, video-tracking software was used to measure locomotion (total distance traveled, in centimeters) during a 30-min period in open field. Two-factor analysis of variance (ANOVA) was done for genotype and diet. Additionally, one-tail t-tests with *P<0.05 are depicted.
RNA extraction and microarray work
Following the locomotor behavioral testing, mice were sacrificed by cervical dislocation, then they were decapitated and blood was collected. Behavioral testing and tissue harvesting were done at the same time of day in all experiments. The brains of the mice were harvested, stereotactically sliced, and hand microdissected using Paxinos mouse anatomical atlas coordinates, to isolate anatomical regions of interest—prefrontal cortex (PFC), amygdala (AMY) and hippocampus (HIP).21, 29 Tissues were flash frozen in liquid nitrogen and stored at −80 °C pending RNA extraction. Approximately 0.5–1 ml of blood per mouse was collected into a PAXgene blood RNA collection tubes (BD Diagnostics, Franklin Lakes, NJ, USA). The PAXgene blood vials were stored in −4 °C overnight, and then at −80 °C until future processing for RNA extraction.
Standard techniques were used to obtain total RNA (22-gauge syringe homogenization in RLT buffer) and to purify the RNA (RNeasy mini kit, Qiagen) from microdissected mouse brain regions. For the whole mouse blood RNA extraction, PAXgene blood RNA extraction kit (PreAnalytiX, a QIAGEN/BD company, BD Diagnostics) was used, followed by GLOBINclear-Mouse/Rat (Ambion/Applied Biosystems, Austin, TX, USA) to remove the globin mRNA. All the methods and procedures were carried out as per the manufacturer's instructions. The quality of the total RNA was confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies). The quantity and quality of total RNA was also independently assessed by 260 nm ultraviolet absorption and by 260/280 ratios, respectively (Nanodrop spectrophotometer, Thermo Scientific, Wilmington, DE, USA). Starting material of total RNA labeling reactions was kept consistent within each independent microarray experiment.
Equal amounts of total RNA extracted from the brain tissue samples or blood from three mice per group was pooled for each experimental condition and used for labeling and hybridization to Mouse Genome 430 2.0 arrays (Affymetrix, Santa Clara, CA, USA). The GeneChip Mouse Genome 430 2.0 Array contains over 45 000 probe sets that analyze the expression level of over 39 000 transcripts and variants from over 34 000 well-characterized mouse genes. Standard Affymetrix protocols were used to reverse transcribe the messenger RNA and generate biotinlylate cRNA (http://www.affymetrix.com/support/downloads/manuals/expression_s2_manual.pdf). The amount of cRNA used to prepare the hybridization cocktail was kept constant within each experiment. Samples were hybridized at 45 °C for 17 h under constant rotation. Arrays were washed and stained using the Affymetrix Fluidics Station 400 and scanned using the Affymetrix Model 3000 Scanner controlled by GCOS software. All sample labeling, hybridization, staining and scanning procedures were carried out as per the manufacturer's recommendations.
Quality control
All arrays were scaled to a target intensity of 1000 using Affymetrix MASv 5.0 array analysis software. Quality control measures including 3′/5′ ratios for glyceraldehyde 3-phosphate dehydrogenase and β-actin, scaling factors, background and Q values were used.
Microarray data analysis
Data analysis was performed using Affymetrix Microarray Suite 5.0 software (MAS v5.0). Default settings were used to define transcripts as present (P), marginal (M) or absent (A). A comparison analysis was performed for DBP KO mice on high-DHA diet, using DBP KO mice on low-DHA diet as the baseline. ‘Signal', ‘Detection', ‘Signal Log Ratio', ‘Change' and ‘Change P-value' were obtained from this analysis. An empirical P-value threshold for change of P<0.0025 was used. Only transcripts that were called Present and that were reproducibly changed in the same direction in two independent experiments were analyzed further.
Gene identification
The identities of transcripts was established using NetAFFX (Affymetrix), and confirmed by cross-checking the target mRNA sequences that had been used for probe design in the Affymetrix Mouse Genome 430 2.0 arrays GeneChip with the GenBank database. Probe sets that did not have a known gene are labeled ‘EST' and their accession numbers kept as identifiers.
Convergent Functional Genomics analyses
Databases
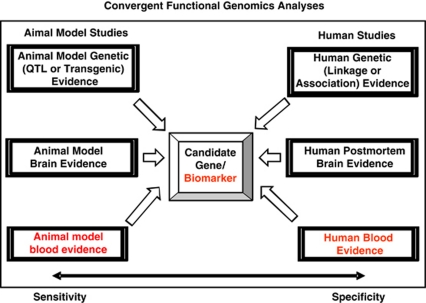
We have established in our laboratory (Laboratory of Neurophenomics, IU School of Medicine) manually curated databases of all the human gene expression (postmortem brain, blood), human genetic (association, linkage) and animal model gene expression studies published to date on psychiatric disorders. These constantly updated large databases have been used in our CFG cross-validation (Figure 2).
Figure 2.
Convergent functional genomics (CFG). Bayesian integration of multiple animal model and human lines of evidence to prioritize disease-relevant genes.
Human genetic evidence (linkage, association)
To designate convergence for a particular gene, the gene had to map within 10 cM (see ref. 19 for detailed discussion) of a microsatellite marker for which at least one published study showed evidence of genetic linkage or a positive association study for the gene itself was reported in the literature (for bipolar disorder, depression, alcoholism, stress and anxiety). The University of Southampton's sequence-based integrated map of the human genome (The Genetic Epidemiological Group, Human Genetics Division, University of Southampton: http://cedar.genetics.soton.ac.uk/public_html/) was used to obtain cM locations for both genes and markers. The sex-averaged cM value was calculated and used to determine convergence to a particular marker. For markers that were not present in the Southampton database, the Marshfield database (Center for Medical Genetics, Marshfield, WI, USA: http://research.marshfieldclinic.org/genetics) was used with the NCBI (National Center for Biotechnology Information) Map Viewer website to evaluate linkage convergence.
Human gene expression evidence (post-mortem brain, blood)
Information about our candidate genes was obtained using GeneCards, the Online Mendelian Inheritance of Man database (http://ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM), as well as database searches using PubMed (http://ncbi.nlm.nih.gov/PubMed) and various combinations of keywords (gene name, bipolar, depression, alcoholism, stress, anxiety, brain, blood, lymphocytes). In addition to our own blood biomarker data for mood disorders,30 we also cross-matched with data for human blood biomarkers for hallucinations and delusions,31 as such symptoms occur in dissociative states related to stress and anxiety.
Mouse genetic evidence (quantitative trait loci (QTLs), transgenic)
To search for mouse genetic evidence—QTLs or transgenic—for our candidate genes, we utilized the MGI_3.54–Mouse Genome Informatics (Jackson Laboratory, Bar Harbor, ME, USA) and used the search menu for mouse phenotypes and mouse models of human disease/abnormal behaviors, using the following subcategories: abnormal emotion/affect behavior and abnormal sleep pattern/circadian rhythm, addiction and drug abuse. To designate convergence for a particular gene, the gene had to map within 10 cM of a QTL marker for the abnormal behavior, or a transgenic mouse of the gene itself displayed that behavior.
Animal model gene expression evidence (brain, blood)
Manually curated databases, developed in our lab, of published gene expression studies in animal models of bipolar disorder, depression, alcoholism, stress and anxiety were used for cross-matching with our list of genes changed in expression by DHA in the DBP KO mice (data from studies published by our own group received 1 point, whereas studies published by other groups received 0.5 points).
Convergent Functional Genomics (CFG) scoring
Only genes reproducibly changed in expression in the same mouse tissue (PFC, AMY, HIP, blood), in the same direction, in two independent experiments, were analyzed further. The six external cross-validating lines of evidence (three animal model, three human) were: animal model genetic data, animal model brain gene expression data, animal model blood gene expression data, human genetic data, human brain gene expression data and human blood gene expression data (see Figure 2). These lines of evidence received a maximum of 1 point each (for animal model genetic data, 0.5 points if it was QTL, 1 point if it was transgenic; for human genetic data, 0.5 points if it was linkage, 1 point if it was association). Thus, the maximum possible CFG score for each gene was 6. It has not escaped our attention that other ways of weighing the scores of line of evidence may give slightly different results in terms of prioritization, if not in terms of the list of genes per se. Nevertheless, we feel this simple scoring system provides a good separation and prioritization of genes and blood biomarkers that may be disease relevant, which is our stated focus.
Pathway analyses
Ingenuity 8.0 (Ingenuity Systems, Redwood City, CA, USA) was employed to analyze the molecular networks, biological functions and canonical pathways of the DHA-modulated genes, as well as identify which genes modulated by DHA are also the target of existing drugs.
Results
Effects of DHA on mood-related behavioral measures in DBP KO mice
Activity levels
DBP (+/+) WT and DBP (−/−) KO mice on a diet either low or high in DHA were subjected to a chronic stress paradigm consisting of isolation (single housing) for 28 days, with an acute stressor (behavioral challenge tests, including FST) at day 21. On day 28, we measured locomotion in open field. Two- factor analysis of variance was carried out (genotype × diet) for FST and Open Field Locomotion.
The FST is a standard test used to measure mood state and response to antidepressant medications in rodents. In female mice (Figure 1), we observed a significant decrease in immobility in the depressed-like WT mice, and an increase in immobility in the manic-like KO mice, on high-DHA diet compared with low-DHA diet. In other words, DHA supplementation seemed to normalize mood state, acting as a mood-stabilizing agent. A slight trend toward reducing immobility in WT male mice was also observed.
Open Field Locomotion is a test that is used as a surrogate for mood state, by extrapolation from human behaviors, with higher locomotion corresponding to higher mood, and lower locomotion to lower mood. In male mice (Figure 1), we observed a significant decrease in locomotion in the manic-like KO mice, and a trend to increased locomotion in the depressed-like WT mice, on high-DHA diet compared with low-DHA diet. Again, DHA supplementation seemed to normalize mood state. Similar trends that did not reach significance were observed in female mice.
Two independent behavioral measures related to mood were normalized by DHA treatment, with interesting gender differences observed. The FST was more significantly changed in female mice, and the open field locomotion in male mice. Similar gender-related differences in behavior have also been reported in other animal models of mood disorders,32 and may be reflective of human gender differences in mood phenotypes.33, 34
PhenoChipping
An unsupervised two-way hierarchical clustering of the mouse open field locomotor behavioral data measures (phenes) using GeneSpring was carried out22 (Supplementary Figure S1). Male stressed (ST) DBP KO mice on the high-DHA diet and male ST DBP KO mice on the low-DHA diet clustered into two distinct groups. Similar to our previous results for male ST DBP KO vs non-ST DBP KO male mice,22 Resting Time was the phene most different between male ST DBP KO mice on high- vs low-DHA diet, being increased in the high-DHA diet group. Center Time (time spent in the center quadrant of the open field), was decreased in mice on the high- vs low-DHA diet. A decrease in Center Time may correlate with a decrease in risk-taking behavior or increased anxiety, as mice generally avoid the potentially dangerous, center area of an open field. Female mice did not separate into two distinct clusters.
Food intake
Food is a hedonic stimulus in mice, and the high-DHA diet may be more appetitive than the low-DHA diet because of higher fat content. Total food intake displayed a minimal trend toward increase in high-DHA vs low-DHA diet, irrespective of genotype. The weight changes were in a similar direction, with the notable exception of female DBP WT mice where there was less increase in weight despite increased food intake (Supplementary Figure S2).
Gene expression effects of omega-3 fatty acids in DBP KO mice
Top genes
At the top of our list for disease-relevant genes modulated by DHA in female mice brain (Tables 1 and 2 and Figure 3) are genes such as GSK3B (in PFC), DRD2 and PPP1R1B/DARRPP-32 (in the AMY) and GRIA2 (in HIP). GSK3B (glycogen synthase kinase 3β) has consistent signals in genome-wide association studies of bipolar disorder.35 GSK3B expression is decreased in mouse PFC by DHA, whereas it is increased in post-mortem human brain in depression.36 Of note, one of the gold standard mood-stabilizing medications for bipolar disorder, lithium, is a GSK3B inhibitor.37 DRD2 (dopamine receptor 2) is a main target for numerous antipsychotic medications (Table 5), and PPP1R1B/DARPP-32 (protein phosphatase 1, regulatory (inhibitor) subunit 1B/dopamine- and cAMP-regulated phosphoprotein, 32 kDa) is at the nexus of signaling pathways by antidepressants and other psychotropic drugs.38 GRIA2 (glutamate receptor, ionotropic, AMPA2) is associated with bipolar disorder,39 and has been reported to be increased in expression in human post-mortem brain from bipolars40 and from suicides,41 whereas DHA decreases the expression in mouse HIP.
Table 1. Top gene expression changes in the brain in female DBP KO ST mice on high-DHA vs low-DHA diet.
| Gene symbol (name) | PFC change | Animal brain evidence | Animal blood evidence | Animal genetic evidence | Human brain evidence | Human blood evidence | Human genetic evidence | CFG score |
|---|---|---|---|---|---|---|---|---|
| GSK3B (glycogen synthase kinase 3 β) | D | (I) Alcohol72 | (Transgenic) Behavioral despair | (I) MDD36 | (I) BP73 | 3q13.33 (association) MDD74 BP75, 76 | 5.0 | |
| ARHGEF9 (CDC42 guanine nucleotide exchange factor (GEF) 9) | D | (Transgenic) Decreased exploration in new environment | (I) Suicide-MDD41 | (I) Hallucinations31 | Xq11.2 (association) Anxiety77 | 4.0 | ||
| GMFB (glia maturation factor, β) | I | (D) DBP-ST PFC22 | (D) DBP-NST Blood22 | (QTL) Addiction/drug abuse Abnormal emotion/affect behavior | (I) BP73 | 14q22.2 (linkage) Anxiety78 | 4.0 | |
| NFIA (nuclear factor I/A) | D | (I) DBP-NST AMY22 | (QTL) Abnormal sleep pattern/ circadian rhythm Abnormal emotion/affect behavior | (I) MDD79 | (I) Alcohol80 | 1p31.3 (linkage) BP81, 82 | 4.0 | |
| KCNMA1 (potassium large conductance calcium-activated channel, subfamily M, α member 1) | D | (D) DBP-ST PFC22 | (QTL) Abnormal emotion/ affect behavior | (I) MDD79 | 10q22.3 (association) Alcohol83 | 3.5 | ||
| MGEA5 (meningioma expressed antigen 5) | I | (D) Alcohol84 | (QTL) Abnormal circadian rhythm | (D) Alcohol85 | (D) Delusion31 | 10q24.32 (linkage) BP86 | 3.5 | |
| RORB (RAR-related orphan receptor β) | D | (D) DBP-ST PFC; (I) DBP-ST AMY22 | (Transgenic) Decreased aggression | 9q21.13 (association) BP53 | 3.0 | |||
|
PTTG1 (pituitary tumor-transforming gene 1) |
D |
(D) DBP-NST AMY22 |
|
|
|
(I) PPD87 |
5q33.3
(linkage)
BP86, 88, 89
PD90 |
2.5 |
|
Gene symbol (name) |
AMY change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
| DRD2 (dopamine receptor 2) | I | (D) BP91 (D) DBP-NST AMY22 | (Transgenic) Increased drinking behavior; decreased anxiety-related response | (D) MDD92 (D) Alcohol93 | (D) Delusions31 | 11q23.2 (association) Alcohol94, 95, 96, 97, 98 Anxiety/social phobia99 BP100 MDD74 PD101 | 5.0 | |
| HSPA1B (heat shock protein 1B) | D | (I) Depression102 (I) DBP-NST AMY22 | BP30 | (QTL) Abnormal eating/drinking behavior; abnormal circadian rhythm | (I) Alcohol103 | (I) Chronic stress104 | 6p21.33 (linkage) Juvenile BP105 Neuroticism106 | 5.0 |
| PPP1R1B (protein phosphatase 1, regulatory (inhibitor) subunit 1B) | I | (D) DBP-NST AMY (I) DBP-ST AMY22 (I) BP PFC29 | (Transgenic) Abnormal alcohol consumption | (D) BP107 | (D) BP108 | 17q12 (association) Alcohol109 | 5.0 | |
| NOS1 (nitric oxide synthase 1, neuronal) | D | (D) BP91 (D) DBP-NST AMY22 | (QTL) Addiction/drug abuse, Abnormal emotion/affect behavior | (I) BP110 | (D) Stress111 | 12q24.22 (association) BP112 | 4.5 | |
| RGS4 (regulator of G-protein signaling 4) | I | (D) BP113 | (Transgenic) Abnormal response to addictive substance | (I) Alcohol114 | 1q23.3 (association) BP112, 115 | 4.0 | ||
| MAL (myelin and lymphocyte protein, T-cell differentiation protein) | I | (D) DBP-NST PFC; (D) DBP-ST PFC22 Alcohol PFC116 | (QTL) Addiction/drug abuse | (D) MDD117 (D) Alcohol114 (D) Suicide-MDD41 | (D) BP118 | 2q11.1 (linkage) MDD-suicide attempts119 BP120, 121 | 4.0 | |
| ADORA2A (adenosine A2a receptor) | I | BP NAC29 (D) DBP-ST PFC22 Alcohol NAC116 | (Transgenic) Decreased exploration in new environment, Abnormal response to addictive substance | (I) Chronic stress104 | 22q11.23 (association) Anxiety122 PD123, 124, 125 | 4.0 | ||
| GALC (galactosylceramidase) | I | (I) DBP-NST AMY22 | (QTL) Addiction/drug abuse | (D) MDD126 (D) BP51 | (D) Chronic stress104 | 14q31.3 (linkage) BP127 OCD128 Simple phobia129 | 4.0 | |
| PRDX2 (peroxiredoxin 2) | I | (D) DBP-ST PFC22 Alcohol PFC116 | BP30 | (QTL) Abnormal emotion/affect behavior Abnormal circadian rhythm | (D) BP130 (I) Response to lithium treatment131 | 19p13.2 (linkage) BP132 MDD119 | 4.0 | |
| TAC1 (tachykinin 1) | I | (I) DBP-ST AMY22 | (Transgenic) Decreased anxiety-related response, increased coping response | (I) MDD79 | 7q21.3 (linkage) BP133 Alcohol134 | 3.5 | ||
| NR4A3 (nuclear receptor subfamily 4, group A, member 3) | D | (I) Alcohol84 (D) MDD-Fluoxetine135 | (QTL) Addiction/drug abuse | (I) Alcohol136 | (I) PTSD43 | 9q22.33 (linkage) Alcohol137 Anxiety, PD138 | 3.5 | |
| ESR1 (estrogen receptor 1 α) | D | (I) Stress50 | (Transgenic) Increased aggression | (I) Alcohol85 (I) MDD139 | 6q25.1 (association) Alcohol140 Childhood onset mood disorder141 | 3.5 | ||
| GABRD (γ-aminobutyric acid (GABA) A receptor, subunit δ) | I | (D) MDD-Fluoxetine135 (I) Anxiety142 (D) Alcohol72 Alcohol CP, HIP116 | (Transgenic) Decreased anxiety-related response | (I) Suicide-MDD41, 143 | 18q12.2 (linkage) BP144 | 3.5 | ||
| CRIM1 (cysteine rich transmembrane BMP regulator 1) | D | (D) Depression102 (D) DBP-ST PFC22 | (QTL) Addiction/drug abuse | (D) Suicide-MDD41 | 2p22.3 (association) PD145 | 3.5 | ||
| PENK (preproenkephalin) | I | (D) Anxiety146 (D) BP91 (D) Stress50 (I) DBP-ST AMY; (D) DBP-ST PFC22 BP PFC29 | (Transgenic) Abnormal emotion/affect behavior, addiction/drug abuse | (D) MDD117 (D) Suicide-MDD41 | 8q12.1 (linkage) BP147 | 3.5 | ||
| ALDH1A (aldehyde dehydrogenase family 1, subfamily A1) | I | (D) Anxiety/Depression148 (I) DBP-ST AMY22 | BP30 | (QTL) Abnormal circadian rhythm | 9q21.13 (association) Alcohol149 | 3.5 | ||
| CADPS2 (Ca2+-dependent activator protein for secretion 2) | D | Alcohol CP116 | (Transgenic) Decreased exploration in new environment, abnormal circadian rhythm, abnormal sleep pattern | (I) Suicide-MDD41 | 7q31.32 (linkage) BP147 | 3.5 | ||
| GFRA2 (glial cell line derived neurotrophic factor family receptor α 2) | D | Alcohol NAC116 | (Transgenic) Abnormal food intake, abnormal water consumption | (I) Suicide-MDD41 | 8p21.3 (linkage) MDD/suicide attempts119 | 3.5 | ||
|
HPCAL1 (hippocalcin-like 1) |
D |
(D) DBP-ST AMY22 |
|
(QTL)
Abnormal circadian rhythm; addiction/drug abuse |
(I) Suicide-MDD41, 150 |
|
2p25.1
(association)
MDD151 |
3.5 |
|
Gene symbol (name) |
HIP change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
| CTNNB1 (catenin (cadherin associated protein), β 1) | D | (I) Anxiety152 | BP30 | (Transgenic) Abnormal suckling behavior | (D) Suicide-MDD41 | (I) Stress111 | 3p22.1 (linkage) Anxiety/PD138 BP153 | 5.0 |
| GRIA2 (glutamate receptor, ionotropic, AMPA2 α 2) | D | (I) Alcohol154 (I) BP91 (I) Stress50 (I) Alcohol72 | BP30 | (Transgenic) Abnormal anxiety-related response | (I) BP40 (I) Suicide-MDD41 | 4q32.1 (association) BP39 | 5.0 | |
| GJA1 (gap junction protein, α 1) | D | (I) Alcohol72 Alcohol, HIP116 | BP30 | (Transgenic) Abnormal suckling behavior | (I) Alcohol103 | 6q22.31 (linkage) BP155, 156 Alcohol157 | 4.5 | |
| GLUL (glutamate-ammonia ligase glutamine synthetase) | D | (I) Stress158 BP PFC, VT29 Alcohol AMY, NAC116 | BP30 | (QTL) Abnormal emotion/affect behavior, Addiction/drug abuse | (D) MDD159, 160 (D) Suicide-MDD41, 143 | 1q25.3 (linkage) Alcohol161 | 4.0 | |
| HOMER1 (homer homolog 1) | D | (D) MDD-Fluoxetine135 (D) Anxiety162 (D) Stress163 Alcohol HIP116 | (Transgenic) Abnormal response to addictive substance | (D) PTSD43 | 5q14.1 (association) MDD164 | 4.0 | ||
| PAM (peptidylglycine α-amidating monooxygenase) | D | (I) DBP-ST PFC22 (I) BP165 | (QTL) Abnormal eating/drinking behavior; Addiction/drug abuse | (I) MDD79 (I) Suicide-MDD150 | (D) Chronic stress104 | 5q21.1 (linkage) MDD119 Alcohol166 BP167 | 4.0 | |
| GABRB3 (γ-aminobutyric acid (GABA) A receptor, subunit β 3) | D | BP AMY, CP29 (D) DBP-ST AMY; (D) DBP-ST PFC22 | (QTL) Addiction/drug abuse | (I) MDD168 (I) Alcohol169 | 15q12 (association) Alcohol170 BP46, 171 | 3.5 | ||
| NCALD (neurocalcin δ) | D | BP AMY, CP29 | (QTL) Addiction/drug abuse | (I) Suicide-MDD41 | 8q22.3 (association) Alcohol172 | 3.5 | ||
| OGT (O-linked N-acetylglucosamine (GlcNAc) transferase) | D | (I) DBP-NST AMY22 | DBP-ST BLOOD (D)22 | (QTL) Abnormal emotion/affect behavior | Xq13.1 (association) BP173 | 3.5 | ||
| PTTG1 (pituitary tumor-transforming gene 1) | D | (D) DBP-NST AMY22 | (I) PPD87 | 5q33.3 (linkage) BP86, 88, 89 PD90 | 2.5 |
Abbreviations: AMY, amygdala; BP, bipolar; CFG, convergent functional genomics; CP, caudate putamen; D, decreased in expression; DBP, D-box binding protein; DHA, docosahexaenoic acid; HIP, hippocampus; I, increased in expression; KO, knockout; MDD, major depressive disorder; NAC, nucleus acumbens; NST, non-stressed; OCD, obsessive compulsive disorder; PD, panic disorder; PFC, prefrontal cortex; PPD, postpartum depression; PTSD, post-traumatic stress disorder; QTL, quantitative trait locus; ST, stressed; VT, ventral tegmentum.
Myelin-related genes are underlined.
Top candidate genes for which there were reproducible changes in expression in high-DHA vs low-DHA mice in PFC (n=7), AMY (n=19) and HIP (n=10) are shown (CFG score of ⩾3.5 points).
Table 2. Top gene expression changes in the brain in male DBP KO ST mice on high-DHA vs low-DHA diet.
| Gene symbol (name) | PFC change | Animal brain evidence | Animal blood evidence | Animal genetic evidence | Human brain evidence | Human blood evidence | Human genetic evidence | CFG score |
|---|---|---|---|---|---|---|---|---|
| PTGDS (prostaglandin D2 synthase) | I | (D) Anxiety49 (D) Stress50 | (Transgenic) Decreased aggression | (D) Alcohol114 | (D) BP48 | 9q34.3 (association) Anxiety47 | 5.0 | |
| ARF3 (ADP-ribosylation factor 3) | I | (D) DBP-ST PFC22 | (QTL) Addiction/drug abuse | (D) Alcohol114 | (I) BP73 (D) Chronic stress104 | 12q13.12 (linkage) PD174 | 4.0 | |
| NFIA (nuclear factor I/A) | I | (I) DBP-NST AMY22 | (QTL) Addiction/drug abuse Abnormal emotion/affect behavior | (I) MDD79 | (I) Alcohol80 | 1p31.3 (linkage) BP81, 82 | 4.0 | |
| KLF4 (Kruppel-like factor 4) | I | Alcohol NAC116 (D) Depression102 | (Transgenic) Abnormal suckling behavior | (D) MDD117 | 9q31.2 (linkage) BP132 Anxiety/PD138 | 3.5 | ||
|
PTTG1 (pituitary tumor-transforming gene 1) |
D |
(D) DBP-NST AMY22 |
|
|
|
(I) PPD87 |
5q33.3
(linkage)
BP86, 88, 89
PD90 |
2.5 |
|
Gene symbol (name) |
AMY change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
| AGT (angiotensinogen) | I | BP NAC29 Alcohol CP, HIP, NAC, PFC116 (D) DBP-NST AMY22 | (D) Alcohol114, 136 | 1q42.2 (association) BP175 | 4.0 | |||
| HPCAL1 (hippocalcin-like 1) | I | (D) DBP-ST AMY22 | (QTL) Abnormal circadian rhythm; addiction/drug abuse | (I) Suicide-MDD41 | 2p25.1 (association) MDD151 | 3.5 | ||
| PNOC (prepronociceptin) | I | (D) DBP-NST AMY22 | (QTL) Abnormal sleep pattern/ circadian rhythm | (D) PTSD176 | 8p21.1 (association) Alcohol177 | 3.5 | ||
| SYT1 (synaptotagmin I) | D | (D) Depression178 (D) Alcohol179 BP AMY29 | (Transgenic) Abnormal suckling behavior | (D) Alcohol180 (D) BP181 | 12q21.2 (linkage) BP182 PD183 Alcohol137 | 3.5 | ||
| PER3 (period homolog 3) | D | (D) BP113 | (Transgenic) Shortened circadian period | 1p36.23 (association) BP184 PD185 Alcohol170 Depression186 | 2.5 | |||
|
PTTG1 (pituitary tumor-transforming gene 1) |
D |
(D) DBP-NST AMY22 |
|
|
|
(I) PPD87 |
5q33.3
(linkage)
BP86, 88, 89
PD90 |
2.5 |
|
Gene symbol (name) |
HIP change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
| FOS (FBJ osteosarcoma oncogene) | I | (I) DBP-ST AMY22 (I) Alcohol84 (D) MDD Fluoxetine135 | BP30 | (Transgenic) Decreased anxiety-related response | (I) BP42 | (I) PTSD43 (I) Stress44 | 14q24.3 (linkage) BP120 Alcohol166 | 5.5 |
| DUSP1 (dual specificity phosphatase 1) | I | Alcohol AMY, CP, NAC, PFC116 (I) Alcohol84 | BP30 | (QTL) Abnormal eating/drinking behavior; Abnormal circadian rhythm | (D) BP, MDD36 | (I) Stress44 | 5q35.1 (linkage) PD187, 188 | 5.0 |
| GABRA1 (γ-aminobutyric acid (GABA) A receptor, subunit α 1) | I | (D) Stress50 (D) Alcohol154 | BP30 | (Transgenic) Impaired passive avoidance behavior | (I) BP189 (I) Suicide-MDD41 | 5q34 (association) BP45, 46 | 5.0 | |
| MBP (myelin basic protein) | I | (I) BP113 (I) Anxiety152 | BP30 | (D) Suicide-MDD41 (D) BP51 | (I) Mood30 | 18q23 (association) BP190 | 5.0 | |
| PTGDS (prostaglandin D2 synthase) | I | (D) Anxiety49 (D) Stress50 | (Transgenic) Decreased aggression toward mice | (D) Alcohol114 | (D) BP48 | 9q34.3 (Association) Anxiety47 | 5.0 | |
| SPP1 (secreted phosphoprotein 1) | I | BP VT29 | BP30 | (QTL) Abnormal emotion/affect behavior Addiction/drug abuse | (D) Alcohol180 (D) Suicide-MDD41 (D) MDD36 | (D) Hallucinations31 | 4q22.1 (linkage) Anxiety78 BP120 Alcohol161 | 5.0 |
| NR4A2 (nuclear receptor subfamily 4, group A, member 2) | I | (D) Anxiety162 (D) Stress50 | (Transgenic) Abnormal suckling behavior | (D) BP, MDD191 | (D) Mood30 | 2q24.1 (linkage) BP81 | 4.5 | |
| CNP (2′,3′-cyclic nucleotide 3′ phosphodiesteras) | I | (D) BP113 | BP30 DBP-NST Blood (D)22 | (QTL) Addiction/drug abuse Abnormal emotion/affect behavior | (D) MDD117 (D) Alcohol114, 136, 192 (D) Suicide-MDD41 | 17q21.2 (linkage) Alcohol193 BP86, 132 | 4.0 | |
| GAD2 (glutamic acid decarboxylase 2) | I | (D) Alcohol179 | (Transgenic) Decreased aggression | (D) BP40, 194, 195 | 10p12.1 (association) Alcohol196 Anxiety/affective disorder197 | 4.0 | ||
| GLS (glutaminase) | I | (QTL) Addiction/drug abuse | (D) BP198 | (D) PTSD43 | 2q32.2 (linkage) Alcohol166, 199 | 4.0 | ||
| GLUL (glutamate-ammonia ligase)a | I | (I) Stress158 BP PFC, VT29 Alcohol AMY, NAC116 | BP30 | (QTL) Abnormal emotion/affect behavior, Addiction/drug abuse | (D) MDD159, 160 (D) Suicide-MDD41, 143 | 1q25.3 (linkage) Alcohol161 | 4.0 | |
| HOMER1 (homer homolog 1) | I | (D) MDD-Fluoxetine135 (D) Anxiety162 (D) Stress163 Alcohol HIP116 | (Transgenic) Abnormal response to addictive substance | (D) PTSD43 | 5q14.1 (association) MDD164 | 4.0 | ||
| NR3C2 (nuclear receptor subfamily 3, group C, member 2) | D | (D) Primate stress-induced200 | (Transgenic) Abnormal response to novel object | (I) MDD139 | 4q31.23 (association) Stress201 | 4.0 | ||
| SLC12A2 (solute carrier family 12, member 2) | I | Alcohol HIP116 | BP30 | (QTL) Abnormal eating/drinking behavior | (D) Alcohol136 | 5q23.2 (linkage) MDD119 | 4.0 | |
| JAK1 (Janus kinase 1) | I | (D) Primate stress-induced200 | (Transgenic) Abnormal suckling behavior | (D) Stress111 | 1p31.3 (linkage) BP81, 82 | 3.5 | ||
| ATRX (α thalassemia/mental retardation syndrome X-linked) | I | (D) Anxiety202 | BP30 | (Transgenic) Abnormal suckling behavior | (D) MDD79 (D) Alcohol85 | Xq21.1 | 3.5 | |
| KCNMA1 (potassium large conductance calcium-activated channel, subfamily M, α member 1) | I | (QTL) Abnormal emotion/affect behavior, addiction/drug abuse | (I) MDD79 | 10q22.3 (association) Alcohol83 | 3.5 | |||
| LPAR1 (lysophosphatidic acid receptor 1) | I | (D) MDD117 (D) BP113 (D) Depression102 | (QTL) Abnormal sleep pattern/circadian rhythm; addiction/drug abuse | (D) MDD117 (D) BP203 (D) Suicide-MDD41 | (I) Mood30 | 9q31.3 (linkage) PD138 BP155 | 3.5 | |
| MAPT (microtubule-associated protein tau) | I | (D) Anxiety204 Alcohol HIP116 | (Transgenic) Decreased anxiety-related response | (D) MDD117 (I) Alcohol180 | 17q21.1 (linkage) Alcohol193 BP86, 132 | 3.5 | ||
| MARCKS (myristoylated alanine rich protein kinase C substrate) | I | (D) BP113, 205 | (Transgenic) Abnormal suckling behavior | (I) MDD206 | 6q21 (linkage) BP81 | 3.5 | ||
| MBNL1 (muscleblind-like 1) | I | BP30 | (QTL) Abnormal emotion/affect behavior; Abnormal circadian rhythm | (I) MDD79 | 3q25.1 (association) BP207 | 3.5 | ||
| NCALD (neurocalcin δ) | I | (QTL) Addiction/drug abuse | (I) Suicide-MDD41 | 8q22.3 (association) Alcohol172 | 3.5 | |||
| NRXN1 (neurexin I) | I | (D) BP113 | (QTL) Addiction/drug abuse Abnormal emotion/affect behavior | (D) BP208 (I) Suicide-MDD41 | 2p16.3 (association) BP173 PD209 | 3.5 | ||
| PIP4K2A (phosphatidylinositol-5-phosphate 4-kinase, type II, α) | I | (D) Stress50 (D) Anxiety204 | (QTL) Addiction/drug abuse | (D) Alcohol136 | 10p12.2 (association) BP210 | 3.5 | ||
| PLXNA2 (plexin A2) | I | (QTL) Addiction/drug abuse Abnormal emotion/affect behavior | (I) Alcohol136 | (D) Mood30 | 1q32.2 (association) Anxiety211 BP207 | 3.5 | ||
| SERPINI1 (serine (or cysteine) peptidase inhibitor, clade I, member 1) | I | (D) Primate stress-induced200 | (Transgenic) Abnormal anxiety-related response | (I) MDD79 | 3q26.1 (linkage) BP81, 212 PD213 | 3.5 | ||
| SNN (stannin) | D | (D) Primate stress-induced200 | (QTL) Addiction/drug abuse | (I) Alcohol136 | 16p13.13 (linkage) Alcohol214 BP86 | 3.5 | ||
| PTTG1 (pituitary tumor-transforming gene 1) | D | (D) DBP-NST AMY22 | (I) PPD87 | 5q33.3 (linkage) BP86, 88, 89 PD90 | 2.5 |
Abbreviations: AMY, amygdala; BP, bipolar; CFG, convergent functional genomics; CP, caudate putamen; D, decreased in expression; DBP, D-box binding protein; DHA, docosahexaenoic acid; HIP, hippocampus; I, increased in expression; KO, knockout; MDD, major depressive disorder; NAC, nucleus acumbens; NST, non-stressed; OCD, obsessive compulsive disorder; PD, panic disorder; PFC, prefrontal cortex; PPD, postpartum depression; PTSD, post-traumatic stress disorder; QTL, quantitative trait locus; ST, stressed; VT, ventral tegmentum.
Blood biomarker. Top candidate genes for which there were reproducible changes in expression in high-DHA vs low-DHA mice in PFC (n=5), AMY (n=5) and HIP (n=29) are shown (CFG score of ⩾3.5 points).
Myelin-related genes are underlined.
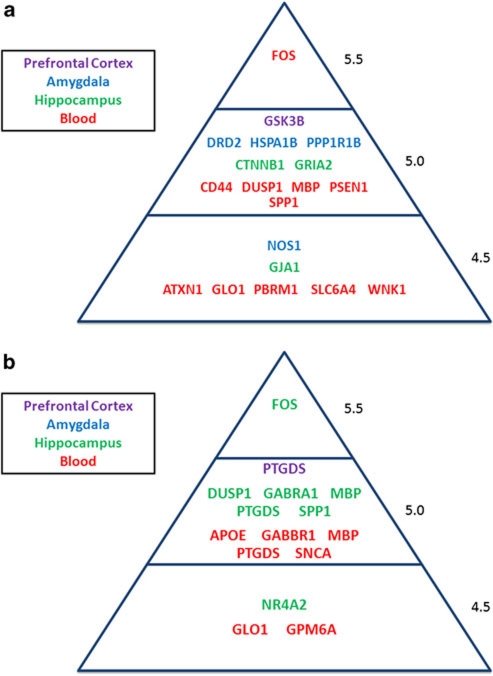
Figure 3.
Top candidate genes changed in DBP knockout (KO) stressed (ST) mice on high- vs low-docosahexaenoic acid (DHA) diet. (a) Female mice and (b) male mice.
At the top of our list for disease-relevant genes modulated by DHA in male mice brain (Tables 1 and 2 and Figure 3) are genes such as FOS, GABRA1, MBP (in HIP) and PTGDS (in HIP and PFC). FOS (FBJ osteosarcoma oncogene) is an immediate response gene involved in response to stress and inflammation. FOS is decreased in the mouse PFC by DHA, an effect in opposite direction to the increase seen in post-mortem brains of bipolar subjects,42 and in blood cells of subjects with stress disorders.43, 44 GABRA1 (γ-aminobutyric acid (GABA) A receptor, subunit α1) is associated with bipolar disorder.45, 46 It is decreased in expression in brains from animal models of alcoholism and stress, whereas DHA increases its expression in DBP mouse HIP. PTGDS (prostaglandin D2 synthase; brain) is associated with anxiety,47 and is decreased in expression in human post-mortem brain from bipolars48 as well as in animal models of anxiety49 and stress,50 whereas DHA increases its expression in the PFC and HIP of DBP KO mice.
Last but not least, MBP (myelin basic protein) is associated with bipolar disorder, and is decreased in expression in human post-mortem brain from bipolars51 and from suicides,41 whereas DHA increases its expression in mouse HIP. Interestingly, a whole series of other myelin-related genes were increased in expression by DHA in DBP male mice (CNP, MOBP, PLP1, MOG) and female mice (MAL, PLP1). Myelin-related gene expression decrease is a common, if nonspecific, denominator of neuropsychiatric disorders,51, 52 and is modeled by the non-DHA-treated DBP KO mice.22 To our knowledge, DHA is the only compound to date to demonstrate such a powerful broad effect on myelin-related genes, and potentially reverse this pathology.
Sex differences and similarities at gene and pathway levels
There are profound sex differences, that is, there is little overlap, at individual gene levels, between the changes induced by DHA in males and in females. For example, in HIP, only five genes are changed in the same direction in males and females: PTTG1 and ADI1 (decreased by DHA) and SCD, HBA-A1 and HBB-B1 (increased by DHA). PTTG1 (pituitary tumor transforming gene 1) is also decreased in all three male brain regions analyzed (PFC, AMY and HIP). PTTG1 is an oncogene involved, among other things, in pituitary tumors. Its downregulation by DHA is indicative of potential anticancer benefits of DHA treatment that merit future exploration. However, at a pathway level, there is more overlap between males and females. For example, two of the five top five canonical pathways in HIP (glutamate receptor signaling, GABA receptor signaling) are shared between males and females, although different genes in these pathways are changed in each sex (Table 3b). Inflammation-related pathways are prominent in the PFC, and signaling pathways (cyclic adenosine monophosphate in females and circadian rhythm in males) in the AMY (Tables 3a and b).
Table 3. Ingenuity pathway analysis of the genes changed in DHA-treated mice: analysis of all differentially expressed genes in (a) female mice and (b) male mice.
| Pathways | P-value | Ratio |
|---|---|---|
| (a) | ||
| Top canonical pathways, female PFC (n=66 genes) | ||
| Primary immunodeficiency signaling | 2.59E−08 | 6/63 (0.095) |
| B-cell development | 1.41E−07 | 5/37 (0.135) |
| Communication between innate and adaptive immune cells | 2.49E−04 | 4/107 (0.037) |
| Autoimmune thyroid disease signaling | 6.39E−04 | 3/61 (0.049) |
| Systemic lupus erythematosus signaling | 1.52E−03 | 4/163 (0.025) |
| Top canonical pathways, female AMY (n=150 genes) | ||
| cAMP-mediated signaling | 3.54E−07 | 10/161 (0.062) |
| G-protein-coupled receptor signaling | 6.51E−07 | 11/222 (0.05) |
| Relaxin signaling | 9.52E−06 | 8/151 (0.053) |
| Cardiac β-adrenergic signaling | 4.27E−04 | 6/142 (0.042) |
| Protein kinase A signaling | 5.61E−04 | 9/318 (0.028) |
| Top canonical pathways, female HIP (n=103 genes) | ||
| Glutamate receptor signaling | 2.67E−04 | 4/70 (0.057) |
| Polyamine regulation in colon cancer | 2.62E−03 | 2/22 (0.091) |
| GABA receptor signaling | 2.49E−02 | 2/55 (0.036) |
| Mitotic roles of polo-like kinase | 3.27E−02 | 2/62 (0.032) |
| TR/RXR activation | 7.64E−02 | 2/99 (0.02) |
|
(b) | ||
| Top canonical pathways, male PFC (n=77 genes) | ||
| CCR5 signaling in macrophages | 2.98E−03 | 3/93 (0.032) |
| Clathrin-mediated endocytosis signaling | 2.52E−02 | 3/169 (0.018) |
| IL-8 signaling | 3.04E−02 | 3/188 (0.016) |
| BMP signaling pathway | 3.36E−02 | 2/80 (0.025) |
| Pathogenesis of multiple sclerosis | 3.49E−02 | 1/9 (0.111) |
| Top canonical pathways, male AMY (n=59 genes) | ||
| Circadian rhythm signaling | 2.16E−03 | 2/35 (0.057) |
| Neuroprotective role of THOP1 in Alzheimer's disease | 4.16E−03 | 2/54 (0.037) |
| Glycine, serine and threonine metabolism | 2.48E−02 | 2/150 (0.013) |
| Glycerophospholipid metabolism | 4.55E−02 | 2/192 (0.01) |
| RAR activation | 4.96E−02 | 2/181 (0.011) |
| Top canonical pathways, male HIP (n=352 genes) | ||
| Aldosterone signaling in epithelial cells | 1.97E−05 | 9/97 (0.093) |
| Glutamate receptor signaling | 7.12E−04 | 6/70 (0.086) |
| GABA receptor signaling | 1.51E−03 | 5/55 (0.091) |
| RAR activation | 2.22E−03 | 9/181 (0.05) |
| 14-3-3-mediated signaling | 2.89E−03 | 7/116 (0.06) |
Abbreviations: AMY, amygdala; BMP, bone morphogenetic protein; cAMP, cyclic AMP; CCR5, chemokine (C–C motif) receptor 5; DHA, docosahexaenoic acid; GABA, g-aminobutyric acid; HIP, hippocampus; IL, interleukin; PFC, prefrontal cortex; RAR, retinoic acid receptor; RXR, retinoid X receptor; THOP1, thimet oligopeptidase 1; TR, thyroid hormone receptor.
Circadian clock genes are also being modulated by DHA, with PER3 (period homolog 3) being decreased in expression in the AMY of males, and RORB (RAR-related orphan receptor β) decreased in expression in PFC of females. Of note, we have previously reported evidence for genetic association of RORB with bipolar disorder in a pediatric bipolar cohort.53
Blood biomarkers
RAB27B (from AMY), and CAP1, CAPZB, GNG2, KLF9, NDUFS5, SSX2IP and VPS13A (from HIP) are co-regulated in the same direction in brain and blood of DBP female mice by DHA (Table 4a). For male mice, TFRC (from PFC), CD24A and FTL1 (from AMY), GLUL, LIMD2, PSME4 and TTR (in HIP) are co-regulated in the same direction in brain and blood by DHA (Table 4b). These genes warrant further studies in human clinical populations as potential gender-specific peripheral biomarkers of DHA treatment response.
Table 4. Brain–blood concordant biomarkers modulated by DHA in (a) female mice and (b) male mice.
|
(a) Females | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gene symbol (name) | AMY and blood change | Animal brain evidence | Animal blood evidence | Animal genetic evidence | Human brain evidence | Human blood evidence | Human genetic evidence | CFG score |
|
RAB27b (member RAS oncogene family) |
D |
(I) Alcohol154 |
|
|
|
|
18q21.2
(linkage)
BP144
MDD119 |
1.0 |
|
Gene symbol (name) |
HIP and blood change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
| KLF9 (Kruppel-like factor 9) | D | BP AMY, CP29 Alcohol NAC116 | (QTL) Abnormal circadian rhythm | (D) Alcohol180 | 9q21.12 (linkage) BP147, 215 Alcohol137, 199 | 3.0 | ||
| CAP1 (adenylate cyclase-associated protein 1) | D | (D) Depression216 | (D) BP216 (D) MDD117 (I) MDD79 | 1p34.2 (linkage) Alcohol161 BP217 | 2.0 | |||
| SSX2IP (synovial sarcoma, X breakpoint 2 interacting protein) | D | (D) Alcohol180 | 1p22.3 (linkage) BP217 Alcohol134, 199 Alcohol/depression218 | 1.5 | ||||
| NDUGA5 (NADH dehydrogenase (ubiquinone) Fe-S protein 5) | I | Alcohol NAC116 | 1.0 | |||||
| VPS13A (vacuolar protein sorting 13A) | D | (I) DBP-ST PFC22 (I) Depression102 | 1.0 | |||||
| CAPZB (capping protein (actin filament) muscle Z-line, β) | D | (I) Alcohol154 | 0.5 | |||||
|
GNG2 (guanine nucleotide binding protein (G protein), γ 2) |
D |
|
|
|
|
|
|
0.0 |
|
(b) Males | ||||||||
|
Gene symbol (name) |
PFC and blood change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
|
TFRC (transferrin receptor) |
I |
Alcohol CP, HIP116 |
|
|
|
|
3q29
(linkage)
BP219
BP/SZ220
SZ, SZA, BP144
Alcohol161 |
1.5 |
|
Gene symbol (name) |
AMY and blood change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
| CD24A (CD24a antigen) | I | (D) DBP-ST AMY22 | BP30 | 2.0 | ||||
|
FTL1 (ferritin light chain 1) |
I |
(D) DBP-ST AMY; (D) DBP-ST PFC22
Alcohol NAC116 |
|
|
|
|
|
1.0 |
|
Gene symbol (name) |
HIP and blood change |
Animal brain evidence |
Animal blood evidence |
Animal genetic evidence |
Human brain evidence |
Human blood evidence |
Human genetic evidence |
CFG score |
| GLUL (glutamate-ammonia ligase) | I | (I) Stress158 BP PFC, VT29 Alcohol AMY, NAC116 | BP30 | (QTL) Abnormal emotion/affect behavior, addiction/drug abuse | (D) MDD159, 160 (D) Suicide-MDD41, 143 | 1q25.3 (linkage) Alcohol161 | 4.0 | |
| LIMD2 (LIM domain containing 2) | D | BP30 | 1.0 | |||||
| TTR (transthyretin) | D | BP CP29 (I) Anxiety/depression148 (I) Anxiety49, 142 (I) Depression221 | 1.0 | |||||
| PSME4 (proteasome (prosome, macropain) activator subunit 4) | I | 0.0 | ||||||
Abbreviations: AMY, amygdala; BP, bipolar; CFG, convergent functional genomics; CP, caudate putamen; D, decreased in expression; DHA, docosahexaenoic acid; HIP, hippocampus; I, increased in expression; MDD, major depressive disorder; NAC, nucleus acumbens; NST, non-stressed; PFC, prefrontal cortex; PPD, postpartum depression; QTL, quantitative trait locus; ST, stressed; SZ, schizophrenia; SZA, schizoaffective disorder; VT, ventral tegmentum.
In addition, a number of other genes are changed in expression by DHA in DBP mouse blood in opposite direction to that seen in human blood in mood disorders and stress disorders (Supplementary Tables S1 and S2). Although not changed in the same direction in the DBP mouse brain, at least in the limited numbers of regions we have assayed so far, they may nevertheless be viable human biomarkers of the therapeutic effects of DHA, upon further study and validation. Notably, one of these candidate markers is SLC6A4 (solute carrier family 6 (neurotransmitter transporter, serotonin), member 4), decreased in expression by DHA in female DBP mouse blood.
Drugs that exert similar effects to DHA
A number of DHA-responsive genes identified by us in mice are modulated by existing drugs (Table 5), notably antipsychotics, benzodiazepines, calcium channel blockers and estrogens in females, respectively valproic acid and ketamine in males. Those classes of medications have a history of mood-modulating effects, use and abuse in bipolar and co-morbid disorders. Recent work has also shown that lithium can modulate DHA metabolism.54
Table 5. DHA-responsive genes in our data set that are the targets of existing drugs.
|
(a) Females |
|
|
|
|---|---|---|---|
| Gene symbol (name) | Type(s) | Drug(s) | CFG score |
| PFC | |||
| GSK3B (glycogen synthase kinase 3 β) | Kinase | Enzastaurin | 5.0 |
| KCNMA1 (potassium large conductance calcium-activated channel, subfamily M, α member 1) | Ion channel | Tedisamil | 3.5 |
| AMY | |||
| DRD2 (dopamine receptor D2) | G-protein-coupled receptor | Paliperidone, risperidone, buspirone, bifeprunox, iloperidone, blonanserin, asenapine, pardoprunox, ocaperidone, abaperidone, SLV-314, RGH-188, rotigotine, opipramol, chloropromazine, metoclopramide, sulpiride, meloxicam, amantadine, trifluoperazine, fluphenazine, pimozide, clozapine, haloperidol, fluoxetine/olanzapine, fluphenazine decanoate, thiothixene, amitriptyline/perphenazine, haloperidol decanoate, molindone, trimethobenzamide | 5.0 |
| NOS1 (nitric oxide synthase 1) (neuronal) | Enzyme | GW 273629, omega-N-methylarginine | 4.5 |
| ALDH1A1 (aldehyde dehydrogenase 1 family, member A1) | Enzyme | Disulfiram, chlorpropamide | 3.5 |
| ESR1 (estrogen receptor 1) | Ligand-dependent nuclear receptor | 17-α-ethinylestradiol, fulvestrant, β-estradiol, estradiol 17-β-cypionate, estrone, estradiol valerate, 3-(4-methoxy)phenyl-4-((4-(2-(1-piperidinyl)ethoxy)phenyl)methyl)-2H-1-benzopyran-7-ol, bazedoxifene, estradiol valerate/testosterone enanthate, TAS-108, ethinyl estradiol/ethynodiol diacetate, estradiol acetate, esterified estrogens, estradiol cypionate/medroxyprogesterone acetate, conjugated estrogens/meprobamate, estradiol/norethindrone acetate, synthetic conjugated estrogens | 3.5 |
| GABRD (γ-aminobutyric acid (GABA) A receptor, δ) | Ion channel | Pagoclone, alphadolone, SEP 174559, tracazolate, sevoflurane, isoflurane, gaboxadol, felbamate, etomidate, muscimol, halothane, fluoxetine/olanzapine, eszopiclone, temazepam, zolpidem, lorazepam, olanzapine, clonazepam, zaleplon, secobarbital, phenobarbital, pentobarbital, D 23129, desflurane, methoxyflurane, enflurane, pregnenolone | 3.5 |
| PDE7B (phosphodiesterase 7B) | Enzyme | Dyphylline, nitroglycerin, aminophylline, anagrelide, milrinone, dipyridamole, tolbutamide, theophylline, pentoxifylline | 1.0 |
| SCN4B (sodium channel, voltage-gated, type IV, β) | Ion channel | Riluzole | 1.0 |
| HIP | |||
| GRIA2 (glutamate receptor, ionotropic, AMPA 2) | Ion channel | Talampanel, Org 24448, LY451395, tezampanel | 5.0 |
| GABRB3 (γ-aminobutyric acid (GABA) A receptor, β 3) | Ion channel | Methohexital, aspirin/butalbital/caffeine, aspirin/butalbital/caffeine/codeine, pagoclone, alphadolone, SEP 174559, acetaminophen/butalbital/caffeine, sevoflurane, isoflurane, gaboxadol, isoniazid, felbamate, etomidate, muscimol, halothane, fluoxetine/olanzapine, amobarbital, atropine/hyoscyamine/phenobarbital/scopolamine, acetaminophen/butalbital, eszopiclone, mephobarbital, hyoscyamine/phenobarbital, acetaminophen/butalbital/caffeine/codeine, butabarbital, temazepam, zolpidem, lorazepam, olanzapine, clonazepam, zaleplon, secobarbital, butalbital, phenobarbital, pentobarbital, thiopental, D 23129, desflurane, methoxyflurane, enflurane, pregnenolone | 4.5 |
| CACNA2D1 (calcium channel, voltage-dependent, α 2/δ subunit 1) | Ion channel | Amlodipine/valsartan/hydrochlorothiazide, amlodipine/telmisartan, bepridil, amlodipine, pregabalin | 1.0 |
|
IFNGR2 (interferon γ receptor 2 (interferon γ transducer 1)) |
Transmembrane receptor |
Interferon γ-1b |
1.0 |
|
(b) Males | |||
| PFC | |||
| COL6A2 (collagen, type VI, α 2) | Other | Collagenase clostridium histolyticum | 1.0 |
| CCR5 (chemokine (C-C motif) receptor 5) | G-protein-coupled receptor | Maraviroc, vicriviroc, SCH 351125 | 0.5 |
| AMY | |||
| GRIN2C (glutamate receptor, ionotropic, N-methyl D-aspartate 2C) | Ion channel | Dextromethorphan/guaifenesin, morphine/dextromethorphan, neramexane, bicifadine, delucemine, CR 2249, besonprodil, UK-240455, ketamine, felbamate, memantine, orphenadrine, cycloserine, N-(2-indanyl)glycinamide, dextromethorphan | 2.0 |
| HIP | |||
| GABRA1 (γ-aminobutyric acid (GABA) A receptor, α 1) | Ion channel | Methohexital, aspirin/butalbital/caffeine, aspirin/butalbital/caffeine/codeine, pagoclone, alphadolone, SEP 174559, acetaminophen/butalbital/caffeine, sevoflurane, isoflurane, gaboxadol, isoniazid, felbamate, etomidate, muscimol, halothane, fluoxetine/olanzapine, amobarbital, estazolam | 5.0 |
| GAD2 (glutamate decarboxylase 2) | Enzyme | Valproic acid | 4.0 |
| NR3C2 (nuclear receptor subfamily 3, group C, member 2) | Ligand-dependent nuclear receptor | Hydrochlorothiazide/spironolactone, fludrocortisone acetate, drospirenone, spironolactone, eplerenone | 4.0 |
| SLC12A2 (solute carrier family 12 (sodium/potassium/chloride transporters), member 2) | Transporter | Bumetanide | 4.0 |
| KCNMA1 potassium large conductance calcium-activated channel, subfamily M, α member 1 | Ion channel | Tedisamil | 3.5 |
| ATP1A2 (ATPase, Na+/K+ transporting, α 2 polypeptide) | Transporter | Digoxin, omeprazole, ethacrynic acid, perphenazine | 2.5 |
| LPL (lipoprotein lipase) | Enzyme | Nicotinic acid, lovastatin/niacin | 2.0 |
| SLC1A3 (solute carrier family 1 (glial high affinity glutamate transporter), member 3) | Transporter | Riluzole | 2.0 |
| SLC6A1 (solute carrier family 6 (neurotransmitter transporter, GABA), member 1) | Transporter | Tiagabine | 2.0 |
| CHUK (conserved helix-loop-helix ubiquitous kinase) | Kinase | Methyl 2-cyano-3,12-dioxoolean-1,9-dien-28-oate | 1.0 |
| PARP1 (poly (ADP-ribose) polymerase 1) | Enzyme | ABT-888, INO-1001 | 1.0 |
| SCN1A (sodium channel, voltage-gated, type I, α subunit) | Ion channel | Articaine/epinephrine, articaine, bupivacaine/lidocaine, chloroprocaine, epinephrine/prilocaine, epinephrine/lidocaine, fosphenytoin, phenytoin, prilocaine, lamotrigine, lidocaine, riluzole | 1.0 |
| TGFB2 (transforming growth factor, β 2) | Growth factor | AP-12009 | 1.0 |
| HTR5A (5-hydroxytryptamine (serotonin) receptor 5A) | G-protein-coupled receptor | Asenapine | 0.5 |
| SCN8A (sodium channel, voltage gated, type VIII, α subunit) | Ion channel | Riluzole | 0.5 |
Abbreviations: AMY, amygdala; CFG, convergent functional genomics; DHA, docosahexaenoic acid; HIP, hippocampus; PFC, prefrontal cortex.
Ingenuity analyses of the genes that are targeted by existing drugs.
Effects of DHA on alcohol consumption in two independent animal models: DBP KO mice and alcohol-preferring P rats
DBP KO mice on high-DHA diet drink less alcohol than DBP KO mice on low DHA
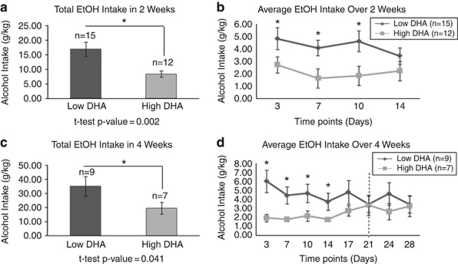
The high rate of co-morbidity between bipolar disorder and alcoholism in humans55 is reflected in our DBP KO mice animal model. We had previously shown that male DBP KO mice subjected to the chronic isolation stress paradigm consume more ethanol than the control DBP WT mice subjected to stress.22 We have now tested if a high-DHA diet would impact the alcohol consumption of these DBP KO mice compared with a low-DHA diet. In two separate analyses, one from a 2-week experiment and one from a 4-week experiment, we found that DHA significantly reduces alcohol consumption (Figure 4). No significant differences in water consumption were observed (data not shown), which shows that mice are showing a preference for alcohol, and not simply drinking more fluids.
Figure 4.
Effects of docosahexaenoic acid (DHA) on male DBP knockout (KO) stressed (ST) mice alcohol (EtOH) consumption: mice on a diet supplemented with either low or high DHA were subjected to alcohol free-choice drinking paradigm. (a, b) Fluid consumption (water or 10% ethanol) monitored for a period of 2 weeks (14 days). (c, d) Fluid consumption (water or 10% ethanol) monitored for a period of 4 weeks (28 days) with an acute stressor (behavioral challenge tests represented by the dotted vertical line) at day 21, as described in the Materials and methods Section. *P<0.05.
P rats on high-DHA diet drink less alcohol than P rats on low-DHA diet
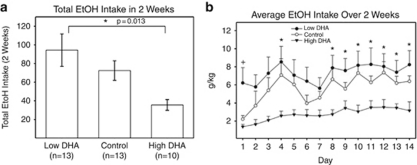
We were able to reproduce our findings in a well-established, independent animal model of alcohol consumption, the alcohol-preferring P rats. These rats are also subjected to single housing, which may induce chronic stress. Additionally, for these experiments, we did not just look at extremes of diet in terms of DHA content, but also used a normal control diet, with an intermediate content of DHA. A dose-dependent effect was observed, where alcohol-preferring P rats on a diet high in DHA drank significantly less alcohol over a 14-day period than did P rats on a normal control diet, and rats on a diet low in DHA (Figure 5).
Figure 5.
Effects of docosahexaenoic acid (DHA) on alcohol (EtOH) consumption in male alcohol-preferring (P) rats. Experimentally naive, male P rats, 4–6 months of age at the start of the experiment, were used as subjects. These rats were placed on one of the three diets: (1) low-DHA diet, (2) control diet or (3) high-DHA diet. Rats were given continuous free-choice access in the home cage to 15% v/v ethanol and water. Ethanol intake was measured daily throughout the experiment. (a, b) Fluid consumption from both bottles was monitored for a period of 2 weeks (14 days). *t-test P<0.05 for rats on low-DHA compared with rats on high-DHA diet.
Discussion
We conducted integrative studies of DHA treatment in animal models as a way of validating the efficacy of DHA as a psychotropic agent, to understand its underlying molecular effects in the brain, and to identify potential blood biomarkers of treatment response. Our work provides evidence on all three counts. Moreover, it identifies a previously unsuspected effect of DHA on decreasing alcohol consumption, which we substantiated in two independent animal models.
DBP KO ST mice as a human disease-relevant animal model
First, the behavioral phenomenology and inferences from molecular changes in the DBP KO mice revealed by our previous work22 bear broad similarities to the DSM (Diagnostic and Statistical Manual of Mental Disorders) criteria for bipolar disorder. Moreover, their switch in phenotype is a cardinal aspect of the human condition. As such, DBP KO mice are arguably one of the first comprehensive genetic animal models of bipolar disorder to be described, complementing earlier elegant pharmacological and genetic manipulations that mimic more restricted endophenotypic aspects of the disorder.19, 29, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 The fact that DBP is a transcription factor directly and indirectly regulating many other genes may explain the surprisingly comprehensive mimicry of a putative polygenic human disorder by a single gene ablation in mouse. Some of the genes identified may be directly regulated by DBP through promoter binding, whereas others may be regulated indirectly by a cascade of gene expression changes set in motion by DBP. Moreover, DBP is a circadian clock regulator, and an emerging body of work53, 66, 67, 68 substantiates the role of clock genes in bipolar and related disorders.
The DBP KO mice are a constitutive KO, and there is always the possibility that compensatory changes can occur during development that may obscure the direct effects of DBP deletion. However, of note, this is a very good equivalent of the human bipolar disorder genetic scenario, where most mutations are likely constitutive rather than acquired, as reflected in the familial inheritance of the disorder. Second, our mice colony is on a mixed genetic background, generated by heterozygote breeding, not on a back-crossed pure mouse-strain background. Although this introduces epistatic variability, it is remarkable that the phenotype remains penetrant across generations and cohorts of mice. Again, however, this is a better model of the human condition, which occurs at a population level in a mixed genetic background, than deriving conclusions from a very particular strain of mice.
Stress is an important trigger of medical and mental illness episodes in humans. Acute overwhelming stress (accidents, illness, loss of employment) on top of the chronic stress of social isolation often precede decompensation in bipolar patients69 and relapse into alcoholism.70 With that in mind, our mice were subjected to a chronic stress paradigm consisting of isolation (single housing) for 1 month, overlaid with an acute stressor (a series of behavioral challenge tests) at the end of the third week of isolation.
Last, the insights into overlapping phenomics, genomics and biomarkers among bipolar, alcoholism, stress and related disorders provided by this mouse model recapitulates in a translational fashion to the issues of complexity, heterogeneity, overlap and interdependence of major psychiatric syndromes as currently defined by DSM71 that are seen in human patients.
The power of the CFG approach
By cross-validating our animal model gene expression data with other lines of evidence, including human data, we were able to extract a shorter list of genes for which there are external corroborating lines of evidence (human genetic evidence, human post-mortem brain data, human blood data, animal model QTL data) linking them to bipolar and related disorders, thus reducing the risk of false positives. This cross-validation also identifies candidate blood biomarkers that are more likely directly related to the relevant disease neuropathology, as opposed to being potential artifactual effects related to a particular animal cohort or indirect effects of mouse colony environment. The power of our CFG approach is exemplified in the fact that our biomarker findings from previous studies have been shown to have good predictive power in independent cohorts,30, 31 a key litmus test in our view, and one that needs to be applied more systematically in this nascent field. The concordant candidate blood biomarkers for response to DHA that we identified in the current study (notably GLUL (glutamate-ammonia ligase glutamine synthetase) in males and KLF9 (Kruppel-like factor 9) in females), as well as some of the blood-only candidates that are changed in reverse direction to that seen in human blood in mood and stress disorders (notably SLC6A4 in females, as well as MBP and GLO1 in both sexes), will need to pass that level of scrutiny in future human studies before being deemed of unambiguous value.
From genes and biomarkers to biology
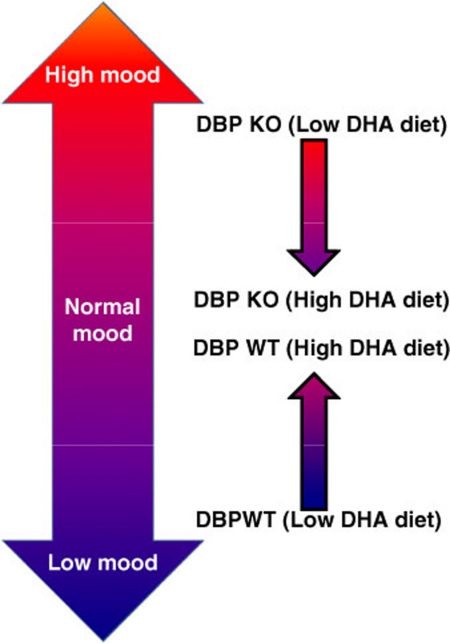
There is little co-directional overlap between the DHA-modulated genes in females and in males identified by us, which is somewhat surprising and quite interesting. However, there is some overlap at a biological pathway level and behavioral level between males and females. A practical implication of this work would be the need to use gender-specific biomarkers of response to treatment. Overall, the model that is emergent from the behavioral and gene expression data is that of DHA acting as a mood-stabilizing agent (Figure 6).
Figure 6.
High-docosahexaenoic acid (DHA) diet has a stabilizing effect on mood in stressed mice. A model integrating the behavioral and genomic data.
Future studies by us and others may focus on understanding at a mechanistic level the novel uncovered effects on alcohol consumption. We also need to test for potential gender differences in the effects of DHA on alcohol consumption.
Conclusions
Taken together, our convergent results provide evidence that DHA modulates and is involved in molecular networks targeted by current psychotropic medications. They also suggest intriguing possible sex differences for the molecular and behavioral effects of DHA, with a more antidepressant-like profile in females and a more antimanic-like profile in males.
The overall case for using DHA in large-scale human clinical trials and empirical clinical practice as an adjuvant mood-stabilizing agent and a novel potential alcoholism treatment, particularly for co-morbid bipolar disorder and alcoholism, is suggested and beginning to be substantiated at a mechanistic level by our work. Other possible therapeutic effects of DHA (in psychosis, anxiety, stress, pain and substance abuse) are pointed at by some of our data, and existing data in the literature. Given the genetic and biological heterogeneity of psychiatric disorders in human populations, it is possible, indeed likely, that not everyone will respond equally well to DHA treatment. Gender distinctions may be important, as our work suggests. The candidate blood biomarkers identified by us merit hypothesis-driven follow-up studies as markers of treatment response in a clinical setting; i.e., to test whether they are able to stratify, predict and differentiate early on in treatment responders from nonresponders.
Acknowledgments
This work was supported by funds from the Indiana University, NARSAD, a VA Merit Award and a NIH Directors' New Innovator Award to ABN.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Cunnane SC, Crawford MA. Survival of the fattest: fat babies were the key to evolution of the large human brain. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:17–26. doi: 10.1016/s1095-6433(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. J Lipid Res. 2006;47:172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR. Omega-3 Fatty acid treatment of women with borderline personality disorder: a double-blind, placebo-controlled pilot study. Am J Psychiatry. 2003;160:167–169. doi: 10.1176/appi.ajp.160.1.167. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- McNamara RK. DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J Nutr. 2010;140:864–868. doi: 10.3945/jn.109.113233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Stanford KE, Hahn CG, et al. Deficits in docosahexaenoic acid and associated elevations in the metabolism of arachidonic acid and saturated fatty acids in the postmortem orbitofrontal cortex of patients with bipolar disorder. Psychiatry Res. 2008;160:285–299. doi: 10.1016/j.psychres.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. Am J Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- Osher Y, Belmaker RH. Omega-3 fatty acids in depression: a review of three studies. CNS Neurosci Ther. 2009;15:128–133. doi: 10.1111/j.1755-5949.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63:1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- Peet M, Stokes C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs. 2005;65:1051–1059. doi: 10.2165/00003495-200565080-00002. [DOI] [PubMed] [Google Scholar]
- Berger GE, Wood SJ, Wellard RM, Proffitt TM, McConchie M, Amminger GP, et al. Ethyl-eicosapentaenoic acid in first-episode psychosis. A 1H-MRS study. Neuropsychopharmacology. 2008;33:2467–2473. doi: 10.1038/sj.npp.1301628. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon MF, Ryan CM, Sheu L, Yao JK, Conklin SM, Manuck SB. Serum phospholipid docosahexaenonic acid is associated with cognitive functioning during middle adulthood. J Nutr. 2010;140:848–853. doi: 10.3945/jn.109.119578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, et al. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–4395. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu AB, III, Segal DS, Kuczenski R, Barrett T, Hauger RL, Kelsoe JR. Identifying a series of candidate genes for mania and psychosis: a convergent functional genomics approach. Physiol Genomics. 2000;4:83–91. doi: 10.1152/physiolgenomics.2000.4.1.83. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bertsch BA, Strother WN, Le-Niculescu H, Balaraman Y, Hayden E, et al. Candidate genes, pathways and mechanisms for alcoholism: an expanded convergent functional genomics approach. Pharmacogenomics J. 2007;7:222–256. doi: 10.1038/sj.tpj.6500420. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Balaraman Y, Patel S, Tan J, Sidhu K, Jerome RE, et al. Towards understanding the schizophrenia code: an expanded convergent functional genomics approach. Am J Med Genet B Neuropsychiatr Genet. 2007;144:129–158. doi: 10.1002/ajmg.b.30481. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, McFarland M, Ogden C, Balaraman Y, Patel S, Tan J, et al. Phenomic, convergent functional genomic, and biomarker studies in a stress-reactive genetic animal model of bipolar disorder and co-morbid alcoholism. Am J Med Genet B. 2008;147B:134–166. doi: 10.1002/ajmg.b.30707. [DOI] [PubMed] [Google Scholar]
- Niculescu AB, Le-Niculescu H. The P-value illusion: how to improve (psychiatric) genetic studies. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:847–849. doi: 10.1002/ajmg.b.31076. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Chacko Y. Evaluation of a depression-related model of alcohol problems in 430 probands from the San Diego prospective study. Drug Alcohol Depend. 2006;82:194–203. doi: 10.1016/j.drugalcdep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Gardner CO, Kendler KS, Prescott CA. The temporal relationship of the onsets of alcohol dependence and major depression: using a genetically informative study design. Psychol Med. 2006;36:1153–1162. doi: 10.1017/S0033291706007860. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O'Connor S, Meyer ET, Reich T, et al. A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, et al. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu AB, Lulow LL, Ogden CA, Le-Niculescu H, Salomon DR, Schork NJ, et al. PhenoChipping of psychotic disorders: a novel approach for deconstructing and quantitating psychiatric phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2006;141:653–662. doi: 10.1002/ajmg.b.30404. [DOI] [PubMed] [Google Scholar]
- Ogden CA, Rich ME, Schork NJ, Paulus MP, Geyer MA, Lohr JB, et al. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–1029. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Kurian SM, Yehyawi N, Dike C, Patel SD, Edenberg HJ, et al. Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry. 2009;14:156–174. doi: 10.1038/mp.2008.11. [DOI] [PubMed] [Google Scholar]
- Kurian SM, Le-Niculescu H, Patel SD, Bertram D, Davis J, Dike C, et al. Identification of blood biomarkers for psychosis using convergent functional genomics. Mol Psychiatry. 2011;16:37–58. doi: 10.1038/mp.2009.117. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Niculescu AB, Akiskal HS.Sex hormones, Darwinism, and depression Arch Gen Psychiatry 2001581083–1084.author reply 5–6. [DOI] [PubMed] [Google Scholar]
- Niculescu AB, III, Akiskal HS. Proposed endophenotypes of dysthymia: evolutionary, clinical and pharmacogenomic considerations. Mol Psychiatry. 2001;6:363–366. doi: 10.1038/sj.mp.4000906. [DOI] [PubMed] [Google Scholar]
- Patel SD, Le-Niculescu H, Koller DL, Green SD, Lahiri DK, McMahon FJ, et al. Coming to grips with complex disorders: genetic risk prediction in bipolar disorder using panels of genes identified through convergent functional genomics. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:850–877. doi: 10.1002/ajmg.b.31087. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Tomita H, Meng F, Bolstad B, Li J, Evans S, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders Mol Psychiatry 200611615663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien WT, Klein PS. Validating GSK3 as an in vivo target of lithium action. Biochem Soc Trans. 2009;37 (Pt 5:1133–1138. doi: 10.1042/BST0371133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, et al. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Smoller JW, Ferreira MA, McQuillin A, Bass N, Lawrence J, et al. A genomewide association study of response to lithium for prevention of recurrence in bipolar disorder. Am J Psychiatry. 2009;166:718–725. doi: 10.1176/appi.ajp.2009.08111633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Subburaju S, Walsh JP. Circuitry-based gene expression profiles in GABA cells of the trisynaptic pathway in schizophrenics versus bipolars. Proc Natl Acad Sci USA. 2008;105:20935–20940. doi: 10.1073/pnas.0810153105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, et al. Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS One. 2009;4:e6585. doi: 10.1371/journal.pone.0006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman RH, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, Shalev AY.Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors Mol Psychiatry 200510500–513.425. [DOI] [PubMed] [Google Scholar]
- Morita K, Saito T, Ohta M, Ohmori T, Kawai K, Teshima-Kondo S, et al. Expression analysis of psychological stress-associated genes in peripheral blood leukocytes. Neurosci Lett. 2005;381:57–62. doi: 10.1016/j.neulet.2005.01.081. [DOI] [PubMed] [Google Scholar]
- Horiuchi Y, Nakayama J, Ishiguro H, Ohtsuki T, Detera-Wadleigh SD, Toyota T, et al. Possible association between a haplotype of the GABA-A receptor alpha 1 subunit gene (GABRA1) and mood disorders. Biol Psychiatry. 2004;55:40–45. doi: 10.1016/s0006-3223(03)00689-9. [DOI] [PubMed] [Google Scholar]
- Breuer R, Hamshere ML, Strohmaier J, Mattheisen M, Degenhardt F, Meier S, et al. Independent evidence for the selective influence of GABA(A) receptors on one component of the bipolar disorder phenotype Mol Psychiatryadvance online publication, 15 June 2010 [e-pub ahead of print]. [DOI] [PubMed]
- Donner J, Pirkola S, Silander K, Kananen L, Terwilliger JD, Lonnqvist J, et al. An association analysis of murine anxiety genes in humans implicates novel candidate genes for anxiety disorders. Biol Psychiatry. 2008;64:672–680. doi: 10.1016/j.biopsych.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann M, Sargin D, Rossner MJ, Bartels C, Theis F, Wichert SP, et al. Episode-specific differential gene expression of peripheral blood mononuclear cells in rapid cycling supports novel treatment approaches. Mol Med. 2008;14:546–552. doi: 10.2119/2008-00053.Begemann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Lee SH, Kim H, Song JS, Yang SD, Paik SG, et al. Progressive cognitive impairment and anxiety induction in the absence of plaque deposition in C57BL/6 inbred mice expressing transgenic amyloid precursor protein. J Neurosci Res. 2004;76:572–580. doi: 10.1002/jnr.20127. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Katsel P, Dracheva S, Stewart DG, Davis KL. Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. Int J Neuropsychopharmacol. 2007;10:565–573. doi: 10.1017/S1461145706007310. [DOI] [PubMed] [Google Scholar]
- McGrath CL, Glatt SJ, Sklar P, Le-Niculescu H, Kuczenski R, Doyle AE, et al. Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry. 2009;9:70. doi: 10.1186/1471-244X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basselin M, Kim HW, Chen M, Ma K, Rapoport SI, Murphy RC, et al. Lithium modifies brain arachidonic and docosahexaenoic metabolism in rat lipopolysaccharide model of neuroinflammation. J Lipid Res. 2010;51:1049–1056. doi: 10.1194/jlr.M002469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ostacher MJ, Perlis RH, Nierenberg AA, Calabrese J, Stange JP, Salloum I, et al. Impact of substance use disorders on recovery from episodes of depression in bipolar disorder patients: prospective data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2010;167:289–297. doi: 10.1176/appi.ajp.2009.09020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaldubina A, Einat H, Szechtman H, Shimon H, Belmaker RH. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. J Neural Transm. 2002;109:433–440. doi: 10.1007/s007020200035. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Einat H, Manji HK, Gould TD, Du J, Chen G. Possible involvement of the ERK signaling cascade in bipolar disorder: behavioral leads from the study of mutant mice. Drug News Perspect. 2003;16:453–463. doi: 10.1358/dnp.2003.16.7.829357. [DOI] [PubMed] [Google Scholar]
- Einat H, Manji HK. Cellular plasticity cascades: genes-to-behavior pathways in animal models of bipolar disorder. Biol Psychiatry. 2006;59:1160–1171. doi: 10.1016/j.biopsych.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Einat H. Establishment of a battery of simple models for facets of bipolar disorder: a practical approach to achieve increased validity, better screening and possible insights into endophenotypes of disease. Behav Genet. 2007;37:244–255. doi: 10.1007/s10519-006-9093-4. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Henter ID, Manji HK. Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry. 2010;15:883–895. doi: 10.1038/mp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M, Kasahara T, Iwamoto K, Komori A, Ishiwata M, Miyauchi T, et al. Therapeutic implications of down-regulation of cyclophilin D in bipolar disorder. Int J Neuropsychopharmacol. 2010;13:1355–1368. doi: 10.1017/S1461145710000362. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes Mol Psychiatry 200611577–593.523. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Sjoholm LK, Partonen T, Schalling M, Forsell Y. PER2 variantion is associated with depression vulnerability. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:570–581. doi: 10.1002/ajmg.b.31021. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Sjoholm LK, Soronen P, Paunio T, Vawter MP, Bunney WE, et al. CRY2 is associated with depression. PLoS One. 2010;5:e9407. doi: 10.1371/journal.pone.0009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoholm LK, Kovanen L, Saarikoski ST, Schalling M, Lavebratt C, Partonen T. CLOCK is suggested to associate with comorbid alcohol use and depressive disorders. J Circadian Rhythms. 2010;8:1. doi: 10.1186/1740-3391-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney WE, Jr, Murphy DL, Goodwin FK, Borge GF. The “switch process” in manic-depressive illness. I. A systematic study of sequential behavioral changes. Arch Gen Psychiatry. 1972;27:295–302. doi: 10.1001/archpsyc.1972.01750270005001. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101 (Suppl 1:23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Niculescu AB., III Polypharmacy in oligopopulations: what psychiatric genetics can teach biological psychiatry. Psychiatr Genet. 2006;16:241–244. doi: 10.1097/01.ypg.0000242195.74268.f9. [DOI] [PubMed] [Google Scholar]
- Sommer W, Arlinde C, Heilig M. The search for candidate genes of alcoholism: evidence from expression profiling studies. Addict Biol. 2005;10:71–79. doi: 10.1080/13556210412331327821. [DOI] [PubMed] [Google Scholar]
- Sugawara H, Iwamoto K, Bundo M, Ishiwata M, Ueda J, Kakiuchi C, et al. Effect of mood stabilizers on gene expression in lymphoblastoid cells. J Neural Transm. 2010;117:155–164. doi: 10.1007/s00702-009-0340-8. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Ng MY, Butler AW, Cohen-Woods S, Uher R, Pirlo K, et al. Genome-wide association study of major recurrent depression in the U.K. population. Am J Psychiatry. 2010;167:949–957. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- Szczepankiewicz A, Skibinska M, Hauser J, Slopien A, Leszczynska-Rodziewicz A, Kapelski P, et al. Association analysis of the GSK-3beta T-50C gene polymorphism with schizophrenia and bipolar disorder. Neuropsychobiology. 2006;53:51–56. doi: 10.1159/000090704. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Pedrosa E, Petruolo OA, Cockerham M, Papolos A, Novak T, et al. Increase in GSK3beta gene copy number variation in bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:259–265. doi: 10.1002/ajmg.b.30498. [DOI] [PubMed] [Google Scholar]
- Kalscheuer VM, Musante L, Fang C, Hoffmann K, Fuchs C, Carta E, et al. A balanced chromosomal translocation disrupting ARHGEF9 is associated with epilepsy, anxiety, aggression, and mental retardation. Hum Mutat. 2009;30:61–68. doi: 10.1002/humu.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaabi B, Gelernter J, Woods SW, Goddard A, Page GP, Elston RC. Genome scan for loci predisposing to anxiety disorders using a novel multivariate approach: strong evidence for a chromosome 4 risk locus. Am J Hum Genet. 2006;78:543–553. doi: 10.1086/501072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiteri C, Guilloux JP, Lewis DA, Sibille E. Altered gene synchrony suggests a combined hormone-mediated dysregulated state in major depression. PLoS One. 2010;5:e9970. doi: 10.1371/journal.pone.0009970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault C, Lai C, Wilke N, Duong B, Olive MF, Rahman S, et al. Expression profiling of neural cells reveals specific patterns of ethanol-responsive gene expression. Mol Pharmacol. 2000;58:1593–1600. doi: 10.1124/mol.58.6.1593. [DOI] [PubMed] [Google Scholar]
- Cichon S, Schumacher J, Muller DJ, Hurter M, Windemuth C, Strauch K, et al. A genome screen for genes predisposing to bipolar affective disorder detects a new susceptibility locus on 8q. Hum Mol Genet. 2001;10:2933–2944. doi: 10.1093/hmg/10.25.2933. [DOI] [PubMed] [Google Scholar]
- Del Zompo M, Severino G, Ardau R, Chillotti C, Piccardi M, Dib C, et al. Genome-scan for bipolar disorder with sib-pair families in the Sardinian population: a new susceptibility locus on chromosome 1p22-p21. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1200–1208. doi: 10.1002/ajmg.b.31092. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin Exp Res. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, McClintick JN, Strother WN, Carr LG, Liang T, et al. Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav. 2009;94:131–147. doi: 10.1016/j.pbb.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov BP, Jiang L, Trivedi NS, Aston C. Transcription profiling reveals mitochondrial, ubiquitin and signaling systems abnormalities in postmortem brains from subjects with a history of alcohol abuse or dependence. J Neurosci Res. 2003;72:756–767. doi: 10.1002/jnr.10631. [DOI] [PubMed] [Google Scholar]
- Etain B, Mathieu F, Rietschel M, Maier W, Albus M, McKeon P, et al. Genome-wide scan for genes involved in bipolar affective disorder in 70 European families ascertained through a bipolar type I early-onset proband: supportive evidence for linkage at 3p14. Mol Psychiatry. 2006;11:685–694. doi: 10.1038/sj.mp.4001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman RH, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, Milwidsky A, et al. Blood mononuclear cell gene expression signature of postpartum depression Mol Psychiatry 20101593–100.2. [DOI] [PubMed] [Google Scholar]
- Sklar P, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, et al. Genome-wide scan in Portuguese Island families identifies 5q31-5q35 as a susceptibility locus for schizophrenia and psychosis. Mol Psychiatry. 2004;9:213–218. doi: 10.1038/sj.mp.4001418. [DOI] [PubMed] [Google Scholar]
- Hong KS, McInnes LA, Service SK, Song T, Lucas J, Silva S, et al. Genetic mapping using haplotype and model-free linkage analysis supports previous evidence for a locus predisposing to severe bipolar disorder at 5q31-33. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:83–86. doi: 10.1002/ajmg.b.20091. [DOI] [PubMed] [Google Scholar]
- Crowe RR, Goedken R, Samuelson S, Wilson R, Nelson J, Noyes R., Jr Genomewide survey of panic disorder. Am J Med Genet. 2001;105:105–109. [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- Kraschewski A, Reese J, Anghelescu I, Winterer G, Schmidt LG, Gallinat J, et al. Association of the dopamine D2 receptor gene with alcohol dependence: haplotypes and subgroups of alcoholics as key factors for understanding receptor function. Pharmacogenet Genomics. 2009;19:513–527. doi: 10.1097/fpc.0b013e32832d7fd3. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Matheson IJ, Flint J. Association of the DRD2 gene Taq1A polymorphism and alcoholism: a meta-analysis of case-control studies and evidence of publication bias. Mol Psychiatry. 2007;12:454–461. doi: 10.1038/sj.mp.4001938. [DOI] [PubMed] [Google Scholar]
- Smith L, Watson M, Gates S, Ball D, Foxcroft D. Meta-analysis of the association of the Taq1A polymorphism with the risk of alcohol dependency: a HuGE gene-disease association review. Am J Epidemiol. 2008;167:125–138. doi: 10.1093/aje/kwm281. [DOI] [PubMed] [Google Scholar]
- Ponce G, Perez-Gonzalez R, Aragues M, Palomo T, Rodriguez-Jimenez R, Jimenez-Arriero MA, et al. The ANKK1 kinase gene and psychiatric disorders. Neurotox Res. 2009;16:50–59. doi: 10.1007/s12640-009-9046-9. [DOI] [PubMed] [Google Scholar]
- Yang BZ, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Haplotypic variants in DRD2, ANKK1, TTC12, and NCAM1 are associated with comorbid alcohol and drug dependence. Alcohol Clin Exp Res. 2008;32:2117–2127. doi: 10.1111/j.1530-0277.2008.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila T, Kananen L, Greco D, Donner J, Silander K, Terwilliger JD, et al. An association analysis of circadian genes in anxiety disorders. Biol Psychiatry. 2010;67:1163–1170. doi: 10.1016/j.biopsych.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Massat I, Souery D, Del-Favero J, Van Gestel S, Serretti A, Macciardi F, et al. Positive association of dopamine D2 receptor polymorphism with bipolar affective disorder in a European Multicenter Association Study of affective disorders. Am J Med Genet. 2002;114:177–185. [PubMed] [Google Scholar]
- Maron E, Nikopensius T, Koks S, Altmae S, Heinaste E, Vabrit K, et al. Association study of 90 candidate gene polymorphisms in panic disorder. Psychiatr Genet. 2005;15:17–24. doi: 10.1097/00041444-200503000-00004. [DOI] [PubMed] [Google Scholar]
- Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Yamamoto M, Ozawa H, Saito T, Kato T. Decreased expression of NEFH and PCP4/PEP19 in the prefrontal cortex of alcoholics. Neurosci Res. 2004;49:379–385. doi: 10.1016/j.neures.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE, Biederman J, Ferreira MA, Wong P, Smoller JW, Faraone SV. Suggestive linkage of the child behavior checklist juvenile bipolar disorder phenotype to 1p21, 6p21, and 8q21. J Am Acad Child Adolesc Psychiatry. 2010;49:378–387. [PMC free article] [PubMed] [Google Scholar]
- Nash MW, Huezo-Diaz P, Williamson RJ, Sterne A, Purcell S, Hoda F, et al. Genome-wide linkage analysis of a composite index of neuroticism and mood-related scales in extreme selected sibships. Hum Mol Genet. 2004;13:2173–2182. doi: 10.1093/hmg/ddh239. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Asada T. Immunohistochemical and immunoblot analysis of Dopamine and cyclic AMP-regulated phosphoprotein, relative molecular mass 32,000 (DARPP-32) in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1177–1181. doi: 10.1016/j.pnpbp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Torres KC, Souza BR, Miranda DM, Nicolato R, Neves FS, Barros AG, et al. The leukocytes expressing DARPP-32 are reduced in patients with schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:214–219. doi: 10.1016/j.pnpbp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Printz M, Flodman P, Hodgkinson C, Goldman D, et al. Genetical genomic determinants of alcohol consumption in rats and humans. BMC Biol. 2009;7:70. doi: 10.1186/1741-7007-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Matzilevich D, Burke RE, Walsh J. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol Psychiatry. 2006;11:241–251. doi: 10.1038/sj.mp.4001758. [DOI] [PubMed] [Google Scholar]
- Kawai T, Morita K, Masuda K, Nishida K, Shikishima M, Ohta M, et al. Gene expression signature in peripheral blood cells from medical students exposed to chronic psychological stress. Biol Psychol. 2007;76:147–155. doi: 10.1016/j.biopsycho.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillin A, Rizig M, Gurling HM. A microarray gene expression study of the molecular pharmacology of lithium carbonate on mouse brain mRNA to understand the neurobiology of mood stabilization and treatment of bipolar affective disorder. Pharmacogenet Genomics. 2007;17:605–617. doi: 10.1097/FPC.0b013e328011b5b2. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin Exp Res. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Cordeiro Q, Talkowski ME, Chowdari KV, Wood J, Nimgaonkar V, Vallada H. Association and linkage analysis of RGS4 polymorphisms with schizophrenia and bipolar disorder in Brazil. Genes Brain Behav. 2005;4:45–50. doi: 10.1111/j.1601-183x.2004.00096.x. [DOI] [PubMed] [Google Scholar]
- Bertsch B, Ogden CA, Sidhu K, Le-Niculescu H, Kuczenski R, Niculescu AB. Convergent functional genomics: a Bayesian candidate gene identification approach for complex disorders. Methods. 2005;37:274–279. doi: 10.1016/j.ymeth.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol Psychiatry. 2005;10:309–322. doi: 10.1038/sj.mp.4001565. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Pato CN, Gentile KL, McGann L, Brown AM, Trauzzi M, et al. Gene expression analysis of peripheral blood leukocytes from discordant sib-pairs with schizophrenia and bipolar disorder reveals points of convergence between genetic and functional genomic approaches. Am J Med Genet B Neuropsychiatr Genet. 2005;136:12–25. doi: 10.1002/ajmg.b.30171. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Maher B, Hughes HB, III, Zubenko WN, Stiffler JS, Kaplan BB, et al. Genome-wide linkage survey for genetic loci that influence the development of depressive disorders in families with recurrent, early-onset, major depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123:1–18. doi: 10.1002/ajmg.b.20073. [DOI] [PubMed] [Google Scholar]
- Cassidy F, Zhao C, Badger J, Claffey E, Dobrin S, Roche S, et al. Genome-wide scan of bipolar disorder and investigation of population stratification effects on linkage: support for susceptibility loci at 4q21, 7q36, 9p21, 12q24, 14q24, and 16p13. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:791–801. doi: 10.1002/ajmg.b.30524. [DOI] [PubMed] [Google Scholar]
- Goes FS, Zandi PP, Miao K, McMahon FJ, Steele J, Willour VL, et al. Mood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11-q14 and 13q21-33. Am J Psychiatry. 2007;164:236–247. doi: 10.1176/ajp.2007.164.2.236. [DOI] [PubMed] [Google Scholar]
- Rogers PJ, Hohoff C, Heatherley SV, Mullings EL, Maxfield PJ, Evershed RP, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology. 2010;35:1973–1983. doi: 10.1038/npp.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Kallassalu K, Tammiste A, Kolde R, Vilo J, Toru I, et al. Peripheral gene expression profiling of CCK-4-induced panic in healthy subjects. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:269–274. doi: 10.1002/ajmg.b.30898. [DOI] [PubMed] [Google Scholar]
- Deckert J, Nothen MM, Franke P, Delmo C, Fritze J, Knapp M, et al. Systematic mutation screening and association study of the A1 and A2a adenosine receptor genes in panic disorder suggest a contribution of the A2a gene to the development of disease. Mol Psychiatry. 1998;3:81–85. doi: 10.1038/sj.mp.4000345. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, Hodge SE, et al. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology. 2004;29:558–565. doi: 10.1038/sj.npp.1300311. [DOI] [PubMed] [Google Scholar]
- Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh SD, Badner JA, Berrettini WH, Yoshikawa T, Goldin LR, Turner G, et al. A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc Natl Acad Sci USA. 1999;96:5604–5609. doi: 10.1073/pnas.96.10.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Wang Y, Shugart YY, Grados M, Fyer AJ, Rauch S, et al. Evidence for potential relationship between SLC1A1 and a putative genetic linkage region on chromosome 14q to obsessive-compulsive disorder with compulsive hoarding. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1000–1002. doi: 10.1002/ajmg.b.30713. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Page GP, Bonvicini K, Woods SW, Pauls DL, Kruger S. A chromosome 14 risk locus for simple phobia: results from a genomewide linkage scan. Mol Psychiatry. 2003;8:71–82. doi: 10.1038/sj.mp.4001224. [DOI] [PubMed] [Google Scholar]
- Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM, et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13:1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- Seelan RS, Khalyfa A, Lakshmanan J, Casanova MF, Parthasarathy RN. Deciphering the lithium transcriptome: microarray profiling of lithium-modulated gene expression in human neuronal cells. Neuroscience. 2008;151:1184–1197. doi: 10.1016/j.neuroscience.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Fullerton JM, Donald JA, Mitchell PB, Schofield PR. Two-dimensional genome scan identifies multiple genetic interactions in bipolar affective disorder. Biol Psychiatry. 2010;67:478–486. doi: 10.1016/j.biopsych.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Lambert D, Middle F, Hamshere ML, Segurado R, Raybould R, Corvin A, et al. Stage 2 of the Wellcome Trust UK-Irish bipolar affective disorder sibling-pair genome screen: evidence for linkage on chromosomes 6q16-q21, 4q12-q21, 9p21, 10p14-p12 and 18q22. Mol Psychiatry. 2005;10:831–841. doi: 10.1038/sj.mp.4001684. [DOI] [PubMed] [Google Scholar]
- Foroud T, Bice P, Castelluccio P, Bo R, Miller L, Ritchotte A, et al. Identification of quantitative trait loci influencing alcohol consumption in the high alcohol drinking and low alcohol drinking rat lines. Behav Genet. 2000;30:131–140. doi: 10.1023/a:1001955205117. [DOI] [PubMed] [Google Scholar]
- Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, et al. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31:1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Yang XR, Bai Y, Beerman MB, Goldstein AM, Goldin LR. Genomic regions linked to alcohol consumption in the Framingham Heart Study. BMC Genet. 2003;4 (Suppl 1:S101. doi: 10.1186/1471-2156-4-S1-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Oskarsson H, Desnica N, Kostic JP, Stefansson JG, Kolbeinsson H, et al. Anxiety with panic disorder linked to chromosome 9q in Iceland. Am J Hum Genet. 2003;72:1221–1230. doi: 10.1086/375141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF.Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances Mol Psychiatry 200813786–799.741. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Cichon S, Ridinger M, Wodarz N, Soyka M, Zill P, et al. Genome-wide association study of alcohol dependence. Arch Gen Psychiatry. 2009;66:773–784. doi: 10.1001/archgenpsychiatry.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Kiss E, Baji I, Kapornai K, Daroczy G, Vetro A, et al. Association study of the estrogen receptor alpha gene (ESR1) and childhood-onset mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1323–1326. doi: 10.1002/ajmg.b.30751. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu YZ, Wong PT, Farook JM, Teo AL, Lee LK, et al. cDNA microarray analysis of gene expression in anxious PVG and SD rats after cat-freezing test. Exp Brain Res. 2003;149:413–421. doi: 10.1007/s00221-002-1369-1. [DOI] [PubMed] [Google Scholar]
- Klempan TA, Rujescu D, Merette C, Himmelman C, Sequeira A, Canetti L, et al. Profiling brain expression of the spermidine/spermine N1-acetyltransferase 1 (SAT1) gene in suicide. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:934–943. doi: 10.1002/ajmg.b.30920. [DOI] [PubMed] [Google Scholar]
- Maziade M, Roy MA, Chagnon YC, Cliche D, Fournier JP, Montgrain N, et al. Shared and specific susceptibility loci for schizophrenia and bipolar disorder: a dense genome scan in Eastern Quebec families. Mol Psychiatry. 2005;10:486–499. doi: 10.1038/sj.mp.4001594. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Czibere L, Roeske D, Lucae S, Unschuld PG, Ripke S, et al. TMEM132D, a new candidate for anxiety phenotypes: evidence from human and mouse studies Mol Psychiatryadvance online publication, 6 April 2010 [e-pub ahead of print]. [DOI] [PubMed]
- Bilkei-Gorzo A, Racz I, Michel K, Zimmer A, Klingmuller D, Zimmer A. Behavioral phenotype of pre-proenkephalin-deficient mice on diverse congenic backgrounds. Psychopharmacology (Berl) 2004;176:343–352. doi: 10.1007/s00213-004-1904-9. [DOI] [PubMed] [Google Scholar]
- Palo OM, Soronen P, Silander K, Varilo T, Tuononen K, Kieseppa T, et al. Identification of susceptibility loci at 7q31 and 9p13 for bipolar disorder in an isolated population. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:723–735. doi: 10.1002/ajmg.b.31039. [DOI] [PubMed] [Google Scholar]
- Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- Sherva R, Rice JP, Neuman RJ, Rochberg N, Saccone NL, Bierut LJ. Associations and interactions between SNPs in the alcohol metabolizing genes and alcoholism phenotypes in European Americans. Alcohol Clin Exp Res. 2009;33:848–857. doi: 10.1111/j.1530-0277.2009.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira A, Klempan T, Canetti L, ffrench-Mullen J, Benkelfat C, Rouleau GA, et al. Patterns of gene expression in the limbic system of suicides with and without major depression. Mol Psychiatry. 2007;12:640–655. doi: 10.1038/sj.mp.4001969. [DOI] [PubMed] [Google Scholar]
- Shyn SI, Shi J, Kraft JB, Potash JB, Knowles JA, Weissman MM, et al. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies. Mol Psychiatry. 2011;16:202–215. doi: 10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrin T, Blank T, Saravana R, Rayner M, Spiess J, Todorovic C. Region specific gene expression profile in mouse brain after chronic corticotropin releasing factor receptor 1 activation: the novel role for diazepam binding inhibitor in contextual fear conditioning. Neuroscience. 2009;162:14–22. doi: 10.1016/j.neuroscience.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Fallin MD, Lasseter VK, Wolyniec PS, McGrath JA, Nestadt G, Valle D, et al. Genomewide linkage scan for bipolar-disorder susceptibility loci among Ashkenazi Jewish families. Am J Hum Genet. 2004;75:204–219. doi: 10.1086/422474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Kimpel MW, Schultz JA, McClintick JN, Edenberg HJ, Bell RL. Changes in gene expression in regions of the extended amygdala of alcohol-preferring rats after binge-like alcohol drinking. Alcohol. 2010;44:171–183. doi: 10.1016/j.alcohol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B, et al. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9:1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Buervenich S, Badner JA, Steele CJ, Detera-Wadleigh SD, Dick D, et al. Loci on chromosomes 6q and 6p interact to increase susceptibility to bipolar affective disorder in the national institute of mental health genetics initiative pedigrees. Biol Psychiatry. 2004;56:18–23. doi: 10.1016/j.biopsych.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Sun F, Cheng R, Flanders WD, Yang Q, Khoury MJ. Whole genome association studies for genes affecting alcohol dependence. Genet Epidemiol. 1999;17 (Suppl 1:S337–S342. doi: 10.1002/gepi.1370170757. [DOI] [PubMed] [Google Scholar]
- Liebl C, Panhuysen M, Putz B, Trumbach D, Wurst W, Deussing JM, et al. Gene expression profiling following maternal deprivation: involvement of the brain Renin-Angiotensin system. Front Mol Neurosci. 2009;2:1. doi: 10.3389/neuro.02.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Pennington K, Behan A, Wait R, Dunn MJ, Cotter D. Proteomic analysis of the anterior cingulate cortex in the major psychiatric disorders: evidence for disease-associated changes. Proteomics. 2006;6:3414–3425. doi: 10.1002/pmic.200500069. [DOI] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression Mol Psychiatryadvance online publication, 13 April 2010 [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- Dick DM, Nurnberger J, Jr, Edenberg HJ, Goate A, Crowe R, Rice J, et al. Suggestive linkage on chromosome 1 for a quantitative alcohol-related phenotype. Alcohol Clin Exp Res. 2002;26:1453–1460. doi: 10.1097/01.ALC.0000034037.10333.FD. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, et al. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsetti M, Di Brisco F, Rinaldi M, Dallorto D, Ghi P. Some molecular effectors of antidepressant action of quetiapine revealed by DNA microarray in the frontal cortex of anhedonic rats. Pharmacogenet Genomics. 2009;19:600–612. doi: 10.1097/FPC.0b013e32832ee573. [DOI] [PubMed] [Google Scholar]
- Ising M, Lucae S, Binder EB, Bettecken T, Uhr M, Ripke S, et al. A genomewide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch Gen Psychiatry. 2009;66:966–975. doi: 10.1001/archgenpsychiatry.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandish PE, Su M, Holder DJ, Hodor P, Szumiloski J, Kleinhanz RR, et al. Regulation of gene expression by lithium and depletion of inositol in slices of adult rat cortex. Neuron. 2005;45:861–872. doi: 10.1016/j.neuron.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S, Zezza N, Hoffman EK, Perlin M, Allan W. A genome wide search for alcoholism susceptibility genes. Am J Med Genet B Neuropsychiatr Genet. 2004;128:102–113. doi: 10.1002/ajmg.b.30013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pato CN, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, et al. Genome-wide scan in Portuguese Island families implicates multiple loci in bipolar disorder: fine mapping adds support on chromosomes 6 and 11. Am J Med Genet B Neuropsychiatr Genet. 2004;127B:30–34. doi: 10.1002/ajmg.b.30001. [DOI] [PubMed] [Google Scholar]
- Choudary PV, Molnar M, Evans SJ, Tomita H, Li JZ, Vawter MP, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc Natl Acad Sci USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama H, Little KY, Sieghart W, Devaud LL, Morrow AL. GABA(A) receptor alpha1, alpha4, and beta3 subunit mRNA and protein expression in the frontal cortex of human alcoholics. Alcohol Clin Exp Res. 1998;22:815–822. [PubMed] [Google Scholar]
- Song J, Koller DL, Foroud T, Carr K, Zhao J, Rice J, et al. Association of GABA(A) receptors and alcohol dependence and the effects of genetic imprinting. Am J Med Genet B Neuropsychiatr Genet. 2003;117:39–45. doi: 10.1002/ajmg.b.10022. [DOI] [PubMed] [Google Scholar]
- Craddock N, Jones L, Jones IR, Kirov G, Green EK, Grozeva D, et al. Strong genetic evidence for a selective influence of GABA(A) receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2010;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PA, Macgregor S, Vink JM, Pergadia ML, Hansell NK, de Moor MH, et al. A genomewide association study of nicotine and alcohol dependence in Australian and Dutch populations. Twin Res Hum Genet. 2010;13:10–29. doi: 10.1375/twin.13.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dushlaine C, Kenny E, Heron E, Donohoe G, Gill M, Morris D, et al. Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol Psychiatry. 2011;16:286–292. doi: 10.1038/mp.2010.7. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Acierno JS, Jr, Rosenbaum JF, Biederman J, Pollack MH, Meminger S, et al. Targeted genome screen of panic disorder and anxiety disorder proneness using homology to murine QTL regions. Am J Med Genet. 2001;105:195–206. doi: 10.1002/ajmg.1209. [DOI] [PubMed] [Google Scholar]
- Meira-Lima IV, Pereira AC, Mota GF, Krieger JE, Vallada H. Angiotensinogen and angiotensin converting enzyme gene polymorphisms and the risk of bipolar affective disorder in humans. Neurosci Lett. 2000;293:103–106. doi: 10.1016/s0304-3940(00)01512-3. [DOI] [PubMed] [Google Scholar]
- Su YA, Wu J, Zhang L, Zhang Q, Su DM, He P, et al. Dysregulated mitochondrial genes and networks with drug targets in postmortem brain of patients with posttraumatic stress disorder (PTSD) revealed by human mitochondria-focused cDNA microarrays. Int J Biol Sci. 2008;4:223–235. doi: 10.7150/ijbs.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuei X, Flury-Wetherill L, Almasy L, Bierut L, Tischfield J, Schuckit M, et al. Association analysis of genes encoding the nociceptin receptor (OPRL1) and its endogenous ligand (PNOC) with alcohol or illicit drug dependence. Addict Biol. 2008;13:80–87. doi: 10.1111/j.1369-1600.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- Kohen R, Kirov S, Navaja GP, Happe HK, Hamblin MW, Snoddy JR, et al. Gene expression profiling in the hippocampus of learned helpless and nonhelpless rats. Pharmacogenomics J. 2005;5:278–291. doi: 10.1038/sj.tpj.6500322. [DOI] [PubMed] [Google Scholar]
- Worst TJ, Tan JC, Robertson DJ, Freeman WM, Hyytia P, Kiianmaa K, et al. Transcriptome analysis of frontal cortex in alcohol-preferring and nonpreferring rats. J Neurosci Res. 2005;80:529–538. doi: 10.1002/jnr.20496. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, van der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S, et al. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. J Neurochem. 2005;93:359–370. doi: 10.1111/j.1471-4159.2004.03021.x. [DOI] [PubMed] [Google Scholar]
- Ryan MM, Lockstone HE, Huffaker SJ, Wayland MT, Webster MJ, Bahn S. Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol Psychiatry. 2006;11:965–978. doi: 10.1038/sj.mp.4001875. [DOI] [PubMed] [Google Scholar]
- Morissette J, Villeneuve A, Bordeleau L, Rochette D, Laberge C, Gagne B, et al. Genome-wide search for linkage of bipolar affective disorders in a very large pedigree derived from a homogeneous population in quebec points to a locus of major effect on chromosome 12q23-q24. Am J Med Genet. 1999;88:567–587. doi: 10.1002/(sici)1096-8628(19991015)88:5<567::aid-ajmg24>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Fyer AJ, Haghighi F, Heiman G, Deng Z, Hen R, et al. Potential panic disorder syndrome: clinical and genetic linkage evidence. Am J Med Genet. 2000;96:24–35. doi: 10.1002/(sici)1096-8628(20000207)96:1<24::aid-ajmg7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Soria V, Martinez-Amoros E, Escaramis G, Valero J, Perez-Egea R, Garcia C, et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010;35:1279–1289. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron E, Hettema JM, Shlik J. Advances in molecular genetics of panic disorder. Mol Psychiatry. 2010;15:681–701. doi: 10.1038/mp.2009.145. [DOI] [PubMed] [Google Scholar]
- Luciano M, Houlihan LM, Harris SE, Gow AJ, Hayward C, Starr JM, et al. Association of existing and new candidate genes for anxiety, depression and personality traits in older people. Behav Genet. 2010;40:518–532. doi: 10.1007/s10519-009-9326-4. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Fyer AJ, Durner M, Heiman GA, Baisre de Leon A, Hodge SE, et al. Further genetic evidence for a panic disorder syndrome mapping to chromosome 13q. Proc Natl Acad Sci USA. 2003;100:2550–2555. doi: 10.1073/pnas.0335669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyer AJ, Hamilton SP, Durner M, Haghighi F, Heiman GA, Costa R, et al. A third-pass genome scan in panic disorder: evidence for multiple susceptibility loci. Biol Psychiatry. 2006;60:388–401. doi: 10.1016/j.biopsych.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Hidaka S, Asada T. Immunohistochemical and immunoblot study of GABA(A) alpha1 and beta2/3 subunits in the prefrontal cortex of subjects with schizophrenia and bipolar disorder. Neurosci Res. 2004;50:77–84. doi: 10.1016/j.neures.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Johnson C, Drgon T, McMahon FJ, Uhl GR. Convergent genome wide association results for bipolar disorder and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:182–190. doi: 10.1002/ajmg.b.30900. [DOI] [PubMed] [Google Scholar]
- Xing G, Zhang L, Russell S, Post R. Reduction of dopamine-related transcription factors Nurr1 and NGFI-B in the prefrontal cortex in schizophrenia and bipolar disorders. Schizophr Res. 2006;84:36–56. doi: 10.1016/j.schres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Etheridge N, Lewohl JM, Mayfield RD, Harris RA, Dodd PR. Synaptic proteome changes in the superior frontal gyrus and occipital cortex of the alcoholic brain. Proteomics Clin Appl. 2009;3:730–742. doi: 10.1002/prca.200800202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Bierut L, Goate A, Rice J, Hinrichs A, et al. Linkage analyses of IQ in the collaborative study on the genetics of alcoholism (COGA) sample. Behav Genet. 2006;36:77–86. doi: 10.1007/s10519-005-9009-8. [DOI] [PubMed] [Google Scholar]
- Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- Heckers S, Stone D, Walsh J, Shick J, Koul P, Benes FM. Differential hippocampal expression of glutamic acid decarboxylase 65 and 67 messenger RNA in bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2002;59:521–529. doi: 10.1001/archpsyc.59.6.521. [DOI] [PubMed] [Google Scholar]
- Lappalainen J, Krupitsky E, Kranzler HR, Luo X, Remizov M, Pchelina S, et al. Mutation screen of the GAD2 gene and association study of alcoholism in three populations. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:183–192. doi: 10.1002/ajmg.b.30377. [DOI] [PubMed] [Google Scholar]
- Unschuld PG, Ising M, Specht M, Erhardt A, Ripke S, Heck A, et al. Polymorphisms in the GAD2 gene-region are associated with susceptibility for unipolar depression and with a risk factor for anxiety disorders. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1100–1109. doi: 10.1002/ajmg.b.30938. [DOI] [PubMed] [Google Scholar]
- Martin MV, Rollins B, Sequeira PA, Mesen A, Byerley W, Stein R, et al. Exon expression in lymphoblastoid cell lines from subjects with schizophrenia before and after glucose deprivation. BMC Med Genomics. 2009;2:62. doi: 10.1186/1755-8794-2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Karssen AM, Her S, Li JZ, Patel PD, Meng F, Bunney WE, Jr, et al. Stress-induced changes in primate prefrontal profiles of gene expression. Mol Psychiatry. 2007;12:1089–1102. doi: 10.1038/sj.mp.4002095. [DOI] [PubMed] [Google Scholar]
- Derijk RH, van Leeuwen N, Klok MD, Zitman FG. Corticosteroid receptor-gene variants: modulators of the stress-response and implications for mental health. Eur J Pharmacol. 2008;585:492–501. doi: 10.1016/j.ejphar.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurata LW, Bukhman YV, Charles V, Capriglione F, Bullard J, Lemire AL, et al. Comparison of microarray-based mRNA profiling technologies for identification of psychiatric disease and drug signatures. J Neurosci Methods. 2004;138:173–188. doi: 10.1016/j.jneumeth.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Youngs RM, Chu MS, Meloni EG, Naydenov A, Carlezon WA, Jr, Konradi C. Lithium administration to preadolescent rats causes long-lasting increases in anxiety-like behavior and has molecular consequences. J Neurosci. 2006;26:6031–6039. doi: 10.1523/JNEUROSCI.0580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Ostrander M, Abplanalp W, Richtand NM, Benoit SC, Clegg DJ. Modulation of phosphoinositide-protein kinase C signal transduction by omega-3 fatty acids: implications for the pathophysiology and treatment of recurrent neuropsychiatric illness. Prostaglandins Leukot Essent Fatty Acids. 2006;75:237–257. doi: 10.1016/j.plefa.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR, et al. Altered expression and phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) in postmortem brain of suicide victims with or without depression. J Psychiatr Res. 2003;37:421–432. doi: 10.1016/s0022-3956(03)00047-5. [DOI] [PubMed] [Google Scholar]
- Hattori E, Toyota T, Ishitsuka Y, Iwayama Y, Yamada K, Ujike H, et al. Preliminary genome-wide association study of bipolar disorder in the Japanese population. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1110–1117. doi: 10.1002/ajmg.b.30941. [DOI] [PubMed] [Google Scholar]
- Chu TT, Liu Y, Kemether E. Thalamic transcriptome screening in three psychiatric states. J Hum Genet. 2009;54:665–675. doi: 10.1038/jhg.2009.93. [DOI] [PubMed] [Google Scholar]
- Maron E, Hettema JM, Shlik J. Advances in molecular genetics of panic disorder. Mol Psychiatry. 2010;15:681–701. doi: 10.1038/mp.2009.145. [DOI] [PubMed] [Google Scholar]
- Stopkova P, Saito T, Fann CS, Papolos DF, Vevera J, Paclt I, et al. Polymorphism screening of PIP5K2A: a candidate gene for chromosome 10p-linked psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:50–58. doi: 10.1002/ajmg.b.20012. [DOI] [PubMed] [Google Scholar]
- Wray NR, James MR, Mah SP, Nelson M, Andrews G, Sullivan PF, et al. Anxiety and comorbid measures associated with PLXNA2. Arch Gen Psychiatry. 2007;64:318–326. doi: 10.1001/archpsyc.64.3.318. [DOI] [PubMed] [Google Scholar]
- Curtis D, Kalsi G, Brynjolfsson J, McInnis M, O'Neill J, Smyth C, et al. Genome scan of pedigrees multiply affected with bipolar disorder provides further support for the presence of a susceptibility locus on chromosome 12q23-q24, and suggests the presence of additional loci on 1p and 1q. Psychiatr Genet. 2003;13:77–84. doi: 10.1097/01.ypg.0000056684.89558.d2. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Bonvicini K, Page G, Woods SW, Goddard AW, Kruger S, et al. Linkage genome scan for loci predisposing to panic disorder or agoraphobia. Am J Med Genet. 2001;105:548–557. doi: 10.1002/ajmg.1496. [DOI] [PubMed] [Google Scholar]
- Foroud T, Bucholz KK, Edenberg HJ, Goate A, Neuman RJ, Porjesz B, et al. Linkage of an alcoholism-related severity phenotype to chromosome 16. Alcohol Clin Exp Res. 1998;22:2035–2042. [PubMed] [Google Scholar]
- Macgregor S, Visscher PM, Knott SA, Thomson P, Porteous DJ, Millar JK, et al. A genome scan and follow-up study identify a bipolar disorder susceptibility locus on chromosome 1q42. Mol Psychiatry. 2004;9:1083–1090. doi: 10.1038/sj.mp.4001544. [DOI] [PubMed] [Google Scholar]
- Nakatani N, Ohnishi T, Iwamoto K, Watanabe A, Iwayama Y, Yamashita S, et al. Expression analysis of actin-related genes as an underlying mechanism for mood disorders. Biochem Biophys Res Commun. 2007;352:780–786. doi: 10.1016/j.bbrc.2006.11.101. [DOI] [PubMed] [Google Scholar]
- Del Zompo M, Severino G, Ardau R, Chillotti C, Piccardi M, Dib C, et al. Genome-scan for bipolar disorder with sib-pair families in the Sardinian population: a new susceptibility locus on chromosome 1p22-p21. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1200–1208. doi: 10.1002/ajmg.b.31092. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Foroud T, Flury L, Su J, Meyer ET, Hu K, et al. Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. Am J Psychiatry. 2001;158:718–724. doi: 10.1176/appi.ajp.158.5.718. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Badner JA, Steele J, Willour VL, Miao K, MacKinnon DF, et al. Genome-wide linkage scan of 98 bipolar pedigrees and analysis of clinical covariates. Mol Psychiatry. 2007;12:630–639. doi: 10.1038/sj.mp.4002027. [DOI] [PubMed] [Google Scholar]
- Bailer U, Leisch F, Meszaros K, Lenzinger E, Willinger U, Strobl R, et al. Genome scan for susceptibility loci for schizophrenia and bipolar disorder. Biol Psychiatry. 2002;52:40–52. doi: 10.1016/s0006-3223(02)01320-3. [DOI] [PubMed] [Google Scholar]
- Sousa JC, Grandela C, Fernandez-Ruiz J, de Miguel R, de Sousa L, Magalhaes AI, et al. Transthyretin is involved in depression-like behaviour and exploratory activity. J Neurochem. 2004;88:1052–1058. doi: 10.1046/j.1471-4159.2003.02309.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.