Abstract
Serotonin reuptake inhibitor (SRI) antidepressants such as fluoxetine (Prozac), promote hippocampal neurogenesis. They also increase the levels of the bcl-2 protein, whose overexpression in transgenic mice enhances adult hippocampal neurogenesis. However, the mechanisms underlying SRI-mediated neurogenesis are unclear. Recently, we identified the microRNA miR-16 as an important effector of SRI antidepressant action in serotonergic raphe and noradrenergic locus coeruleus (LC). We show here that miR-16 mediates adult neurogenesis in the mouse hippocampus. Fluoxetine, acting on serotonergic raphe neurons, decreases the amount of miR-16 in the hippocampus, which in turn increases the levels of the serotonin transporter (SERT), the target of SRI, and that of bcl-2 and the number of cells positive for Doublecortin, a marker of neuronal maturation. Neutralization of miR-16 in the hippocampus further exerts an antidepressant-like effect in behavioral tests. The fluoxetine-induced hippocampal response is relayed, in part, by the neurotrophic factor S100β, secreted by raphe and acting via the LC. Fluoxetine-exposed serotonergic neurons also secrete brain-derived neurotrophic factor, Wnt2 and 15-Deoxy-delta12,14-prostaglandin J2. These molecules are unable to mimic on their own the action of fluoxetine and we show that they act synergistically to regulate miR-16 at the hippocampus. Of note, these signaling molecules are increased in the cerebrospinal fluid of depressed patients upon fluoxetine treatment. Thus, our results demonstrate that miR-16 mediates the action of fluoxetine by acting as a micromanager of hippocampal neurogenesis. They further clarify the signals and the pathways involved in the hippocampal response to fluoxetine, which may help refine therapeutic strategies to alleviate depressive disorders.
Keywords: antidepressant, hippocampus, locus coeruleus, microRNA, neurogenesis, raphe
Introduction
Adult neurogenesis in the hippocampus is enhanced by antidepressant therapies.1, 2, 3, 4, 5 This hippocampal response to antidepressants likely involves multiple effectors, whose identification and roles are the focus of intense research.2, 6
Recently, we identified the microRNA miR-16, which targets the serotonin transporter (SERT), as an important effector of serotonin reuptake inhibitor (SRI) antidepressant action in raphe and locus coeruleus (LC).7 In particular, we showed that systemic fluoxetine treatment can promote either decreases or increases in the level of miR-16 depending upon the region of the brain. In serotonergic raphe, the level of miR-16 is low and increases in response to fluoxetine. On the other hand, upon fluoxetine treatment, raphe promotes a decrease in miR-16 in the noradrenergic LC.7 This action of fluoxetine on the LC is relayed by the neurotrophic protein S100β, which is released by raphe in response to SRI treatment.7
Another well described target of miR-16 in tumors is the bcl-2 (B-cell chronic lymphocytic lymphoma 2) protein.8 Beyond its well-established anti-apoptotic role, bcl-2 also exerts neurotrophic functions.3 Accordingly, overexpression of bcl-2 in transgenic mice results in increased adult hippocampal neurogenesis.9 Moreover, antidepressant therapies do increase the levels of bcl-2 protein in the hippocampus.3, 10 However, the link between antidepressants, bcl-2 expression and neurogenesis remains unclear.
Because the hippocampus is highly innervated by raphe- and LC-originating fibers11 and bcl-2 is a known target of miR-16, we hypothesized that SRI antidepressants such as Prozac (fluoxetine) enhance neurogenesis in the hippocampus by regulating the level of bcl-2 via miR-16. Using two different experimental paradigms, that is, local infusion into raphe or systemic injection, we obtained evidence that fluoxetine downregulates miR-16 in the hippocampus, which promotes neurogenesis. Further, we have examined the impact of a neutralization of hippocampal miR-16 in behavioral tests. Finally, using serotonergic neuronal cells, we have screened the signaling molecules that are secreted in response to fluoxetine and that participate in the miR-16-mediated hippocampal changes induced by this antidepressant. The relevance of our findings was assessed by investigating these signaling factors in the cerebrospinal fluid (CSF) of mice and depressed patients exposed to fluoxetine.
Materials and methods
Materials
Dibutyryl cyclic AMP and cyclohexane carboxylic acid were purchased from Sigma-Aldrich (St Louis, MO, USA). [3H]-paroxetine (0.98–1.01 TBq mmol−1) was from NEN Life Science Products (Boston, MA, USA). Fluoxetine was kindly provided by Dr M Bouhassira (Eli Lilly, Indianapolis, IN, USA). S100β siRNA oligo was from Invitrogen (Carlsbad, CA, USA). Recombinant mouse brain-derived neurotrophic factor (BDNF) was from R&D systems (Minneapolis, MN, USA). 15-Deoxy-delta12,14-prostaglandin J2 (15d-PGJ2) was from Cayman Biochemical (Ann Harbor, MI, USA).
Intracerebroventricular injections
Adult 6–8 week-old male Swiss-Kunming mice (25–30 g), were housed at 22±0.5 °C with food and water ad libitum and a reversed 12:12 h light cycle. All animal procedures were performed in accordance with National Institutes of Health guidelines for care and were approved by the Animal Care and Use Committee at Basel University.
CMA/11 microdialysis guide cannulas (CMA Microdialysis, Stockholm, Sweden) were stereotaxically implanted into the raphe or the hippocampus of avertin-anaesthetized mice as in Baudry et al.7 They were connected to syringe pumps and perfusion was performed at a rate of 2 μl min−1 with a solution of artificial CSF containing 1 μ fluoxetine as in Baudry et al.,7 1 n S100β, BDNF (1–100 ng ml−1), Wnt2 (0–5 ng ml−1) or 15d-PGJ2 (0–1 μ). miR-16 or anti-miR16 (1 μl, 2 μM) or S100β siRNA (2 μg, 1 μg μl−1) were directly injected at 36 h intervals as in Baudry et al.7 S100β antibodies were injected at 1 μg ml−1. After 1 day (S100β antibodies, S100β protein, BDNF, Wnt2 or 15d-PGJ2), 20 days (S100β siRNA) or 3 days (other treatments), mice were anesthetized with isoflurane, decapitated and the hippocampus was collected for RNA extraction and miR-16 expression analysis by real-time PCR as well as radioligand binding and western blot experiments.
Lesion of the noradrenergic system
Selective degeneration of LC noradrenergic fibers was carried out with two intraperitoneal injections of DSP4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine)12 (50 mg kg−1 each at an interval of 7 days).
RNA isolation and quantitative real-time analysis
Total RNA was isolated and mature miR-16 expression was detected as in Baudry et al.7
Radioligand binding studies
Binding experiments were performed on cell membranes as described in Launay et al.13
Immunoblot analysis
Hippocampal extracts were lysed in a buffer containing 50 m Tris HCl pH 8, 150 m NaCl, 0.1% SDS, 1% nonidet P40, 0.5% sodium deoxycholate, 0.02% sodium azide, 100 μg ml−1 phenylmethylsulfonyl fluoride and 1 μg ml−1 aprotinin. The protein concentration was measured using the bicinchoninic acid method (Pierce, Rockford, IL, USA). Proteins (80 μg) were resolved by 12% SDS–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane. Immunoblotting was carried out using antibodies to Bcl-2 (1:200; Santa Cruz, Santa Cruz, CA, USA).
Immunostaining
Mice were anesthetized with avertin and perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline. After overnight postfixation and cryoprotection in 30% sucrose for at least 4 h, serial cryostat sections (40 μm) were cut through the entire hippocampus and stored in phosphate-buffered saline. Sections were processed with a standard immunohistochemical procedure as in Baudry et al.7 to visualize Doublecortin (Dcx) (Santa cruz; 1:500), SERT (1:1000; Millipore, Temecula, CA, USA), V-GLUT-1 or V-GLUT-2 (both from Synaptic Systems, 1:2000, Goettingen, Germany). Biotinylated or fluorescent secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) was used. Images were obtained with a Zeiss LSM 510 Meta confocal microscope (Zeiss, Goettingen, Germany). For Dcx, results are expressed as the mean number of Dcx-positive cells per hippocampus as in Egeland et al.14 For double-label immunohistochemical imaging, the two channels were collected separately with single wavelength excitation and then merged to produce the composite image.
Behavioral tests
The forced swimming test was carried out as in Baudry et al.7 In unpredictable chronic mild stress experiments, male mice were repeatedly subjected to various socio-environmental stressors according to a ‘random' schedule for a total period of 6 weeks.7, 15 Treatment was administered for the last 5 weeks, as in Baudry et al.7
Patients
In all, 11 medication-free outpatients with major depressive disorder (mean±s.d. age 35.3±11.4 years, body mass index 26.7±6.5 kg m−2, seven men) gave informed consent to participate in the study, using ethical procedures approved by the Assistance Publique—Hôpitaux de Paris, Comité de Protection des Personnes. Patients were physically healthy, had received no psychotropic medications for at least 6 weeks before the lumbar puncture, and did not meet the criteria for alcohol or substance abuse or dependence for at least 6 months before the study. The study was conducted between January 2009 and August 2010. Major depressive disorder diagnosis was established using the Structured Clinical Interview or DSM-IV Axis I Disorders, Clinical Version (SCID-CV). Severity of depressive and anxiety symptoms was assessed using the Inventory of Depressive Symptomatology and Hamilton Anxiety Rating Scale. Raters were experienced mental health research professionals, who served as the case-managing clinicians. All patients were offered a 12-week course of open-label treatment with fluoxetine, and agreed to participate. Of these 11, 9 completed the treatment course. Treatment was started at a dose of 20 mg per day and increased to 40 mg per day if clinical improvement was not satisfactory after 1 month of treatment (mean±s.d. fluoxetine dose=33.3±6.5 g per day). All completers were responders and achieved remission with a 64% decrease in Inventory of Depressive Symptomatology score. In all, 2 of the 9 completers refused the second, post-treatment, lumbar puncture procedure.
Cell culture
1C11 cells were grown and induced to differentiate toward the serotonergic pathway, as described previously.16
Determination of BDNF, Wnt2 and 15d-PGJ2 levels
BDNF levels were determined through enzyme-linked immunosorbent assay quantification (Insight Genomics, Nashville, TN, USA), according to the manufacturer's protocol. Wnt2 was identified in cell culture medium and in CSF (collected (from mouse cisterna magna and via lumbar puncture of depressed patients) and frozen at −80 °C until batch assayed) through liquid chromatography-electrospray ionization mass spectrometry on time-of-flight instruments. Briefly, cell supernatant or CSF were filtered through low speed centrifugation on a 0.45 μm BAS polyacetate filter (Bioanalytical Systems, West Lafayette, IN, USA) and separated through high-pressure liquid chromatography. Chromatographic fractions were freeze-dried and analyzed by fast-atom bombardment mass spectrometry, using a VG instruments Model ZAB-E spectrometer (VG Analytical, Manchester, UK). 1H and 13C NMR spectra were obtained with a Varian model 300XL spectrometer (Agilent Technologies, Foster City, CA, USA). Following identification, the levels of Wnt2 were quantified as above. Calibration curves were established with mouse recombinant Wnt2 produced in NIH3T3 cells stably transfected with a pCEV/WNT-2-HFc plasmid as previously described.17 15d-PGJ2 levels were determined by liquid chromatography coupled to mass spectrometry, as in Bell-Parikh et al.18 using pure standards from Cayman Biochemical. Combined injections were carried out using concentrations selected according to the combination index method.19
Statistics
The results are reported as the means±s.e.m. Due to the small sample size avoiding to test the distributions, non-parametric (Wilcoxon and Kruskal–Wallis) tests were used for comparisons. A P-value <0.05 was considered significant.
Results
miR-16 mediates the hippocampal response to fluoxetine
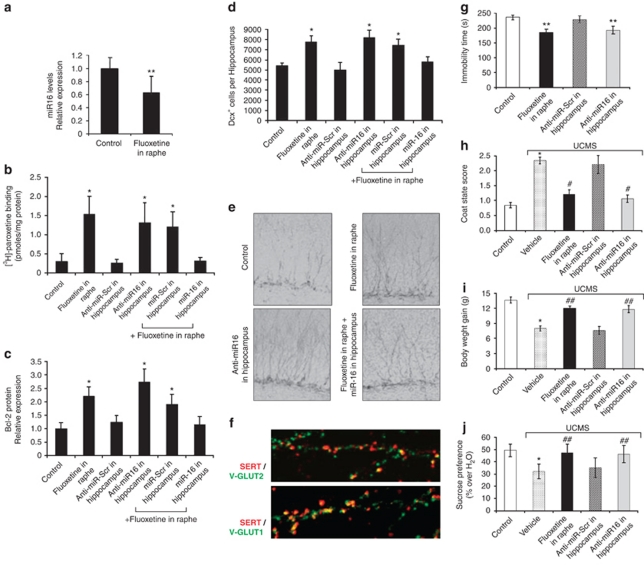
To investigate whether fluoxetine could regulate the level of miR-16 in the hippocampus, we collected hippocampal extracts from mice after a 3-day stereotaxic injection of fluoxetine in raphe. We found that infusion of fluoxetine into raphe resulted in a decrease in the endogenous level of miR-16 in the hippocampus (Figure 1a). This change was accompanied by an increase in the expression of SERT (fivefold) and bcl-2 (2.2-fold) proteins (Figures 1b and c). The fluoxetine-induced SERT molecules were mainly implemented on glutamatergic neurons of the hippocampus20 (V-GLUT-positive cells) as revealed by confocal microscopy (Figure 1f). Injection of fluoxetine into raphe also increased (1.5-fold) the number of hippocampal cells immunoreactive for Dcx, a marker for cells undergoing neuronal maturation21, 22 (Figures 1d and e). Neutralization of endogenous miR-16 by direct injection of anti-miR-16 in the hippocampus increased the levels of SERT and bcl-2 and the number of Dcx-positive cells, similarly to fluoxetine injection in the raphe (Figures 1b–e). Conversely, the hippocampal changes induced by fluoxetine were eliminated under concomitant exposure of the hippocampus to miR-16 (Figures 1b–e).
Figure 1.
Infusion of fluoxetine into raphe decreases miR-16 in the hippocampus, which in turn, increases serotonin transporter (SERT) and bcl-2 levels, promotes neurogenesis and exerts an antidepressant effect. (a–e) Mice received a chronic perfusion of fluoxetine into raphe (1 μ, 2 μl min−1, 3 days) in combination (n=11) or not (n=6) with direct injection of miR-16 (1 μl, 2 μ, every 36 h) into the hippocampus. Alternatively, a direct injection of anti-miR-16 (1 μl, 2 μ, every 36 h, n=6 mice) alone into the hippocampus was performed. Scrambled miRNAs (n=7) or anti-miRNAs (n=10) were used as controls. Control values were obtained in n=13 mice. All measurements were made on hippocampus samples: miR-16 level (real-time PCR) (a), SERT expression ([3H]-paroxetine binding) (b), Bcl-2 protein expression (western blot) (c) and neurogenesis (Doublecortin (Dcx) immunolabeling) (d, e). (f) Immunolabeling of SERT (red) in hippocampal cells positive for V-GLUT 1 (bottom) or V-GLUT-2 (top) (green) after infusion of fluoxetine into raphe for 3 days. (g) Injection of fluoxetine into raphe or anti-miR16 into the hippocampus similarly reduced the time of immobility in the forced swimming test. (h–j) Six-week unpredictable chronic mild stress (UCMS)-induced deterioration of the coat state score (h) and reductions in body weight gain (i) and sucrose preference (j) were alleviated by injection of fluoxetine into raphe or anti-miR16 into the hippocampus (n=9 mice in each group). Values are means±s.e.m. *P<0.01 and **P<0.05 vs control, #P<0.01 and ##P<0.05 vs vehicle UCMS.
Further, in behavioral tests, the injection of anti-miR-16 in the hippocampus reduced the time of immobility in the forced swimming test (Figure 1g). It also alleviated, to the same extent as chronic infusion of fluoxetine into raphe, the deterioration of coat state (Figure 1h) and the reductions in body weight gain (Figure 1i) and sucrose preference (Figure 1j) that were observed following a 6-week regimen of unpredictable chronic mild stress. These results indicate that the fluoxetine-induced downregulation of miR-16 in the hippocampus has an antidepressant effect.
S100β released by the raphe in response to fluoxetine partly relays the action of this SRI on the hippocampus via the LC
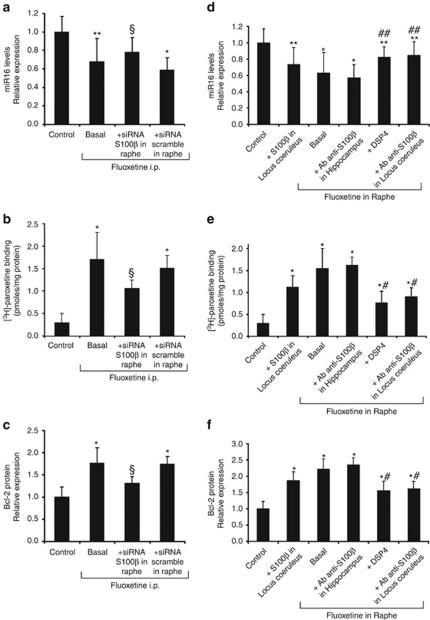
Similar to local infusion of fluoxetine into raphe, systemic fluoxetine treatment (20 days) promoted a decrease in miR-16 in the hippocampus and an increase in SERT and bcl-2 proteins (Figures 2a–c). The fluoxetine-induced downregulation of miR-16 in the hippocampus is reminiscent of that monitored in the LC.7 Because the action of fluoxetine on the LC is mediated by the secretion of the neurotrophic S100β protein by the raphe,7 we investigated whether the fluoxetine-induced secretion of S100β by raphe acts on the hippocampus. The fluoxetine-induced changes in hippocampal miR-16, SERT and bcl-2 were partly reversed (40–50%) upon siRNA-mediated knockdown of S100β in the raphe (Figures 2a–c), whereas insensitive to injection of S100β antibodies in the hippocampus (Figures 2d–f). One explanation that may account for these observations is that the LC relays, in part, the action of fluoxetine onto the hippocampus. We tested this hypothesis by directly infusing S100β at the LC, to mimic the effect of fluoxetine treatment on this brain region, without acting at the raphe. The stereotaxic injection of S100β at the LC triggered in the hippocampus a decrease in miR-16 and an increase in SERT and bcl-2. These changes were, however, weaker than those measured upon injection of fluoxetine at the raphe (Figures 2d–f). Further, upon selective degeneration of noradrenergic fibers using the DSP4 neurotoxin,12 there was only a partial (about 50%) effect of fluoxetine on hippocampal miR-16, SERT and bcl-2 levels (Figures 2d–f). Concomitant infusion of fluoxetine at the raphe and anti-S100β antibodies at the LC also reduced by 50% the hippocampal response (Figures 2d–f). We conclude that the action of fluoxetine on the hippocampus is relayed, in part, by S100β via the noradrenergic neurons of the LC. Other signal(s) emanating from the raphe must thus account for the LC-independent effect of fluoxetine on the hippocampus.
Figure 2.
S100β released by raphe upon fluoxetine treatment does not act directly on the hippocampus but partially relays the fluoxetine response via the locus coeruleus. (a–c) Mice were chronically exposed to fluoxetine for 20 days (daily intraperitoneal injection, 5 mg kg−1, ‘basal' n=7). During the treatment, two groups of mice also received stereotaxic injection into the raphe of S100β-siRNA (2 μg, 1 μg μl−1, n=8) or scrambled oligonucleotides every 36 h (n=9). Control values were obtained in n=13 mice. The downregulation of miR-16 (real-time PCR) (a) and the upregulation of SERT ([3H]-paroxetine binding) (b) and bcl-2 protein levels (western blot analysis) (c) in the hippocampus after a 20-day intraperitoneal injection of fluoxetine in mice were partially abolished by siRNA-mediated knockdown of S100β in raphe. (d–f) Mice received a stereotaxic injection of S100β (1 n, 2 μl min−1, 1 day, n=7) in the locus coeruleus (LC). Alternatively, fluoxetine was perfused into the raphe either alone (‘basal' n=8) or combined with injection of S100β antibodies (1 μg ml−1) into the hippocampus (n=8) or the LC (n=7), or with degeneration of noradrenergic fibers with the DSP4 neurotoxin (n=7). Control values were obtained in n=12 mice. Hippocampal extracts from these different groups of mice were collected to quantify the miR-16 level (d), SERT (e) and bcl-2 (f) protein expression. The values are the means±s.e.m, *P<0.01 and **P<0.05 vs control, §P<0.05 vs scramble, #P<0.01 and ##P<0.05 vs basal fluoxetine in raphe.
BDNF, Wnt2 and 15d-PGJ2 synergize to relay the action of fluoxetine from the raphe onto the hippocampus
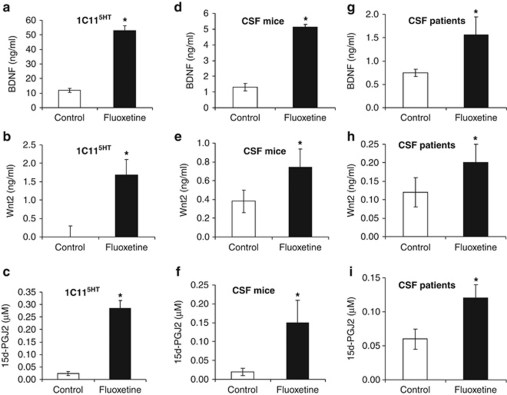
To address the question of the raphe-released signal molecule(s) that account for the S100β-independent effect of fluoxetine on the hippocampus, we exploited the 1C11 cell line, which was instrumental in uncovering the functional interplay between S100β, miR-16 and the SERT.7. A mass spectrometry-wide analysis of cell culture media indicated that 1C115−HT serotonergic cells exposed to fluoxetine (50 n) for 2 days released BDNF, the frizzled ligand Wnt2 and the anti-inflammatory 15d-PGJ2 (Figures 3a–c). These three molecules were also found to be increased in the CSF of mice receiving a systemic fluoxetine treatment (20 days) (Figures 3d–f) and that of naive depressed patients after a 12-week fluoxetine treatment (Figures 3g–i). Both BDNF and Wnt2 are known to be secreted in response to neuronal activity23, 24 and to exert an antidepressant-like effect in the hippocampus.25, 26, 27 15d-PGJ2 is an endogenous ligand of the nuclear receptor PPARγ, the agonists of which exert beneficial effects in neuropsychiatric diseases.28
Figure 3.
Brain-derived neurotrophic factor (BDNF), Wnt2 and 15-Deoxy-delta12,14-prostaglandin J2 (15d-PGJ2) are secreted by serotonergic neurons in response to fluoxetine and are increased in cerebrospinal fluid (CSF) of mice or depressed patients following fluoxetine treatment. (a–c) Treatment of 1C115−HT cells with fluoxetine (50 n, 2 days) induced the release of BDNF (a), Wnt2 (b) and 15d-PGJ2 (c) (n=6). (d–i), Treatment of mice (d–f) or depressed patients (g–i) with fluoxetine induced an increase of BDNF (d, g), Wnt2 (e, h) and 15d-PGJ2 (f, i) in CSF. The values are the means±s.e.m (n=5 for mice, n=7 for patients), *P<0.01 vs control.
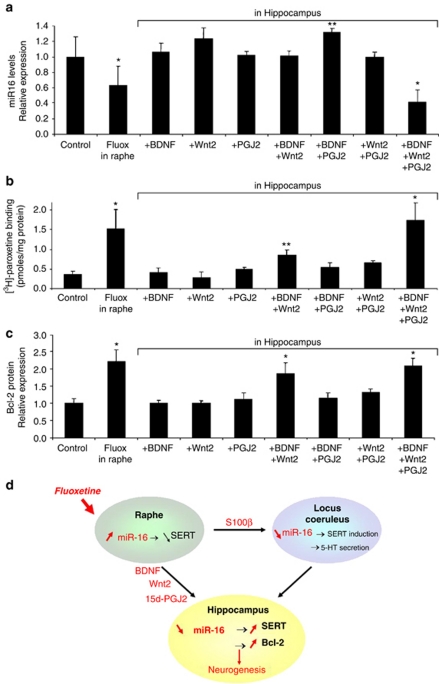
We next evaluated the impact of an infusion of these signaling molecules into the hippocampus, at concentrations encompassing those found with 1C115−HT cells (see Materials and methods). Administration of one or two out of these three molecules had null or weak effects. In contrast, if all three molecules were injected simultaneously, hippocampal miR-16 markedly decreased, with ensuing pronounced induction of SERT and bcl-2 (Figures 4a–c). Under the optimal conditions, which were obtained with a combination of 50 ng ml−1 BDNF, 1.5 ng ml−1 Wnt2 and 0.25 μ 15d-PGJ2, the changes recapitulated those measured upon infusion of fluoxetine into the raphe.
Figure 4.
Brain-derived neurotrophic factor (BDNF), Wnt2 and 15-Deoxy-delta12,14-prostaglandin J2 (15d-PGJ2) act synergistically on the hippocampus by decreasing miR-16 and increasing serotonin transporter (SERT) and bcl-2 levels. (a–c) Mice received a chronic perfusion of fluoxetine (fluox) into raphe (n=6) or a direct stereotaxic perfusion of BDNF, Wnt2 or 15d-PGJ2 into the hippocampus (n=7). Combined injections were carried out using concentrations selected according to the combination index method of Chou-Talalay.19 An optimal response was reached with a combination of 50 ng ml−1 BDNF, 1.5 ng ml−1 Wnt2 and 0.25 μ 15d-PGJ2. (a) The miR-16 level (real-time PCR), (b) SERT expression ([3H]-paroxetine binding) and (c) bcl-2 protein expression (western blot analysis) were measured in hippocampus extracts. Control values were obtained in n=9 mice. The values are the means±s.e.m. *P<0.01 and **P<0.05 vs control. (d) Prozac treatment induces the secretion of S100β, BDNF, Wnt2 and 15d-PGJ2 from raphe serotonergic neurons. These signals relay the action of fluoxetine by downregulating miR-16, which acts as a micromanager in the hippocampal response to SRI antidepressants.
Discussion
In this study, we identify miR-16 as the missing link between SRI treatment and hippocampal neurogenesis. Indeed, the reduction of miR-16 in the hippocampus is sufficient to trigger increases in bcl-2 and SERT levels as well as in the number of Dcx-positive cells and to exert beneficial effects in a mouse model of depression. On another hand, neutralizing the decrease in miR-16 directly in the hippocampus counteracts all the above changes induced by fluoxetine. These experiments establish that miR-16 behaves as a micromanager that sustains the hippocampal response to SRI antidepressants. On this basis, miR-16 can now be viewed as a readout for the action of SRIs in the hippocampus, which may help design new strategies or refine existing therapeutic protocols.
This work also shows that the release of S100β by the raphe induces significant changes at the hippocampus by mobilizing the LC. Indeed, degeneration of the LC using the DSP4 neurotoxin, knockdown of S100β at the raphe, or antibody-mediated neutralization of S100β at the LC, all reduce by 50% the hippocampal changes induced by fluoxetine. Such observations may provide some molecular clue as to the altered responses to antidepressant drugs reported in norepinephrine-deficient mice.29
Finally, we establish here that the cooperation between three molecules, BDNF, Wnt2 and 15d-PGJ2, secreted from the raphe and acting on the hippocampus, is necessary to relay the action of fluoxetine. The identification of this combination of signals was rendered possible through mass spectrometry-wide analysis of the culture medium of 1C115−HT serotonergic neurons exposed to fluoxetine. These three factors are also increased in the CSF of mice treated with fluoxetine. An important observation is that any factor on its own is unable to mimic the action of fluoxetine on the hippocampus. By showing that there is a synergy between the three signaling molecules to sustain the hippocampal response to fluoxetine, our work sheds some light on the intricate interplay of signals that relay the action of antidepressants. From a translational research point of view, the therapeutic relevance of the release by the raphe of BDNF, Wnt2 and 15d-PGJ2 is substantiated by our observation that these three factors systematically increase in the CSF of naive depressed patients after a 12-week fluoxetine treatment.
To conclude, our study shows that fluoxetine treatment induces the secretion of various signaling molecules from raphe serotonergic neurons (Figure 4d). BDNF, Wnt2 and 15d-PGJ2 act cooperatively on the hippocampus, whereas S100β controls the LC-dependent hippocampal response to fluoxetine. These signals relay the action of fluoxetine by downregulating miR-16 in the hippocampus. By providing an integrated view of the pathways originating from the raphe that are involved in the hippocampal response to fluoxetine, our study may pave the way towards a better understanding of the physiopathology of depression.
Acknowledgments
We thank P Weil-Malherbe, V Mutel, F d'Agostini, G Zürcher, E Borroni, Z Lam, M Bühler, N Pieron and R Hochköppler for skillful methodological assistance, M Leboyer for providing human samples, and S Blanquet, L Aggerbeck and M Briley for critical reading of the manuscript. OK is a professor at the Université Paris Sud. This work was funded by the ANR and INSERM.
The authors declare no conflict of interest.
References
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death. Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Drzyzga LR, Marcinowska A, Obuchowicz E. Antiapoptotic and neurotrophic effects of antidepressants: a review of clinical and experimental studies. Brain Res Bull. 2009;79:248–257. doi: 10.1016/j.brainresbull.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Macqueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research. Mol Psychiatry. 2010;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- Perera TD, Dwork AJ, Keegan KA, Thirumangalakudi L, Lipira CM, Joyce N, et al. Necessity of hippocampal neurogenesis for the therapeutic action of antidepressants in adult nonhuman primates. PLoS One. 2011;6:e17600. doi: 10.1371/journal.pone.0017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Launay JM, Kellermann O. New views on antidepressant action. Curr Opin Neurobiol. 2011;21:1–8. doi: 10.1016/j.conb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. MiR-16 Targets the Serotonin Transporter: A New Facet for Adaptive Responses to Antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Biebl M, Wilhelm D, Li M, Friedlander RM, Winkler J. Increased generation of granule cells in adult Bcl-2-overexpressing mice: a role for cell death during continued hippocampal neurogenesis. Eur J Neurosci. 2005;22:1907–1915. doi: 10.1111/j.1460-9568.2005.04377.x. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagg T. Molecular regulation of adult CNS neurogenesis: an integrated view. Trends Neurosci. 2005;28:589–595. doi: 10.1016/j.tins.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Selective effects of DSP-4 on locus coeruleus axons: are there pharmacologically different types of noradrenergic axons in the central nervous system. Prog Brain Res. 1991;88:257–268. doi: 10.1016/s0079-6123(08)63815-7. [DOI] [PubMed] [Google Scholar]
- Launay JM, Schneider B, Loric S, Da Prada M, Kellermann O. Serotonin transport and serotonin transporter-mediated antidepressant recognition are controlled by 5-HT2B receptor signaling in serotonergic neuronal cells. FASEB J. 2006;20:1843–1854. doi: 10.1096/fj.06-5724com. [DOI] [PubMed] [Google Scholar]
- Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Neurogenic effects of fluoxetine are attenuated in p11 (S100A10) knockout mice. Biol Psychiatry. 2010;67:1048–1056. doi: 10.1016/j.biopsych.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34:1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillet-Richard S, Mutel V, Loric S, Tournois C, Launay JM, Kellermann O. Regulation by neurotransmitter receptors of serotonergic or catecholaminergic neuronal cell differentiation. J Biol Chem. 2000;275:9186–9192. doi: 10.1074/jbc.275.13.9186. [DOI] [PubMed] [Google Scholar]
- Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest. 2003;112:945–955. doi: 10.1172/JCI18012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma. 2008;49:2059–2080. doi: 10.1080/10428190802353591. [DOI] [PubMed] [Google Scholar]
- Gutierrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, et al. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, et al. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry. 2010;68:521–527. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bueno B, Perez-Nievas BG, Leza JC. Is there a role for the nuclear receptor PPARgamma in neuropsychiatric diseases. Int J Neuropsychopharmacol. 2010;13:1411–1429. doi: 10.1017/S1461145710000970. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci USA. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]