Abstract
Genotype–phenotype correlations of common monogenic diseases revealed that the degree of deviation of mutant genes from wild-type structure and function often predicts disease onset and severity. In complex disorders such as schizophrenia, the overall genetic risk is still often >50% but genotype–phenotype relationships are unclear. Recent genome-wide association studies (GWAS) replicated a risk for several single-nucleotide polymorphisms (SNPs) regarding the endpoint diagnosis of schizophrenia. The biological relevance of these SNPs, however, for phenotypes or severity of schizophrenia has remained obscure. We hypothesized that the GWAS ‘top-10' should as single markers, but even more so upon their accumulation, display associations with lead features of schizophrenia, namely positive and negative symptoms, cognitive deficits and neurological signs (including catatonia), and/or with age of onset of the disease prodrome as developmental readout and predictor of disease severity. For testing this hypothesis, we took an approach complementary to GWAS, and performed a phenotype-based genetic association study (PGAS). We utilized the to our knowledge worldwide largest phenotypical database of schizophrenic patients (n>1000), the GRAS (Göttingen Research Association for Schizophrenia) Data Collection. We found that the ‘top-10' GWAS-identified risk SNPs neither as single markers nor when explored in the sense of a cumulative genetic risk, have any predictive value for disease onset or severity in the schizophrenic patients, as demonstrated across all core symptoms. We conclude that GWAS does not extract disease genes of general significance in schizophrenia, but may yield, on a hypothesis-free basis, candidate genes relevant for defining disease subgroups.
Keywords: Cambridge neurological inventory, cognition, GWAS, PGAS, positive and negative syndrome scale, prodrome
Introduction
In complex disorders lacking clearly identifiable disease-causing factors, such as schizophrenia, the overall genetic risk is often >50%1 but genotype–phenotype relationships are obscure. Recent genome-wide association studies (GWAS) on schizophrenia, building on very large cohorts of cases and controls, have uncovered and replicated a risk for several single-nucleotide polymorphisms (SNPs). Among the 10 ‘top hits', reaching genome-wide significance in different populations after multiple-testing correction (even though with low odds ratios), are markers in the major histocompatibility complex region and in TCF4, ZNF804A and NRGN genes.2, 3, 4, 5, 6 The biological relevance of these SNPs, however, for phenotypes or severity of schizophrenia has remained unclear.
All current GWAS data on schizophrenia rely on endpoint diagnosis only and do not allow for genotype–phenotype correlations. In an approach complementary to GWAS, we employed the ‘top-10' schizophrenia-associated SNPs that have emerged as genome-wide significant from several GWAS, and explored their potential contribution to the disease phenotype, including positive and negative symptoms, cognitive deficits and neurological signs (including catatonia), and age of onset of the prodrome as developmental readout and predictor of disease severity. For this phenotype-based genetic association study (PGAS), we utilized the GRAS (Göttingen Research Association for Schizophrenia) Data Collection.7, 8
Materials and methods
Schizophrenic patients
The GRAS study was approved by the Ethics Committees of the Georg-August-University of Göttingen and of participating centers, and comprises at present 1041 patients with confirmed Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)9 diagnosis of schizophrenia (82.2%) or schizoaffective disorder (17.8%), examined between 2005 and 2010 in 23 collaborating centers all over Germany (Supplementary Table 1).7, 8
Healthy subjects
Healthy subjects for the case–control study were blood donors (n=1144), recruited according to national guidelines for blood donation.7 Comparable to the patient population (Caucasian 95.5% other ethnicities 1.8% unknown 2.7%), almost all controls were of European–Caucasian descent (Caucasian 97.8% other ethnicities 2% unknown 0.2%).
Phenotyping
Comprehensive interviews, testing and clinical ratings were conducted by an invariable team of trained examiners (psychiatrists and psychologists) using the ‘GRAS Manual'.7, 8 Diagnoses of schizophrenia/schizoaffective disorders were based on the structured clinical interview for DSM-IV (SCID),10 substantiated by information from medical records/discharge letters of all the patients. Psychopathological state, symptom severity and functional outcome were evaluated by clinical ratings (positive and negative syndrome scale (PANSS)11 and global assessment of functioning (GAF).9) Neuropsychological testing (subtest 3 of Leistungsprüfsystem,12 Trail-Making Test,13, 14 Verbal Learning and Memory test15) including pre-morbid intelligence (Mehrfachwahl-Wortschatz-lntelligenz test-B16) and neurological examination (Cambridge Neurological Inventory17) yielding respective composite scores, are described in detail elsewhere.7, 8
Genotyping
SNP genotyping was performed with SimpleProbes (TIB Molbiol, Berlin, Germany) on LightCycler480 (Roche, Mannheim, Germany). All markers fulfilled the Hardy–Weinberg equilibrium.
Statistical methods
PGAS: Phenotype data were standardized to be normally distributed with expectation zero and variance one, and presented such that higher values always indicate better performance. Expected values of univariate and multivariate phenotypes were analyzed with linear models (Rv2.12.0), covariate adjusted and tested for additive effects of GWAS risk alleles with single-locus models (Table 1) and with a 10-loci model (Table 2). The latter simultaneously estimates for all the 10 loci regression coefficients with number of respective GWAS risk alleles. To assess statistical significance, the commonly used Bonferroni correction was employed; however, raw P-values are given.
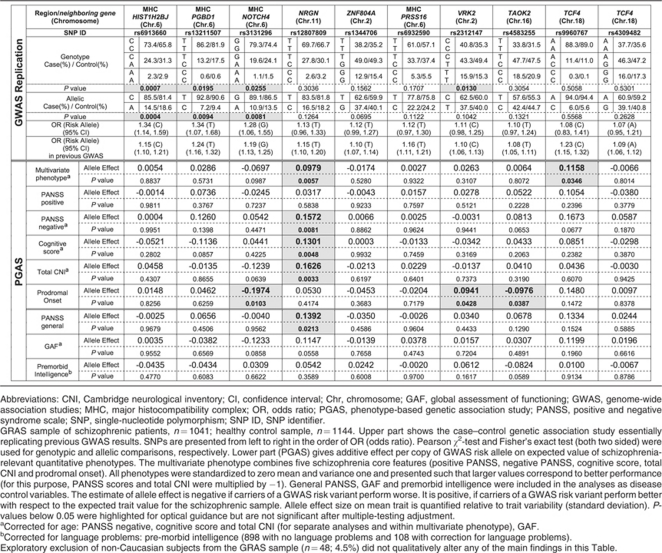
Table 1. GWAS replication and PGAS single-locus quantitative association analysis.
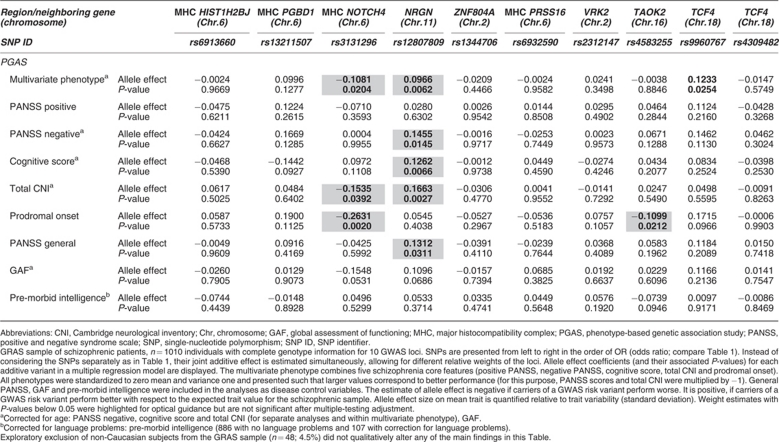
Table 2. PGAS multilocus quantitative association analysis.
Results
First, we proved in a case–control study (cases n=1041; healthy controls n=1144) that the GRAS population (for patient characteristics see Supplementary Table 1) provides a ‘genetic data matrix' that essentially replicates the GWAS results (Table 1, upper part). In fact, screening of the 10 genome-wide hits resulted in a significant association of markers rs6913660, rs13211507 and rs3131296 in the major histocompatibility complex region (chromosome 6), and marker rs2312147 in chromosome 2 (VRK2 gene) with an increased risk for the disease. Due to the smaller sample size compared with the GWAS studies, leading to lower power (power 70–90% for the SNPs found to reach allelic P-values ⩽0.05, average power of 43% over all markers), not all SNPs turned out to be significantly associated with the schizophrenia risk. Nevertheless, all of them exhibit the same direction of association as reported in GWAS.2, 3, 4, 5, 6 In fact, the GRAS sample has been included in a recent large GWAS follow-up study.6
We next conducted PGAS single-locus and PGAS multilocus quantitative association analyses. In both the procedures we searched for the hypothesized association of markers with the lead symptoms of schizophrenia including developmental readouts/disease severity, both in the form of a composite construct (multivariate phenotype) and of its components separately (individual phenotypes), that is, positive and negative symptoms (PANSS), cognitive deficits (composite of executive function, reasoning, verbal learning and memory) and neurological signs (including catatonia), and/or with the age of onset of the disease prodrome. We further hypothesized that, if the markers were disease specific, they should not be associated with the schizophrenia-unrelated, general disease control variables. Hence, such variables referring to symptoms that are not in any way specific for schizophrenia were also included PANSS general psychopathology (depression, anxiety and others), global assessment of functioning and pre-morbid intelligence (basic cognitive capabilities of an individual before disease onset) (Tables 1 and 2; Supplementary Table 2).
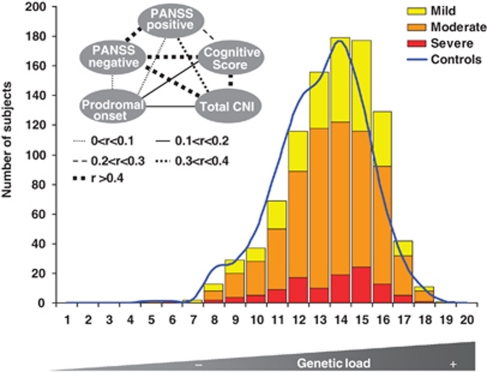
With >1000 patients, the single-locus analysis found none of the schizophrenia phenotypes, neither individual nor multivariate, significantly influenced by any of the markers after multiple-testing correction by Bonferroni (Table 1). We thus wondered whether—instead of single genotypes—the ‘genetic load' of a patient, that is, the accumulation of risk genotypes, would give us a clearer signal regarding the contribution of genetic risk to disease phenotype and severity. As illustrated in Figure 1, the composite severity score, built on the basis of the five core symptom variables (see inset), shows an essentially equal distribution of schizophrenia severity from the lowest to the highest genetic load group. As more symptoms do not necessarily reflect more severe disease and certain symptom groups may better associate with genetic load, all five variables were considered separately, too. But again, for the 10 top hits of GWAS, none of the schizophrenia symptom groups was dependent regarding its severity on the increase in the ‘genetic load' (Supplementary Table 2, and Supplementary Figure 1). This becomes evident when the composite score data are presented alternatively as percentage severity over all the genetic load groups; there is no tendency of an increase in severe cases with an increase in the number of risk markers (Supplementary Figure 2). Interestingly, grouping the distribution of accumulated risk genotypes in the Icelandic GWAS sample (n=582 schizophrenic individuals) yields a pattern of overall risk distribution that is comparable to that of the GRAS database (Supplementary Figure 3), further supporting the validity of our sample for the PGAS approach to the GWAS hits.
Figure 1.
Distribution of phenotype severity and cumulative genetic load with respect to the number of GWAS-identified ‘top-10' risk SNP alleles in the GRAS population (bar graph). Phenotype severity is based on a composite score of the five core features of schizophrenia, displayed as inset (including intercorrelations between items). Score range in the GRAS sample is divided into three equal parts and ranked as mild, medium and severe disease phenotype. The blue line denotes the comparison of the risk SNP allele distribution in the healthy control sample.
We now pursued the question whether better insight into the phenotypical contribution of the GWAS hits could be obtained by performing a multilocus additive joint model analysis. This analysis weighs each marker according to its estimated relative importance as a risk genotype. Also this approach failed to uncover statistically significant associations after multiple-testing correction. Close to significance are two associations, one between the marker rs3131296 in NOTCH4 (chromosome 6) and prodromal onset (developmental readout), the other between rs12807809 in chromosome 11 (near NRGN gene) and severity of the neurological signs (Table 2). In fact, when ignoring the multiple-testing issue, the latter marker which has a relatively low odds ratio in our case–control study (OR 1.13 here and 1.15 in the GWAS study of Stefansson et al.5), shows several association ‘signals' that make it attractive for follow-up in future subgroup analyses. However, this marker did not associate with the cognition of schizophrenic subjects in a recent study.18
Discussion
How can the overall negative result regarding the phenotypical significance of basically all GWAS ‘top-10' genotypes be explained? First, in clear contrast to monogenic diseases,19 the genetic risk for schizophrenia may not simply be reflected by phenotypical disease severity or by core symptoms of the disease. Here, an array of environmental risk factors that cannot easily be controlled for might also have a modulating role.20 Second, the genotype –to phenotype translation may only be visible and valid for a relatively small subgroup of individuals, but still leads to significant (even though low) genetic risk odds ratios in very large GWAS samples. Third, one risk genotype may partly ‘neutralize' another one, resulting in risk reduction upon combination rather than accumulation of the genetic load. This latter, seemingly paradox interaction is supported by the observation that the few effects on phenotypes found here in marker rs12807809 (chromosome 11; near NRGN) unexpectedly go into the opposite direction (risk genotype shows less severity). Fourth and finally, we cannot rule out that for some analyses the GRAS sample may not have enough power to detect the (certainly weak if any) phenotypical consequences of the ‘top 10' GWAS hits. In this context, however, the general question arises of how much clinical significance a genetic association may have if several thousand patients are needed to reveal a tiny effect on disease severity or phenotype. Thus, building on >1000 patients, one would at least have expected some more signals to pop up (that is, more nominal P-values around 0.05 in the PGAS part of Table 1). In fact, the estimated power assessed according to Lettre et al.,21 who performed simulations in a similar context (sample size, normally distributed phenotype), amounts to overall around 80% at the α=0.05 level for the PGAS approach. The presented additive model, chosen to match our hypothesis of cumulated risk, performed similarly to a co-dominant one but appeared to be slightly more powerful.
After all, we note that entirely different genotypes that were never found to be significantly associated with any schizophrenia risk in GWAS, still may profoundly modulate the schizophrenic phenotype, for example, of genes encoding neuregulin-1, complexin 2 or COMT.7, 22, 23 On the other hand, GWAS finds may be of general rather than disease-specific significance. Several studies have for instance suggested that schizophrenia and affective disorders are on a continuum of liability. Genetic linkage and association studies have proposed common disease loci for both the disorders.2, 24 Likewise, family studies show that first-degree relatives of bipolar patients have a higher risk for schizophrenia compared with first-degree relatives of healthy controls.25, 26 Also other psychiatric diseases like alcoholism or major depression have been found to be associated with certain schizophrenia risk genes, for example, DISC1.27 Thus, exploration of many other phenotypes available in the GRAS database, including candidate intermediate phenotypes28, 29 or those reflecting a more dimensional approach to the disease,30 might potentially be interesting. Purpose of the present study, however, was to cover the lead symptoms of schizophrenia in the first place.
To conclude, GWAS approaches in diseases as complex as schizophrenia do not lead to the reconstruction of a ‘common disease mechanism' or to the discovery of ‘classical disease genes', as such genes obviously do not exist. What makes our study important for the clinician is that we can show, for the first time, that the combination of a whole battery of genetic pre-disposing factors (the ‘top 10' GWAS finds in schizophrenia) in individual patients will not make their schizophrenic phenotype any different or worse than that of those patients who do not carry these genetic factors. Importantly, however, GWAS results may guide, on a hypothesis-free basis, to the identification of totally unexpected candidate genes involved in certain disease aspects in subgroups of patients, as they can be defined by PGAS. In order to get closer to understanding the disorders as complex and heterogeneous as schizophrenia, GWAS and PGAS will have to go hand in hand.
Acknowledgments
This study was supported by the Max Planck Society. We are indebted to all patients for their participation in the GRAS study and to all colleagues in the collaborating centers who contributed to the GRAS data collection. Author Contributions: H.E. and S.P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The study concept and design was given by S.P. and H.E. Data were acquired by M.B., A.K., S.P., S.Sp., H.S. and H.E. Analysis and interpretation of data was done by S.P., D.M., A.K., H.B., H.S. K-A.N. and H.E. Manuscript was drafted by H.E., S.P. and K-A.N. Critical revision of the manuscript for important intellectual content was done by S.P., D.M., A.K., S.Sp., M.B., H.S., H.B., K-A.N. and H.E. Statistical analyses were carried out by D.M. and H.B. Administrative, technical and material support was given by S.Sp. and M.B. The study was supervised by H.E.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet. 2008;40:1053–1055. doi: 10.1038/ng.201. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, de Jong S, Andreassen OA, Werge T, Borglum AD, Mors O, et al. Common variants at VRK2 and TCF4 conferring risk of schizophrenia Hum Mol Genet 2011. advance online publication 26 July 2011; doi: 10.1093/hmg/ddr325 [DOI] [PMC free article] [PubMed]
- Begemann M, Grube S, Papiol S, Malzahn D, Krampe H, Ribbe K, et al. Modification of cognitive performance in schizophrenia by complexin 2 gene polymorphisms. Arch Gen Psychiatry. 2010;67:879–888. doi: 10.1001/archgenpsychiatry.2010.107. [DOI] [PubMed] [Google Scholar]
- Ribbe K, Friedrichs H, Begemann M, Grube S, Papiol S, Kastner A, et al. The cross-sectional GRAS sample: a comprehensive phenotypical data collection of schizophrenic patients. BMC Psychiatry. 2010;10:91. doi: 10.1186/1471-244X-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association: Washington; 2000. [Google Scholar]
- Wittchen H-U, Zaudig M, Fydrich T. SKID-I (Strukturiertes Klinisches Interview für DSM-IV; Achse I: Psychische Störungen) Hogrefe: Göttingen; 1997. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Horn W.Leistungsprüfsystem (LPS)2 edn.Hogrefe: Goettingen; 1983 [Google Scholar]
- Reitan RM. The validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- War-Department . Army Individual Test Battery. Manual of directions and scoring. War Department, Adjutant General's Office: Washington, DC; 1944. [Google Scholar]
- Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest (VLMT) Beltz: Goettingen; 2001. [Google Scholar]
- Lehrl S. Mehrfachwahl-Wortschatz-Intelligenz test MWT-B. Spitta Verlag: Balingen; 1999. [Google Scholar]
- Chen EY, Shapleske J, Luque R, McKenna PJ, Hodges JR, Calloway SP, et al. The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiatry Res. 1995;56:183–204. doi: 10.1016/0165-1781(95)02535-2. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Walters J, Morris DW, Da Costa A, Rose E, Hargreaves A, et al. A neuropsychological investigation of the genome wide associated schizophrenia risk variant NRGN rs12807809. Schizophr Res. 2010;125:304–306. doi: 10.1016/j.schres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat Genet. 2003;33 (Suppl:228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Lettre G, Lange C, Hirschhorn JN. Genetic model testing and statistical power in population-based association studies of quantitative traits. Genet Epidemiol. 2007;31:358–362. doi: 10.1002/gepi.20217. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, et al. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the consortium on the genetics of schizophrenia. Am J Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48:531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- Sham PC, Jones P, Russell A, Gilvarry K, Bebbington P, Lewis S, et al. Age at onset, sex, and familial psychiatric morbidity in schizophrenia. Camberwell collaborative psychosis study. Br J Psychiatry. 1994;165:466–473. doi: 10.1192/bjp.165.4.466. [DOI] [PubMed] [Google Scholar]
- Valles V, Van Os J, Guillamat R, Gutierrez B, Campillo M, Gento P, et al. Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophr Res. 2000;42:83–90. doi: 10.1016/s0920-9964(99)00117-6. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders--cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner M. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes and Genes. Springer: Dordrecht/ The Netherlands; 2009. [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.