Abstract
Caveolin-1 (Cav-1) is a scaffolding protein important for regulating receptor signaling cascades by partitioning signaling molecules into membrane microdomains. Disruption of the CAV1 gene has recently been identified as a rare structural variant associated with schizophrenia. Although Cav-1 knockout (KO) mice displayed no baseline behavioral disruptions, Cav-1 KO mice, similar to schizophrenic individuals, exhibited increased sensitivity to the psychotomimetic N-methyl--aspartate receptor antagonist phencyclidine (PCP). Thus, PCP disruption of prepulse inhibition (PPI) and PCP-induced mouse locomotor activity were both enhanced by genetic deletion of Cav-1. Interestingly, genetic deletion of Cav-1 rendered the atypical antipsychotics clozapine and olanzapine and the 5-HT2A-selective antagonist M100907 ineffective at normalizing PCP-induced disruption of PPI. We also discovered that genetic deletion of Cav-1 attenuated 5-HT2A-induced c-Fos and egr-1 expression in mouse frontal cortex and also reduced 5-HT2A-mediated Ca2+ mobilization in primary cortical neuronal cultures. The behavioral effects of the 5-HT2A agonist (2,5-dimethoxy-4-iodoamphetamine) including head twitch responses and disruption of PPI were also attenuated by genetic deletion of Cav-1, indicating that Cav-1 is required for both inverse agonist (that is, atypical antipsychotic drug) and agonist actions at 5-HT2A receptors. This study demonstrates that disruption of the CAV1 gene—a rare structural variant associated with schizophrenia—is not only pro-psychotic but also attenuates atypical antipsychotic drug actions.
Keywords: antipsychotic, caveolin, clozapine, 5-HT2A, schizophrenia
Introduction
Schizophrenia—affecting 1% of the world's population—is a genetically heterogeneous disease with devastating consequences to affected individuals.1, 2, 3, 4 It now appears that schizophrenia is due to both common and rare gene mutations as well as copy-number variations. The identification and study of exceedingly rare mutations such as DISC1 has had a major impact on our understanding of the potential genetic underpinnings of psychiatric disease.5, 6 Recent analyses of genomic structural variations in individuals with schizophrenia indicated that the gene caveolin-1 (CAV1) is disrupted by an insertional mutation, thereby identifying CAV1 as a rare structural variant associated with schizophrenia.7
The CAV1, -2 and -3 genes encode caveolins, which represent a family of multi-functional scaffolding proteins and cholesterol-binding proteins that mediate cholesterol trafficking to lipid microdomains at the plasma membrane.8 Caveolins also function as protein scaffolds to organize proteins and lipids together in membrane domains and thereby control various cellular functions including vesicular trafficking, lipid homeostasis and signal transduction.9, 10 Cav-1 and Cav-2 are widely expressed in the central and peripheral nervous systems mainly in cortical and hippocampal neurons as well as brain microvessels, astrocytes, oligodendrocytes, Schwann cells and dorsal root ganglia cells10, 11, 12 while Cav-3 is muscle-specific. Transcriptional profiling studies of mRNAs demonstrate that both Cav-1 and Cav-2 are expressed in relevant central nervous system cells including layer III and V pyramidal cortical neurons.13
The relevant functional role(s) of Cav-1 vis-a-vis the pathogenesis of schizophrenia is undefined. As many G protein-coupled neurotransmitter receptors, heterotrimeric G proteins and signaling effectors are known Cav-1 interacting partners,10, 11, 14 disruption of CAV1 will likely impair neuronal signaling thereby leading to symptoms of schizophrenia in susceptible individuals. Targeted disruption of the CAV1 gene in mice provides a knockout (KO) animal model, which recapitulates the life-long disruption of CAV1 seen as a rare structural variant associated with schizophrenia. Here, we report that genetic deletion of Cav-1 in mice induces a propsychotic-like condition whereby mice are supersensitive to the psychotomimetic actions of the N-methyl--aspartate (NMDA)-receptor antagonist phencyclidine (PCP). Furthermore, Cav-1 KO mice are also resistant to the actions of atypical antipsychotic drugs and selected serotonergic agents implying an underlying disruption of serotonergic signaling also is present. These findings suggest the disruption in CAV1 arising in human schizophrenia could lead to treatment non-responsiveness to atypical antipsychotics because of intrinsic alterations in serotonergic signaling.
Materials and methods
See Supplementary Information for additional materials and methods.
In vivo studies in mice
All experiments were approved by the Institutional Animal Care and Use Committees at the University of North Carolina, Chapel Hill, NC, USA. Cav-1 KO mice were obtained from the Jackson Laboratories (Bar Harbor, ME, USA) and were housed under standard conditions—12-h light–dark cycle with food and water provided ad libitum. Adult, age-matched (10–15 week) male and female littermate wild-type and Cav-1 KO drug-naive mice were used for all behavioral testing.
Prepulse inhibition
Prepulse inhibition (PPI) testing and analysis was performed as described previously.15, 16 Adult male and female littermate wild-type and Cav-1 KO mice were pre-administered (10 ml kg−1, intraperitoneally (i.p.)) vehicle (0.9% saline; 50 m tartaric acid), clozapine (0.5, 1.0 mg kg−1), olanzapine (0.5 mg kg−1) or M100907 (0.5, 1.0 mg kg−1) and returned to their home cage. After 30 min, animals were administered phencyclindine (2.0, 4.0 or 6 mg kg−1, i.p.) and immediately placed into the PPI apparatus (SR-LAB Startle Response System, San Diego Instruments, San Diego, CA, USA). The auditory pulse protocol, test trials used and PPI calculation have been reported previously.15, 16 The data were analyzed with SR-LAB programs and are presented as means±s.e.m. The calculated PPI values from all groups were analyzed using a one-way analysis of variance followed by Newman–Keuls multiple comparison test with Graphpad Prism 5.0 (Graphpad Software Inc., La Jolla, CA, USA). In all cases, P<0.05 was considered statistically significant.
Open field activity
Locomotor activity was assessed under standardized environmental conditions in 25.8 × 25.8 cm Plexiglas chambers with photo-beams spaced at 1.52 cm (AccuScan Instruments, Columbus, OH, USA) as previously described.15, 16 For dose responses to PCP, mice were placed into the open field and 30 min later, PCP (2.0, 4.0 6.0 mg kg−1) was administered and animals were immediately returned to the open field for 90 min. For antipsychotic studies, mice were injected (i.p.) with vehicle (0.9% saline, 50 m tartaric acid), clozapine (1.0 mg kg−1), M100907 (0.1, 1.0 mg kg−1) or haloperidol (0.1 mg kg−1) and placed into the open field. After 30 min, PCP (6.0 mg kg−1) was administered and mice were immediately returned to the open field for 90 min. Activity was monitored throughout this entire period. Horizontal activity was measured as the total distance traveled in centimeters. Stereotypy counts reported correspond to the number of times that stereotypic behavior was observed in the animal. A break in stereotypy of 1 s or more was required to separate one stereotypic episode from the next. If the animal breaks the same beam (or set of beams) repeatedly, the monitor considers that the animal is exhibiting stereotypy and each event is counted. The means±s.e.m. of the locomotor responses and stereotypy counts were analyzed using Graphpad Prism 5.0. Cumulative responses were analyzed using a one-way analysis of variance followed by Newman–Keuls multiple comparison test and statistical significant was set at P<0.05.
DOI-induced head twitch
Wild-type (WT) and Cav-1 KO littermate mice were injected i.p. with saline, or 0.25 to 1.25 mg kg−1 of DOI (2,5-dimethoxy-4-iodoamphetamine). The number of head twitches was counted and recorded in 5 min bins for a 35-min period immediately after injection. A subset of the 1.0 and 1.25 mg kg−1 injections were counted by two observers, one of whom was blinded to the genotype; similar results were obtained by both observers (data not shown). All the other head twitch experiments were performed by one blinded observer. The means±s.e.m. of the head twitch responses were analyzed using Graphpad Prism 5.0 and statistical differences analyzed using a one-way analysis of variance followed by Newman–Keuls multiple comparison test and statistical significant was set at P<0.05.
Quantitative reverse transcriptase-PCR for c-FOS and egr-1 mRNA expression
WT and Cav-1 KO littermates were injected with the 5-HT2-selective agonist DOI (1.0 mg kg−1, i.p.) and after 1 h, mice were euthanized, and total RNA was immediately isolated from micro-dissected frontal cortex tissue using Trizol (Invitrogen, Carlsbad, CA, USA). In all, 10 μg of RNA was treated with DNAse (DNA-free, Ambion, Austin, TX, USA), and first strand complementary DNA was synthesized from 2 μg of the DNase-treated RNA using the Superscript III RNase H Reverse Transcriptase kit (Invitrogen), with Oligo-(dT) primers (Invitrogen). Mouse c-Fos and egr-1 complementary DNA was amplified using Power SYBR Green PCR master mix (Applied Biosystems, Invitrogen, Carlsbad, CA, USA) in the 7500 Real-time PCR System (Applied Biosystems). The relative amount of c-Fos or egr-1-specific mRNA was determined by normalizing with β-actin mRNA present in the same samples and is expressed as fold above the saline-treated littermate WT, heterozygous (HET) or Cav-1 KO samples. The following gene-specific primers were used in PCR—mouse C-Fos: sense 5′-CAACACACAGGACTTTTGCG-3′, anti-sense 5′-GGAGATAGCTGCTCTACTTTG-3′ mouse β-actin: sense 5′-TGTTACCAACTGGGACGACA-3′ anti-sense 5′-CTGGGTCATCTTTTCACGGT-3′. Mouse egr-1: sense 5′-TCCTCTCCATCACATGCCTG-3′ anti-sense 5′-CACTCTGACACATGCTCCAG-3′. Statistical comparisons of the means±s.e.m. of the transcript levels were analyzed using Graphpad Prism 5.0 using a one-way analysis of variance followed by Newman–Keuls multiple comparison test.
Results
Cav-1 KO mice exhibit increased sensitivity to the behavioral effects of the psychotomimetic PCP
PCP is a psychotomimetic NMDA glutamate receptor antagonist and PCP administration models in rodents various behavioral disruptions similar to those seen in humans with schizophrenia.17, 18 Additionally, in non-psychotic individuals, PCP induces psychosis, cognitive disruptions and negative symptoms similar to those observed in schizophrenia thus providing evidence for the NMDA hypofunction hypothesis of schizophrenia.19 Notably, administration of PCP or its analog ketamine to individuals with schizophrenia greatly exacerbates preexisting symptoms.20, 21 Accordingly, we investigated the effects of PCP in WT and Cav-1 KO mice. Cav-1 KO mice were generated previously by targeted disruption of exons 1 and 2 in the CAV1 gene by insertion of a neomycin cassette using homologous recombination22 thereby recapitulating the insertional deletion observed as a rare variant in schizophrenia.
PCP disruption of PPI is a well-established pharmacological approach to model the disrupted sensory motor gating seen in schizophrenia and related disorders.18, 23 Cav-1 KO mice exhibited an enhanced response to PCP compared with WT mice (Figure 1a). At all prepulse levels tested, 4 mg kg−1 PCP significantly disrupted PPI in Cav-1 KO mice but not in WT mice. At a higher dose of 6 mg kg−1, PCP significantly disrupted PPI in both genotypes. PCP disrupted PPI in Cav-1 KO mice to a larger extent than in WT mice, particularly at the 4 and 8 dB prepulse levels. In behavioral phenotyping control studies, WT and Cav-1 KO mice exhibited similar startle intensities to all auditory stimuli tested (Supplementary Figure S1a) and no significant difference in baseline PPI was determined between genotypes (Supplementary Figure S1b).
Figure 1.
Genetic deletion of caveolin-1 (Cav-1) sensitizes mice to the behavioral effects of the psychotomimetic phencyclidine (PCP). (a) Cav-1 knockout (KO) mice exhibit increased sensitivity to PCP disruption of sensory motor gaiting. Littermate wild-type (WT) and Cav-1 KO mice were injected with saline, 4 or 6 mg kg−1 PCP and immediately placed into acoustic startle response chambers. In all, 6 mg kg−1 PCP significantly decreased (disrupted) prepulse inhibition (PPI) at all prepulse levels in WT and Cav-1 KO mice. However, a lower dose of 4 mg kg−1 PCP significantly disrupted PPI in only Cav-1 KO but not WT mice (mean±s.e.m., n=8 to 12 littermate pairs; *P<0.05 versus WT saline or Cav-1 KO saline at each prepulse level). (b, c) PCP-induced hyperlocomotion is increased in Cav-1 KO mice. Littermate WT and Cav-1 KO mice were placed into open field locomotion chambers and allowed to acclimate; 30 min later mice were injected with PCP and the total distance traveled (cm) was determined. Cav-1 KO mice exhibited a greater sensitivity to 4 mg kg−1 PCP than WT mice (panel b, mean±s.e.m., n=9 littermate pairs; *P<0.05 versus WT mice at time indicated). Cav-1 KO mice also displayed increased total distance traveled in response to 4 and 6 mg kg−1 PCP compared with WT mice (panel c, mean±s.e.m., n=8 to 12 littermate pairs; *P<0.05 versus WT mice in each group). (d, e) Stereotypy movement is increased in Cav-1 KO mice in response to 4 mg kg−1 PCP (panel d, mean±s.e.m., n=9 littermate pairs). Cav-1 KO mice displayed increased total stereotypy counts in response to 4 and 6 mg kg−1 PCP compared with WT mice (panel e, mean±s.e.m., n=8 to 12 littermate pairs; *P<0.05 versus WT mice in each group).
Next, we examined the effect of PCP on mouse locomotor activity in the open field locomotion test. Before drug administration mice were acclimated to the novel environment for 30 min; the novelty-induced locomotion during this initial 30-min period was similar in WT and Cav-1 KO mice (Figure 1b). Similar results were observed when mice were examined for novelty-induced locomotion over a 60-min period (Supplementary Figures S1c and d). Strikingly, administration of 4 mg kg−1 PCP significantly increased locomotor activity in Cav-1 KO mice in comparison with WT mice (Figures 1b and c). In dose response studies, Cav-1 KO mice displayed increased total distance traveled in response to 4 and 6 mg kg−1 PCP compared with WT mice. In addition, we also discovered that Cav-1 KO mice showed an increased number of stereotypic episodes in response to PCP compared with WT mice (Figures 1d and e). In the PCP dose response studies, the stereotypy counts (measured as repetitive beam breaks) in response to 4 and 6 mg kg−1 PCP was significantly increased in Cav-1 KO mice. Collectively, these findings indicate genetic loss of Cav-1 sensitizes mice to the effects of PCP in a manner reminiscent of the enhanced sensitivity individuals with schizophrenia display to PCP's psychotomimetic actions.
CAV1 KO mice show reduced sensitivity to atypical but not typical antipsychotic drugs
Cav-1 is a scaffold for both D2-dopamine and 5-HT2A serotonin receptors—both of which represent canonical targets for typical (for example, D2) and most atypical (for example, 5-HT2A and D2) antipsychotic drugs.14, 24, 25, 26 Therefore, we assessed antipsychotic drug activity in WT and Cav-1 KO littermate mice. As can be seen in Figure 2a, clozapine normalized the PCP-induced disruption of PPI in WT mice at all prepulse levels and doses tested. In contrast, clozapine was ineffective at normalizing PCP disrupted PPI in Cav-1 KO mice at the 4 and 8 dB prepulse levels. To further confirm this phenomenon of attenuated atypical antipsychotic activity, mice were also administered the structurally related drug olanzapine. As observed with clozapine, olanzapine was also ineffective at normalizing PCP disrupted PPI in Cav-1 KO mice (Supplementary Figure S2). To determine if this altered antipsychotic activity in Cav-1 KO mice is due to altered serotonin signaling, mice were administered the 5-HT2A-selective antagonist M100907, which is known to reverse PCP-induced disruption of PPI.15 Importantly, M100907 was also ineffective at normalizing PCP disrupted PPI in Cav-1 KO mice, particularly at the 4 and 8 dB prepulse levels (Figure 2b). The observation that M100907 is ineffective in Cav-1 KO mice suggests antagonism of 5-HT2A receptors is ineffective in these mice; ineffective 5-HT2A antagonism because of loss of Cav-1 also likely explains the ineffectiveness of clozapine and olanzapine in this behavioral index of atypical antipsychotic drug action.
Figure 2.
Caveolin-1 (Cav-1) knockout (KO) attenuates atypical antipsychotic and 5-HT2A antagonist drug actions in the phencyclidine (PCP) mouse model of psychosis. (a, b) Cav-1 KO renders clozapine and M100907 less effective at normalizing disrupted sensory motor gaiting. Littermate wild-type (WT) and Cav-1 KO mice received injections of vehicle, vehicle +6 mg kg−1 PCP, clozapine (0.5 or 1.0 mg kg−1) +PCP or M100907 (0.5 or 1.0 mg kg−1) + PCP followed by measurement of acoustic startle responses. 6 mg kg−1 PCP significantly decreased (disrupted) prepulse inhibition (PPI) in both genotypes at all prepulse levels. Clozapine normalized the disrupted PPI to near vehicle levels in WT mice; however, clozapine was ineffective at normalizing PCP disrupted PPI in Cav-1 KO mice at 4 and 8 dB prepulse levels (panel a, mean±s.e.m., n=12 littermate pairs; *P<0.05 versus vehicle-treated WT or vehicle-treated Cav-1 KO mice). Similarly, M100907 normalized the disrupted PPI to near vehicle levels in WT mice but this was prevented in Cav-1 KO mice at 4 and 8 dB prepulse levels (panel b, mean±s.e.m., n=10 littermate pairs, *P<0.05 versus vehicle-treated WT or vehicle-treated Cav-1 KO mice). (c–f) Clozapine and M100907, but not haloperidol, are less effective at normalizing PCP-induced hyperlocomotion in Cav-1 KO mice. Clozapine treatments significantly decreased the total distance traveled after PCP in WT but not Cav-1 KO mice; however, haloperidol was equally effective in both genotypes (panels c and d, mean±s.e.m., n=8 littermate pairs; *P<0.05 versus vehicle WT mice or vehicle Cav-1 KO mice). M100907 was also less effective at normalizing PCP-induced hyperlocomotion in Cav-1 KO mice compared with the drug activity in WT animals (panels e and f; mean±s.e.m., n=8 littermate pairs; *P<0.05 versus WT 0.1 M100 + PCP; #P<0.05 versus WT M100 + PCP).
In a complementary behavioral assessment, we examined the effects of these medications to normalize PCP-induced hyperlocomotion. WT and Cav-1 KO mice were administered vehicle followed by PCP, clozapine followed by PCP, or haloperidol followed by PCP and locomotor activity was measured. Clozapine significantly decreased PCP-induced locomotion and total distance traveled in WT but not Cav-1 KO mice (Figures 2c and d), further indicating clozapine efficacy is decreased in Cav-1 KO mice.
Interestingly, the D2-dopamine receptor antagonist and typical antipsychotic haloperidol was equally effective in both genotypes and significantly decreased PCP-induced locomotion (Figures 2c and d). This effectiveness of haloperidol in both genotypes indicates antagonism of D2-dopamine receptors is preserved in Cav-1 KO mice. This also suggests the attenuated clozapine and olanzapine effects observed in Cav-1 KO mice are not due to alterations in D2 receptor antagonist actions. Antagonism of 5-HT2A receptors by M100907 provided additional insights. WT and Cav-1 mice were administered vehicle or M100907 followed by PCP and locomotor activity was determined as before. M100907 significantly decreased PCP-induced locomotion in WT and Cav-1 KO mice; however, M100907 was less effective in Cav-1 KO mice compared with WT mice (Figures 2e and f). Together, these results indicate genetic deletion of Cav-1 attenuates atypical antipsychotic and 5-HT2A antagonist efficacy and that Cav-1 is required for the actions of these drugs to normalize the psychotomimetic-like effects of PCP in mice.
Mouse behavioral and biochemical responses to 5-HT2A receptors are attenuated by genetic deletion of CAV1
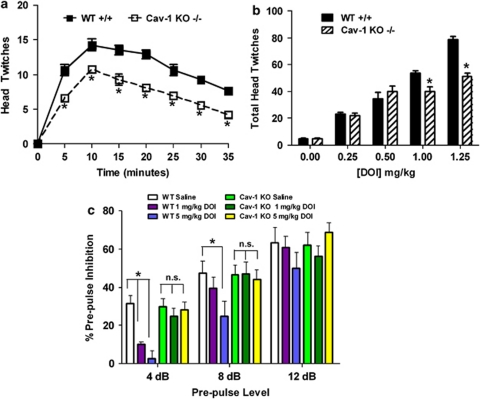
The observation that both atypical antipsychotic drugs and 5-HT2A receptor inverse agonists are ineffective in Cav-1 KO mice prompted us to examine 5-HT2A agonist related behaviors and signaling responses. One reliable and extensively validated behavioral readout for 5-HT2A agonism is the head twitch response.16, 27, 28 Thus, littermate WT and Cav-1 KO mice were administered 1.25 mg kg−1 DOI and head twitches scored over a 35-min period; DOI-induced head twitches were attenuated in Cav-1 KO mice at all-time points (Figure 3a). In a dose response trial of DOI (Figure 3b), total mouse head twitches were attenuated in Cav-1 KO mice at concentrations above 1.0 mg kg−1, further indicating that genetic loss of Cav-1 attenuates agonist activity at 5-HT2A receptors. 125I-DOI receptor autoradiography (Supplementary Figure S3) and radioligand binding (Supplementary Figure S4) in frontal cortex revealed no difference in 5-HT2A receptor-binding sites between the genotypes. Therefore, although a reduction in atypical antipsychotic drug action as well as the behavioral responses to DOI are seen in Cav-1 KO mice, these are not due to detectable changes in the overall number of cortical 5-HT2A receptors, but rather could be due to a decrement in the signaling function of the receptors.
Figure 3.
Mouse behavioral responses to the 5-HT2A agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) are attenuated by genetic deletion of caveolin-1 (Cav-1). (a) Mouse head twitch responses to the 5-HT2A agonist DOI are decreased by Cav-1 knockout (KO). Littermate wild-type (WT) and Cav-1 KO mice were injected with 1.25 mg kg−1 DOI and head twitches scored over a 35-min period; DOI-induced head twitches were attenuated in Cav-1 KO mice at all-time points (panel a, mean±s.e.m., n=12 littermate pairs; *P<0.05 versus WT mice). (b) Dose responses of DOI on total mouse head twitches over a 35-min period are shown; head twitches were attenuated in Cav-1 KO mice only at higher concentrations (panel b, mean±s.e.m., n=6 to 8 littermate pairs; *P<0.05 versus WT mice at each concentration). (c) DOI disruption of sensory motor gaiting in mice is prevented by Cav-1 KO. Littermate WT and Cav-1 KO mice were injected with saline, 1 or 5 mg kg−1 DOI and 10 min later placed into acoustic startle response chambers to assess prepulse inhibition (PPI). DOI significantly decreased (disrupted) PPI at 4 and 8 dB prepulse levels in WT but not Cav-1 KO mice (panel c, mean±s.e.m., n=8 littermate pairs; *P<0.05 versus WT saline-treated mice at each prepulse level). NS, nonsignificant.
Given the aforementioned findings, we next determined whether Cav-1 might directly modulate 5-HT2A signaling. 5-HT2A agonists such as DOI are known to selectively induce the expression of the immediate early response genes c-Fos and egr-1 in the mouse cortex.16, 29 In WT animals, DOI significantly increased cortical c-Fos and egr-1 mRNA expression. Notably, DOI-treated Cav-1 KO mice exhibited no significant induction of c-Fos or egr-1, indicating genetic loss of Cav-1 attenuates signaling activity at 5-HT2A receptors in the mouse cortex in vivo (Figures 4a and b). In comparison, animals heterozygous for the Cav-1 gene exhibited a reduced c-Fos induction in response to DOI, suggesting a gene dosage effect. As 5-HT2A receptors couple principally to Gαq and on ligand activation, signal via the Gαq/phospholipase Cβ/IP3 pathway to mobilize calcium release from intracellular stores, we tested if Cav-1 altered 5-HT2A-induced calcium signaling in primary cortical neurons isolated from WT and Cav-1 KO embryonic mice. As previously detailed,30 DOI does not elicit a detectable calcium response in primary neurons probably due to a low expression of endogenous 5-HT2A receptors; however, WT neurons expressing virally transduced 5-HT2A receptors exhibit a robust increase in intracellular calcium dye fluorescence after addition of DOI (Figure 4c). By contrast, Cav-1 KO neurons normalized for expression of equivalent 5-HT2A receptor levels show attenuated responses. Quantification of fluorescence in individual cells determined the peak calcium response after DOI (Emax) as well as the total calcium response (area under curve) was significantly decreased by Cav-1 KO (Figure 4d) (WT cells peak calcium Emax=0.72±0.04, Cav-1 KO cells peak calcium Emax=0.57±0.4*; WT cells total calcium response=36.7±1.2, Cav-1 KO cells total calcium response=24.9±1.6*; *P<0.05 versus WT cells). Taken together, these results indicate genetic loss of Cav-1 attenuates neuronal 5-HT2A signaling in vitro and in vivo.
Figure 4.
5-HT2A signaling responses to the agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) are attenuated in vivo and in vitro by genetic deletion of caveolin-1. (a, b) Littermate wild-type (WT) and caveolin-1 (Cav-1) knockout (KO) mice were injected with 1.0 mg kg−1 DOI, 1 h later frontal cortex was isolated, mRNA extracted and transcript levels of c-Fos and egr-1 determined by real-time PCR. DOI-induced c-Fos and egr-1 gene expression in frontal cortex was decreased in mice heterozygous for Cav-1; DOI-induced gene expression was prevented in KO mice (panels a and b, mean±s.e.m., n=5 littermate mice for each group; *P<0.05 versus saline-treated mice in each group). (c) Neuronal 5-HT2A receptor calcium signaling is decreased by genetic deletion of Cav-1. Primary cortical neurons were isolated from WT and Cav-1 KO mice and cultured in 96-well plates. Neurons were infected with lentiviruses encoding 5-HT2A receptors and intracellular calcium release was determined by fluorescence calcium dye imaging using high-content imaging microscopy. Images of cells are shown before (left panels) and after (middle panels) an automated addition of the 5-HT2A agonist DOI. WT neurons expressing 5-HT2A receptors (middle panels) exhibited a robust increase in intracellular calcium dye fluorescence after DOI. In comparison, KO neurons expressing 5-HT2A receptors (lower panels) displayed a reduced intracellular calcium dye fluorescence after agonist. The calcium dye fluorescence in individual responding cells in the wells (segmenting) was determined. (d) Quantification of calcium dye fluorescence in individual cells is shown and expressed as fold over initial fluorescence; KO neurons exhibited a reduced peak and total calcium response (Figure 4d, mean fluorescence intensity±s.e.m. from >90 cells per group from n=3 cell preparations).
Discussion
The major finding of this study is that genetic deletion of CAV1—a rare structural variant associated with schizophrenia—leads to an enhanced susceptibility to the psychotomimetic actions of PCP and reduced sensitivity to atypical but not typical antipsychotic drugs. This study also provides the first in vivo evidence that the scaffolding protein Cav-1 influences both central nervous system receptor function and psychoactive drug activity.
Scaffolding-related control mechanisms are now clearly important for the function of many receptors and ion channels and scaffolding proteins in general have been implicated in schizophrenia as well as many other neuropsychiatric diseases.31, 32, 33 Thus, for example, in the 22q11.2 microdeletions arising in DiGeorge syndrome, which results in cognitive deficits and a high risk of developing schizophrenia, the protein ZDHHC8 has been identified as a palmitoyltransferase for PSD95 that controls PSD95 function and its ability to scaffold proteins in the postsynaptic density.34 Genetic deletion of ZDHHC8 in DiGeorge syndrome may therefore disrupt synaptic glutamatergic and G protein-coupled receptor (GPCR) scaffolding with PSD95 and dysregulate receptor functions contributing to pathologies associated with schizophrenia.16 Here, we show that disruptions of Cav-1, an important neuronal signaling scaffold, may contribute to the mechanisms causing schizophrenia and possibly other neuropsychiatric disorders.
Our identification here of Cav-1-mediated regulation of 5-HT2A receptors raises the intriguing possibility for altered 5-HT2A receptor function in individuals with schizophrenia that possess the CAV1 mutation. The observation that Cav-1 KO mice exhibit increased sensitivity to the psychotomimetic effects of PCP (Figure 1), a phenomenon also observed in humans with schizophrenia,20, 21 is a compelling behavioral observation bridging the mouse model and human schizophrenia. The mechanisms underlying this enhanced PCP sensitivity in Cav-1 KO mice are unknown, although our findings implicate dysfunctional serotonergic signaling. Recent studies have also indicated altered mGluR function in Cav-1 KO mice as well as alterations in synaptic plasticity.11, 35 NMDA receptors are not currently known to directly interact with caveolins, but both proteins do colocalize in hippocampal neurons where Cav-1 is required for NMDA receptor signaling.36 However, hippocampal NMDA-mediated long-term depression is not altered in Cav-1 KO mice, although the mGluR-mediated long-term depression is altered.35 Therefore, this enhanced response to PCP observed in Cav-1 KO mice may involve changes in mGluR-NMDA receptor function, or, possibly, altered NMDA receptor scaffolding/trafficking by lack of Cav-1, both possibilities warrant future study.
Caveolins are thought to regulate GPCR signaling cascades by two distinct mechanisms (1) Caveolin scaffolding of GPCRs, G proteins and their associated effectors in membrane microdomains to regulate protein–protein interactions and therefore signal transduction and, (2) caveolin regulation of receptor trafficking as caveolins can control a distinct form of clathrin-independent endocytosis.9, 10 However, exactly how caveolins function in the central nervous system remains unclear, although recent evidence indicates Cav-1 controls constitutive receptor endocytosis in hippocampal neurons for some GPCRs such as the mGluR1/511 and signaling by hippocampal mGluR1/5 receptors to control hippocampal long-term depression is abnormal in Cav-1 KO mice.35 This is particularly interesting as both mGluR1/5 and 5-HT2A receptors couple primarily through Gαq mechanisms, suggesting multiple GPCR-Gαq signaling cascades may require Cav-1. Consistent with this notion, in our previous investigations, we determined that Cav-1 and Cav-2 interact with 5-HT2A receptors and stable knockdown of caveolins profoundly inhibits Gαq-mediated calcium signaling.14 We also previously determined that the Cav/5-HT2A interaction promotes a complex between Gαq and the receptor and this may be a mechanism by which caveolin influences 5-HT2A receptor signaling and pharmacology.
Cav-1 scaffolding also seems essential for the salutary actions of atypical antipsychotic drugs in mice. Using the PCP mouse model of psychosis, we determined that genetic loss of Cav-1 attenuated the ability of the atypical antipsychotics clozapine and olanzapine to normalize the PCP-disrupted PPI and the PCP-induced hyperlocomotion (Figure 2). Cav-1 KO similarly attenuated the activity of the selective 5-HT2A antagonist M100907 in both behavioral tests. However, the typical antipsychotic haloperidol, which acts primarily as a D2-dopamine receptor antagonist, was equally effective in WT and Cav-1 KO mice. In addition, the increased potency of PCP in Cav-1 KO mice (Figure 1) cannot thoroughly explain the reduced efficacy of these atypical antagonists in behavioral studies. This is because the relative difference in PCP alone effects between WT and Cav-1 KO mice is not proportional to those differences observed between WT and Cav-1 KO mice after treatment with PCP and antipsychotics (Figure 2). These results indicate atypical antipsychotics, acting via antagonism at 5-HT2A and other receptors, require the expression of Cav-1 for their full efficacy in mice. As total receptor number is unchanged by Cav-1 KO, this requirement for Cav-1 could be explained by an attenuated 5-HT2A receptor signaling. We recently determined that the presynaptic component of the serotonergic neuronal system is required for clozapine's antipsychotic activity (that is, normal 5-HT2A signaling via serotonin must be intact for effective antagonism by clozapine in these PCP behavioral studies).15 Therefore, the reduced 5-HT2A signaling evident in Cav-1 KO mouse cortex (Figure 4a) in cultured cortical neurons (Figure 4b) and in behavioral responses (Figure 3) is an indication that receptor activity is also reduced; this could make antagonism of the receptors less effective. Also consistent with this notion, we recently determined that PSD95 KO mice exhibit reduced agonist-induced 5-HT2A signaling, which is associated with decreased clozapine activity in the KO mice.16 The findings here that Cav-1 is required for antipsychotic activity, along with our recent PSD95 KO mice results, indicate that 5-HT2A signaling via scaffolding proteins appears to be a requirement for atypical antipsychotic activity in mouse models.
In summary, we have found that Cav-1 KO mice are supersensitive to the psychotomimetic actions of PCP. We also discovered that genetic loss of CAV1 attenuates the biochemical and behavioral actions of atypical antipsychotic drugs and that 5-HT2A agonists and inverse agonists in vitro and in vivo have attenuated effects in Cav-1 KO mice. These findings provide fresh insights into the roles of caveolin in the central nervous system and provide support for the involvement of caveolin in the etiology of schizophrenia. Given that Cav-1 KO mice show resistance to the apparent antipsychotic actions of clozapine and olanzapine, it appears that caveolin is also required for atypical antipsychotic drug actions.
Acknowledgments
This work was supported by research grants from the National Institutes of Health, PHS RO1MH61887, U19MH82441, RO1DA017204 and HHSN-271-2008-00025-C to BLR MF was supported by an NIH National Research Service Award F31MH091921-01. JAA was supported by NIH National Research Service Award training grant T32HD040127-07 and the UNC-Carolina Institute for Developmental Disabilities.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro-Peled H, Ayhan Y, Pletnikov MV, Sawa A. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophr Bull. 2010;36:301–313. doi: 10.1093/schbul/sbp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–416. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Ostrom RS. New determinants of receptor-effector coupling: trafficking and compartmentation in membrane microdomains. Mol Pharmacol. 2002;61:473–476. doi: 10.1124/mol.61.3.473. [DOI] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29:3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A, Sheffler DJ, Kroeze WK, Compton-Toth B, Roth BL. Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem. 2004;279:34614–34623. doi: 10.1074/jbc.M404673200. [DOI] [PubMed] [Google Scholar]
- Yadav PN, Abbas AI, Farrell MS, Setola V, Sciaky N, Huang XP, et al. The presynaptic component of the serotonergic system is required for clozapine's efficacy. Neuropsychopharmacology. 2011;36:638–651. doi: 10.1038/npp.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, et al. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB. The NMDA receptor hypofunction model of psychosis. Ann NY Acad Sci. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Linn GS, Javitt DC. Phencyclidine (PCP)-induced deficits of prepulse inhibition in monkeys. Neuroreport. 2001;12:117–120. doi: 10.1097/00001756-200101220-00031. [DOI] [PubMed] [Google Scholar]
- Genedani S, Guidolin D, Leo G, Filaferro M, Torvinen M, Woods AS, et al. Computer-assisted image analysis of caveolin-1 involvement in the internalization process of adenosine A2A-dopamine D2 receptor heterodimers. J Mol Neurosci. 2005;26:177–184. doi: 10.1385/JMN:26:2-3:177. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, et al. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA. Do classical hallucinogens act as 5-HT2 agonists or antagonists. Neuropsychopharmacology. 1990;3:509–517. [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, et al. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan RT, Allen JA, Sheffler DJ, Roth BL. p90 Ribosomal S6 kinase 2, a novel GPCR kinase, is required for growth factor-mediated attenuation of GPCR signaling. Biochemistry. 2010;49:2657–2671. doi: 10.1021/bi901921k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen MJ, Williams HJ, O'Donovan MC. Schizophrenia genetics: advancing on two fronts. Curr Opin Genet Dev. 2009;19:266–270. doi: 10.1016/j.gde.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Collins MO, Uren RT, Kopanitsa MV, Komiyama NH, Croning MD, et al. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol Syst Biol. 2009;5:269. doi: 10.1038/msb.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayes A, van de Lagemaat LN, Collins MO, Croning MD, Whittle IR, Choudhary JS, et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat Neurosci. 2011;14:19–21. doi: 10.1038/nn.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu Y, Takeuchi K, Kumari R, Bennett MV, Zukin RS, Francesconi A. Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proc Natl Acad Sci U S A. 2010;107:21778–21783. doi: 10.1073/pnas.1015553107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, et al. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 2008;22:828–840. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.