Abstract
Dopaminergic projections to the prefrontal cortex support higher-order cognitive functions, and are critically involved in many psychiatric disorders that involve memory deficits, including schizophrenia. The role of prefrontal dopamine in long-term memory, however, is still unclear. We used an imaging genetics approach to examine the hypothesis that dopamine availability in the prefrontal cortex selectively affects the ability to suppress interfering memories. Human participants were scanned via functional magnetic resonance imaging while practicing retrieval of previously studied target information in the face of interference from previously studied non-target information. This retrieval practice (RP) rendered the non-target information less retrievable on a later final test—a phenomenon known as retrieval-induced forgetting (RIF). In total, 54 participants were genotyped for the catechol-O-methyltransferase (COMT) Val108/158Met polymorphism. The COMT Val108/158Met genotype showed a selective and linear gene-dose effect on RIF, with the Met allele, which leads to higher prefrontal dopamine availability, being associated with greater RIF. Mirroring the behavioral pattern, the functional magnetic resonance imaging data revealed that Met allele carriers, compared with Val allele carriers, showed a greater response reduction in inhibitory control areas of the right inferior frontal cortex during RP, suggesting that they more efficiently reduced interference. These data support the hypothesis that the cortical dopaminergic system is centrally involved in the dynamic control of human long-term memory, supporting efficient remembering via the adaptive suppression of interfering memories.
Keywords: COMT, episodic memory, imaging genetics, inhibition, retrieval-induced forgetting
Introduction
It is generally accepted that the prefrontal cortex is the major site of top–down control over thoughts and actions, enabling us to maintain task-relevant processing in the face of distraction.1, 2 Computational models of cognitive control have emphasized the role of the neurotransmitter dopamine in controlling interference from task-irrelevant information.1, 3, 4, 5 These models suggest that high sustained dopaminergic activity in prefrontal cortex promotes cognitive stability and resistance to distraction. Deficient prefrontal dopaminergic functioning, as found in psychiatric conditions like schizophrenia,6 is known to impair performance on higher-order cognitive tasks.7, 8 Although the role of dopamine signaling in the core memory structures, such as the hippocampus, is well established,9, 10, 11 the role of prefrontal dopamine has thus far been primarily investigated in relation to attention and working memory rather than long-term memory.12, 13 Therefore, using an imaging genetics approach, we here investigated the role of prefrontal dopamine in long-term memory retrieval.
The catechol-O-methyltransferase (COMT) gene expresses an enzyme that degrades cortical dopamine. A common variant of the COMT gene, the Val108/158Met polymorphism, renders the enzyme thermolabile, resulting in lower COMT activity and ultimately in higher dopamine concentrations in brain regions with low dopamine transporter expression, particularly prefrontal cortex.14, 15, 16 Individuals carrying the Met allele typically perform better on a variety of complex cognitive tasks,15 but the impact of COMT Val108/158Met on long-term memory has not yet been conclusively established. Although some authors have reported better recognition memory in Met carriers than in Val carriers,17 others have found an effect only on free or cued recall tests,18 or found no behavioral COMT effect at all.19, 20, 21 Functional neuroimaging studies have consistently demonstrated COMT effects on prefrontal activation during long-term memory encoding and retrieval, but the functional significance of these effects is not yet clear.17, 20, 21 One important control mechanism that might be affected by prefrontal dopamine levels is the ability to inhibit conflicting responses, an assumption based on the hypothesized role of the dopaminergic system,1, 3, 4, 5 as well as empirical findings.22, 23 On the basis of this idea, we hypothesized that during the retrieval of target information from long-term memory, the increased level of tonic dopamine associated with the COMT Met allele might enhance the ability to adaptively suppress interfering memories.
To test this hypothesis, brain activity measures were recorded from three groups of participants, 18 from each possible COMT Val108/158Met genotype, during the retrieval practice paradigm. Typically, practicing recall of target information, while enhancing later memory for that information,24, 25 also produces later forgetting of interfering information that is associated with the same semantic or contextual cues.26, 27 This characteristic recall impairment is called retrieval-induced forgetting, and has been attributed to a cognitive control mechanism that adaptively suppresses the interfering information during retrieval practice, leaving it suppressed during later recall attempts.27, 28, 29 We predicted that participants with high levels of prefrontal dopamine (COMT Met/Met carriers) would be most efficient at suppressing interfering information, and would therefore show the highest levels of retrieval-induced forgetting. Imaging and electrophysiological evidence has shown that this adaptive memory suppression is mediated by prefrontal brain areas during retrieval practice,28, 30, 31 and has supported the claim that this suppression persists during later recall attempts.29 On the basis of this previous research, we further predicted that Met/Met carriers should show a more pronounced response reduction in prefrontal inhibitory control areas,32 consistent with a more efficient suppression process.30
Materials and methods
Participants
Fifty-four healthy volunteers (mean age 25.6 years, range 21–38 years) were recruited from a larger participant database at the Leibniz Institute for Neurobiology in Magdeburg; participants were chosen in such a manner that 18 were homozygous for the Met allele, 18 were heterozygous and 18 were homozygous for the Val allele. In each group, half of the participants were male and half female. All participants had normal or corrected-to-normal vision, and none of them reported any history of neurological or psychiatric disease. Participants received financial compensation for participation. All experimental procedures were conducted in accordance with the Declaration of Helsinki and the guidelines of the Ethics Committee of the University of Magdeburg Medical Faculty, with written informed consent obtained from all participants. The experiment was run double-blind, such that participants and experimenters were naïve with respect to genotype until all behavioral data were scored.
Genotyping
Genomic DNA was extracted from venous whole blood using the GeneMole automated DNA extraction system (Mole Genetics, Lysaker, Norway) according to the manufacturer's protocol. The DNA fragment containing the COMT Val108/158Met polymorphism (NCBI dbSNP: rs4680) on human chromosome 22q11.2 was amplified out using PCR (details available upon request). The amplicons were submitted to site-specific restriction analysis with the NlaIII isoschizomer Hin1II (Fermentas, St Leon-Rot, Germany), and the resulting fragments were separated on ethidium bromide-stained agarose gels and visualized under ultraviolet light.
Paradigm and procedure
Materials were 216 German nouns from 36 different semantic categories drawn from published association norms.33, 34 The six exemplars from the same category had unique first letters, such that the category plus the first letter of an exemplar (e.g., FRUIT—M____) always constituted an unambiguous cue.
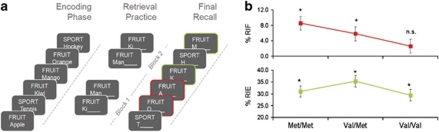
Each participant completed nine consecutive runs consisting of a study phase, a retrieval practice (RP) phase and a final recall phase (Figure 1a). The scanner was stopped after every third run, allowing for a short break. In each study phase, participants intentionally studied 24 words from four different categories, each item being presented for 2000 ms after 1500 ms of fixation (+). A simple version of the Eriksen flanker task was conducted after study for about 35 s in order to preclude recall from working memory, followed by another 20 s of fixation (+). During RP, participants actively retrieved half of the items from half of the studied categories. On each trial, following 1000 ms of fixation (+), a cue consisting of a category plus an item-specific word stem remained on screen for 2500 ms. Consistent with previous studies,28, 29, 31 in order to avoid movement artefacts, participants were asked to retrieve the corresponding item from the preceding study list covertly. Behavioral data from this phase of the experiment are therefore not available; however, later retrieval-induced forgetting (RIF) and enhancement (RIE) demonstrate that participants followed the RP instructions. Retrieval practice was repeated in different random order after recall of all six (2 × 3) to-be-practiced items. Three fixation trials (+) of 3500 ms duration were interspersed during each of the two RP blocks. The items with the normatively weakest association to the category cue were always chosen as to-be-practiced to maximize competition from the stronger non-practiced items during RP. During final recall, participants were asked to retrieve all previously studied items, cued by the category and an item's initial letter. A test trial began with 1000 ms fixation (+), followed by the cue for 2000 ms, followed by three exclamation marks (!!!), prompting participants to overtly respond with the corresponding word from the preceding study list. Oral responses were recorded via an MR-compatible microphone fixed to the head coil. Final recall phases were followed by another 20 s of fixation (+).
Figure 1.
(a) Schematic depiction of the experimental procedure. Participants first studied categorized word lists, and then selectively retrieved half of the items from half of the categories via a category plus word-stem cue. A final recall test assessed the effects of previous retrieval practice on the recall of practiced target items (P+, green) and related, but non-practiced non-target items (P−, red), compared with unrelated control (C) items. (b) Behavioral data (means±s.e.) from the final recall test. Retrieval-induced forgetting (RIF), an index of successful interference reduction, was significant in Met/Met and Val/Met, but not in Val/Val carriers. Retrieval-induced enhancement (RIE), the beneficial effect of retrieval practice, was significant in all three genotypes.
This procedure resulted in 54 (9 × 6) normatively weak items that were practiced in the RP phase (P+ items, green in Figure 1), 54 normatively strong items that were not practiced, but shared their category with the practiced items and thus interfered during RP (P− items, red in Figure 1), 54 normatively weak control items (C+ items) and 54 normatively strong control items (C− items). RIF was calculated as the difference in final recall between P− and the corresponding C− items, whereas retrieval-induced enhancement (RIE) was calculated as the difference in final recall between P+ and the corresponding C+ items.27, 29 RIF and RIE were tested for significance using one-tailed, one-sample t-tests threshold at P<0.05. Two-tailed t-tests (P<0.05) were used to test for differences between the two homozygous groups, and polynomial contrasts (P<0.05) were calculated to test for linear gene-dose effects. Relationships between two behavioral measures were assessed using partial correlation analysis, calculating the correlation across the whole participant sample while controlling for the effects of genotype.
Functional magnetic resonance imaging data acquisition and analysis
Imaging data were obtained on a General Electric Signa 1.5T system located at the Department of Neurology in Magdeburg. T2*-weighted functional images sensitive to blood-oxygenation-level-dependent contrast were acquired in an interleaved, bottom-to-top echo-planar imaging sequence (repetition time=2000 ms; echo time=35 ms, flip angle=90°). Each whole-brain volume consisted of 23 axial slices of 5 mm thickness and a 1-mm gap between slices, oriented parallel to the anterior–posterior commissure plane, with an in-plane resolution of 3.15 by 3.15 mm. The first six images of each scanner run were discarded to allow for stable tissue magnetization. T1-weighted, high-resolution structural images using a standard magnetization-prepared rapid gradient echo sequence (192 sagittal slices, 1 mm resolution) were acquired after the functional scanning runs.
Statistical parametric mapping (SPM5, http://www.fil.ion.ucl.ac.uk/spm/) was used for preprocessing and statistical analyses. The 23 slices of each functional volume were corrected for differences in slice acquisition time; volumes were then realigned and unwarped to correct for movement-related changes across time, and co-registered to the bias-corrected structural image (using the bias correction algorithm implemented in the SPM5 segmentation routine). Individual T1-images were normalized to a T1-weighted template in standard stereotactic MNI space (Montreal Neurological Institute, http://www2.bic.mni.mcgill.ca/), and the resulting parameters were then applied to all functional images before smoothing them with an 8-mm full width at half maximum Gaussian kernel.
For statistical analysis at the single participant level, a vector containing delta functions at the onset of each event, or boxcar functions of 20 s length at the onset of a fixation block, was convolved with a first-order canonical hemodynamic response function.35 Study phase time series were modeled by eight event-related conditions (corresponding to the onsets of P+, P−, C+ and C− words, later remembered or forgotten), RP phases by two conditions (cue onsets during the first and second RP, RP1 and RP2), and final recall phases eight conditions (cue onsets of remembered or forgotten P+, P−, C+ and C+ words). Movement parameters acquired during realignment were also included in the model. Maximum likelihood estimates of the model parameters were obtained using an autoregressive AR(1) model plus white noise assumption for the error, and a 1/128 Hz high-pass filter on time series. Linear contrasts (t-maps) were calculated on the resulting parameter estimates of interest, in this case, corresponding to the contrasts between first RP events and fixation blocks (RP1 vs fixation), and between second RP events and fixation blocks (RP2 vs fixation). For group-level analysis, the resulting individual contrast maps were entered into a 3 × 2 full-factorial analysis of variance, with factors GENOTYPE (Met/Met, Val/Met or Val/Val, between-participants) and RETRIEVAL PRACTICE BLOCK (first or second, within-participants). For within-group comparisons, an uncorrected voxel height threshold of P<0.001 was applied in combination with a cluster extent threshold (k) of at least 10 adjacent voxels. For between-group comparisons (interaction contrasts), the uncorrected voxel height threshold was lowered to P<0.005 (k>20). Mean parameter estimates (eigenvalues) from functional clusters of interest were extracted using the EasyROI software (http://www.sbirc.ed.ac.uk/cyril/). To verify reliability of the between-group differences, we performed confidence interval estimation on the differences of the eigenvalues between the first and second RP blocks, using bootstrap re-sampling and the percentile-t method.20 Only between-group differences with non-overlapping confidence intervals were considered reliable. To assess the relationship between RIF and the repetition-related decrease in a given functional cluster of interest, we computed partial correlations between RIF and the difference in eigenvalues between the first and second RP block across the whole subject sample, controlling for the effects of genotype.
Results
Behavioral data
At final test, significant RIF (Figure 1b, upper row; Table 1) was found in the Met/Met (8.5%, t17=4.02, P<0.05) and the Val/Met (5.7%, t17=3.97, P<0.05), but not in the Val/Val (2.6%, t17=1.43, P=0.09) group. The difference in RIF between the homozygous groups was significant (t34=2.14, P<0.05). Polynomial contrast analysis of the between-participants factor GENOTYPE revealed a significant linear effect of Met allele load on RIF (mean contrast estimate c=4.22, s.e.=1.81, P<0.05). RIE (Figure 1b, lower row; Table 1) was significant in all three genetic groups (Met/Met: 31.0%, t17=10.92, P<0.05; Val/Met: 35.3%, t17=17.64, P<0.05; Val/Val: 29.4%, t17=12.64, P<0.05). RIE did not differ significantly between the homozygous groups (t34=0.43, P=0.34), nor was there a significant linear gene-dose effect (c=1.09, s.e.=2.41, P=0.65). Finally, overall memory performance, assessed by mean recall performance for control items (averaged across C+ and C− items), was similar in all three groups (Met/Met: 59.4%, s.e.=3.25; Val/Met: 54.7%, s.e.=3.35; Val/Val: 61.1%, s.e.=2.93), with no significant difference between the homozygous groups (t34=0.53, P=0.60). Across all participants, RIF showed no significant partial correlation (using genotype as a covariate) with mean recall performance (ρ51=0.09, P=0.55) or with RIE (ρ51=0.02, P=0.90).
Table 1. Mean percent final recall (with standard errors in brackets) of non-practiced items from practiced categories (P−), and the corresponding strong control items (C−) from non-practiced categories, as well as practiced items (P+) and the corresponding weak control items from non-practiced categories (C+).
| P− | C− | RIF | P+ | C+ | RIE | |
|---|---|---|---|---|---|---|
| Met/Met | 59.77 (2.60) | 68.31 (2.17) | 8.54 (2.12) | 81.38 (2.25) | 50.41 (3.32) | 30.97 (2.84) |
| Val/Met | 57.72 (2.85) | 63.48 (2.52) | 5.76 (1.45) | 77.21 (1.94) | 43.74 (2.99) | 35.29 (2.00) |
| Val/Val | 66.15 (1.94) | 68.73 (2.01) | 2.57 (1.80) | 82.82 (1.65) | 53.40 (2.57) | 29.42 (2.33) |
Abbreviations: RIE, retrieval-induced enhancement; RIF, retrieval-induced forgetting.
Imaging data
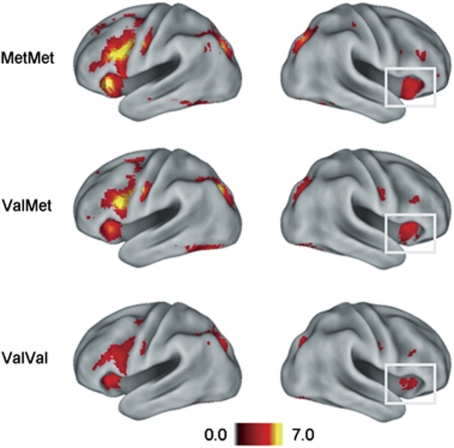
Planned comparisons contrasting the first and second RP blocks (RP1, RP2), separately in each genotype (Supplementary Table 1), showed that all three groups revealed the same basic pattern of a significant blood-oxygenation-level-dependent signal decrease across blocks, with the most prominent decrease in bilateral middle and inferior frontal lobe (Brodmann area, BA 44/45/47) and superior parietal lobe (BA 7). This pattern of a repetition-related decrease was most prominent in Met/Met carriers, and least pronounced in Val/Val carriers (Figure 2).
Figure 2.
Functional magnetic resonance imaging results showing the contrast between the first and the second retrieval practice blocks (P<0.001, k>10) in the three genotype groups on a flattened cortical surface (PALS-B12 atlas from Caret software;54). Gray boxes highlight the region in which the repetition-related decrease across retrieval practice blocks showed a significant interaction with genotype (Met/Met vs Val/Val, Figure 3).
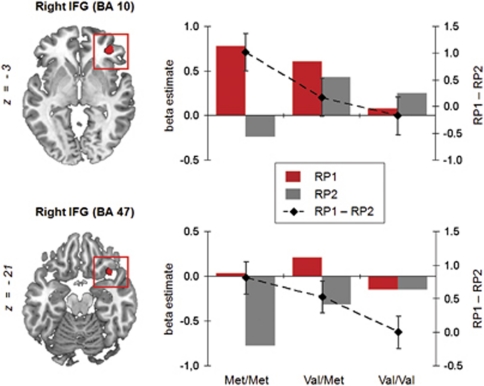
The GENOTYPE × RETRIEVAL PRACTICE BLOCK interaction tested formally for a difference in the repetition-related decrease between the two homozygous genotypes. This analysis gave a significant interaction in two right inferior frontal regions (BA 10 at x,y,z=39, 42, –3; k=32; and BA 47 at x,y,z=39, 18, –18; k=29). In both regions, the interaction was caused by a larger practice-related decrease in the Met/Met than in the Val/Val group (Figure 3). More specifically, Met/Met carriers showed strong initial BA 10 activation during RP1, with a large decrease from RP1 to RP2. Val/Val carriers, by contrast, showed little initial BA 10 activation and no corresponding decrease across blocks. A similar pattern emerged in BA 47, but was mainly driven by an activation decrease in RP2 relative to fixation. Across all three groups, polynomial contrast analysis of the eigenvalues of the RP1 vs RP2 contrast revealed a significant linear effect of Met allele load in both BA 10 (c=0.84, s.e.=0.29, P<0.005) and BA 47 (c=0.57, s.e.=0.19, P<0.005). Confidence interval estimation for the RP1 vs RP2 differences revealed non-overlapping intervals for Val and Met homozygotes in both regions.
Figure 3.
Regions in which the activation decrease across retrieval practice blocks (RP1, RP2) was significantly larger in Met/Met than in Val/Val carriers (P<0.005, k>10). Bar plots show the mean activation (beta estimates) of the corresponding BA 10 and BA 47 clusters during RP1 and RP2, compared with fixation. Dotted lines depict the differences between the two retrieval blocks (secondary y axis, error bars correspond to standard errors of the difference), showing a linear increase with Met allele load in both right regions.
Finally, we computed partial correlations between RIF and the practice-related decreases (RP1-RP2) in BA 10 and BA 47, with genotype as a covariate. This analysis revealed a significant partial correlation between RIF and the activation decrease in BA 10 (ρ51=0.33, P<0.05), but not in BA 47 (ρ51=0.13, P=0.37).
Discussion
In this experiment, we tested the hypothesis that genetic determinants of prefrontal dopamine are related to a participant's ability to adaptively suppress interfering information when recalling target information from long-term memory. Our data show a gene-dose-dependent influence of COMT Val108/158Met genotype on behavioral and brain activity indices of memory suppression.27, 30 Retrieval-induced forgetting, a behavioral index of memory suppression,27 increased linearly with Met allele load, suggesting a positive relationship between cortical dopamine availability and inhibitory control (Figure 1b). This relationship was specific to suppression, because no group differences were found with respect to retrieval-induced enhancement or overall memory performance. The behavioral data, thus, point to a selective advantage of Met carriers in attenuating memory interference during recall, in line with previous findings that the suppressing and enhancing effects of retrieval practice are separable on behavioral36 and neural29 levels.
Mirroring the linear effect of genotype on behavior, functional imaging data revealed that the beneficial effects of memory suppression, as assessed by a decrease in prefrontal brain activity across retrieval practice blocks,30 also increased with Met allele load (Figure 2). In particular, the homozygous groups differed significantly in their dynamic recruitment of the right inferior frontal cortex,32 a brain area that has been shown to mediate inhibitory control not only over unwanted motor actions32 but also over interfering long-term memories in the retrieval practice paradigm.30 Met/Met individuals showed a larger beneficial decrease than Val/Val carriers, indicating that the Met allele is associated with more efficient memory suppression. In addition, brain–behavior correlations, controlling for the influence of genotype, revealed that the extent of this decrease in one of the two right inferior frontal gyrus regions (BA 10, see Figure 3) significantly predicted the behavioral outcome of memory suppression (i.e., RIF), again consistent with previous imaging data from a similar paradigm.30
We were thus not only able to replicate the finding that the right inferior frontal gyrus is centrally involved in the suppression of interfering memories30 but also able to explain individual differences in the degree to which people dynamically engage this region in terms of genetic variability of prefrontal dopamine levels. Together, our data suggest a selective influence of COMT Val108/158Met genotype on memory control and its underlying neural mechanism,30 without affecting practice-related improvements or overall memory performance. Regarding the latter finding, the importance of controlling the retrieval process for overall memory performance might grow with increasing levels of interference, such as in recognition tests with highly similar old and new test items, or when recall test cues do not closely specify the properties of to-be-recalled items. Some of the extant COMT research18 lends support to this interference hypothesis.
In addition to demonstrating a selective dopaminergic modulation of dynamic control processes in human long-term memory, our results clearly support the more general theory that high prefrontal dopamine levels are beneficial for cognitive stability and resistance to distraction.1, 3, 5 The COMT Val108/158Met polymorphism, with its well-established relationship to prefrontal dopamine concentrations, has offered a new route for studying the impact of dopaminergic signaling on human cognition (for reviews, see refs. 15 and 14). Consistent with theoretical models, Met carriers appear to perform better on N-back2, 37 or Stroop-like38 tasks that place high demands on cognitive stability. Therefore, we tentatively suggest that this enhanced cognitive stability is attributable to higher levels of inhibitory control over interfering representations, allowing for more stable, ‘tuned' cognitive representations.39 In this context, we note that previous imaging studies on COMT have shown that the directionality of the brain activity differences can vary across tasks and brain regions,40 with Val carriers showing hyperactivation in some tasks and areas,12, 13, 41, 42 and hypoactivation in others.17, 19, 21, 43 Moreover, some studies have reported a U-shaped,8 rather than a linear,2, 12 relationship between prefrontal dopamine concentrations and cognitive functions.8 Our prediction of a linear COMT effect was thus derived from theoretical models1, 3, 5 rather than the mixed empirical findings. However, we cannot rule out that a U-shaped pattern might emerge with our paradigm when additional functional genetic variants associated with changes in COMT activity are considered.44
Dysfunctional prefrontal dopamine circuits have been considered to be at the heart of the cognitive deficits in psychiatric conditions like schizophrenia,24 and the COMT Val108/158Met polymorphism was hence early investigated as a potential risk factor for schizophrenia.14 Although later meta-analyses failed to confirm a straightforward association between the COMT Val108/158Met polymorphism and schizophrenia (for review see ref. 45), the association between COMT and prefrontal cognitive functioning is well established,46 and the polymorphism therefore remains an important neuronal model for cognitive deficits in schizophrenia. Among these deficits are pronounced long-term memory impairments, although the neural basis of these impairments is poorly understood.47 Our results suggest that prefrontal dopamine affects the ability to suppress irrelevant memories during retrieval. Thus, the memory deficits in schizophrenia might partially reflect a failure to control the retrieval process by protecting target from interfering information (ref. 48, but see ref. 49). Deficient memory suppression has been demonstrated in other psychiatric disorders associated with impulsivity or impaired prefrontal control, like in attention-deficit hyperactivity disorder.50 Future clinical studies will show whether this deficiency is due to an inefficient recruitment of inhibitory brain areas.
In summary, we propose that sustained levels of cortical dopamine modulate the ability to selectively activate target memories in the face of interference, thus adaptively reducing future interference. A future challenge is to establish whether the COMT effect on the suppression of interfering information generalizes to different materials, and to domains other than long-term memory. Investigating the effects of COMT genetic variation on retrieval-induced forgetting for emotional stimuli will likely be of particular interest, as the Met allele has been associated with increased neural reactivity to emotional information,16 and the effects observed here might be attenuated or even reversed when using emotional study material. Regarding the possible domain generality of the cognitive control mechanism modulated by prefrontal dopamine, recent data suggest that high levels of retrieval-induced forgetting are associated with high working-memory capacity,51 and studies of working memory have found that individuals with high working-memory capacity are more capable of selectively activating task-relevant items.52, 53 Jointly, these findings point to a common factor underlying these different cognitive measures. Dopamine might be the major neuromodulatory transmitter regulating access to working memory, and COMT investigations have reported a positive effect of Met allele load on working memory performance.5, 15 Therefore, we speculate that the ability to suppress interfering information is a domain-general mechanism that is mediated by the prefrontal dopaminergic system.
Acknowledgments
We thank Christine Esslinger and Simon Hanslmayr for providing valuable comments on the manuscript, and Pierre Kurby, Ilona Wiedenhöft and Kerstin Möhring for assistance with data acquisition. This research was supported by the German Science Foundation (Deutsche Forschungsgemeinschaft), Grants DFG RI1847/1-1 and DFG SFB779TPA7 (both to AR-K), and by the Alexander von Humboldt Foundation (to AR-K).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- O'Reilly RC. Biologically based computational models of high-level cognition. Science. 2006;314:91–94. doi: 10.1126/science.1127242. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Herd SA, Pauli WM. Computational models of cognitive control. Curr Opin Neurobiol. 2010;20:257–261. doi: 10.1016/j.conb.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Düzel E, Bunzeck N, Guitart-Masip M, Wittmann B, Schott BH, Tobler PN. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32:321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Schott BH, Düzel E.The multiple roles of dopaminergic neurotransmission in episodic memoryIn: Dere E, Easton A, Nadel L, Huston HP (eds).Handbook of Behavioral Neuroscience : Handbook of Episodic Memory Elsevier: Amsterdam; 2008379–396. [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–328. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci. 2006;17:359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- De Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson L. COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav Genet. 2004;34:533–539. doi: 10.1023/B:BEGE.0000038491.06972.8c. [DOI] [PubMed] [Google Scholar]
- Krach S, Jansen A, Krug A, Markov V, Thimm M, Sheldrick AJ, et al. COMT genotype and its role on hippocampal-prefrontal regions in declarative memory. Neuroimage. 2010;53:978–984. doi: 10.1016/j.neuroimage.2009.12.090. [DOI] [PubMed] [Google Scholar]
- Schott BH, Seidenbecher CI, Fenker DB, Lauer CJ, Bunzeck N, Bernstein H, et al. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J Neurosci. 2006;26:1407–1417. doi: 10.1523/JNEUROSCI.3463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Need AC, LaBar KS, Waters-Metenier S, Cirulli ET, Kragel J, et al. COMT val108/158 met genotype affects neural but not cognitive processing in healthy individuals. Cereb Cortex. 2010;20:672–683. doi: 10.1093/cercor/bhp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, et al. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpicke JD, Roediger HL. The critical importance of retrieval for learning. Science. 2008;319:966–968. doi: 10.1126/science.1152408. [DOI] [PubMed] [Google Scholar]
- Roediger HL, Butler AC. The critical role of retrieval practice in long-term retention. Trends Cogn Sci. 2011;15:20–27. doi: 10.1016/j.tics.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Bjork RA, Bjork EL. Remembering can cause forgetting: retrieval dynamics in long-term memory. J Exp Psychol Learn Mem Cogn. 1994;20:1063–1087. doi: 10.1037//0278-7393.20.5.1063. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends Cogn Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S, Bäuml KHT. Theta oscillations reflect the dynamics of interference in episodic memory retrieval. J Neurosci. 2010;30:11356–11362. doi: 10.1523/JNEUROSCI.0637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimber M, Bäuml KHT, Bergström Z, Markopoulos G, Heinze H, Richardson-Klavehn A. Neural markers of inhibition in human memory retrieval. J Neurosci. 2008;28:13419–13427. doi: 10.1523/JNEUROSCI.1916-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Dudukovic NM, Kahn I, Wagner AD. Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat Neurosci. 2007;10:908–914. doi: 10.1038/nn1918. [DOI] [PubMed] [Google Scholar]
- Wimber M, Rutschmann RM, Greenlee MW, Bäuml KHT. Retrieval from episodic memory: neural mechanisms of interference resolution. J Cogn Neurosci. 2009;21:538–549. doi: 10.1162/jocn.2009.21043. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Battig WF, Montague WE. Category norms of verbal items in 56 categories: a replication and extension of the Connecticut category norms. J Exp Psychol. 1969;80:1–64. [Google Scholar]
- Scheithe K, Bäuml KHT. Deutschsprachige Normen für Vertreter von 48 Kategorien [German norms for representatives of 48 categories] Sprache Kognition. 1995;14:39–43. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith C, Frackowiak RSJ. Statistical parametric maps functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Roman P, Soriano MF, Gomez-Ariza CJ, Bajo MT. Retrieval-induced forgetting and executive control. Psychol Sci. 2009;20:1053–1058. doi: 10.1111/j.1467-9280.2009.02415.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Asper CM, Goldberg TE, Kolachana BS, Straub RE, Egan MF, Weinberger DR. Genetic variation in catechol-O-methyltransferase: effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biol Psychiatry. 2008;63:72–79. doi: 10.1016/j.biopsych.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa EC, Dickinson D, Apud J, Weinberger DR, Elvevåg B. COMT Val158Met polymorphism, cognitive stability and cognitive flexibility: an experimental examination. Behav Brain Funct. 2010;6:53. doi: 10.1186/1744-9081-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Weinberger DR, Malhotra AK, Goldberg TE.The role of COMT Val158Met in cognition Biol Psychiatry 200965e1–e2.author reply e3–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Callicott JH, Weinberger DR. Prefrontal cognitive systems in schizophrenia: towards human genetic brain mechanisms. Cogn Neuropsychiatry. 2009;14:277–298. doi: 10.1080/13546800903091665. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Constable RT, Lesch KP, Canli T. Influence of SLC6A3 and COMT variation on neural activation during response inhibition. Biol Psychol. 2009;81:144–152. doi: 10.1016/j.biopsycho.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, et al. Impact of complex genetic variation in COMT on human brain function. Mol Psychiatry. 2006;11:867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Williams HJ, Owen MJ, O'Donovan MC. Is COMT a susceptibility gene for schizophrenia. Schizophr Bull. 2007;33:635–641. doi: 10.1093/schbul/sbm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Minzenberg MJ, Ragland JD. The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry. 2008;64:18–25. doi: 10.1016/j.biopsych.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano MF, Jiménez JF, Román P, Bajo MT. Inhibitory processes in memory are impaired in schizophrenia: evidence from retrieval induced forgetting. Br J Psychol. 2009;100:661–673. doi: 10.1348/000712609X418912. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Piech R, Allen C, Niznikiewicz M, Shenton M, McCarley RW. Retrieval-induced forgetting in schizophrenia. Schizophr Res. 2005;75:199–209. doi: 10.1016/j.schres.2005.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm BC, White HA. ADHD and retrieval-induced forgetting: evidence for a deficit in the inhibitory control of memory. Memory. 2010;18:265–271. doi: 10.1080/09658210903547884. [DOI] [PubMed] [Google Scholar]
- Aslan A, Bäuml KHT. Individual differences in working memory capacity predict retrieval-induced forgetting. J Exp Psychol Learn Mem Cogn. 2010;36:54–65. doi: 10.1037/a0021324. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.