Abstract
We find that a common mutation that increases angiotensin I-converting enzyme activity occurs with higher frequency in male patients suffering from refractory temporal lobe epilepsy. However, in their brains, the activity of the enzyme is downregulated. As an explanation, we surprisingly find that carbamazepine, commonly used to treat epilepsy, is an inhibitor of the enzyme, thus providing a direct link between epilepsy and the renin–angiotensin and kallikrein–kinin systems.
Keywords: angiotensin I-converting enzyme, carbamazepine, epilepsy, TLE
Introduction
Epilepsy is a chronic neurological disease characterized by abnormal neural activity leading to epileptic seizures afflicting ∼1% of the population worldwide. We have recently found that temporal lobe epilepsy (TLE) patients show increased expression of the Angiotensin II (Ang II) AT1 and AT2 receptors1 as well as the kinin B1 and B2 receptors in their brain,2 thus providing a link between one of the most common forms of epilepsy, TLE associated to mesial sclerosis, to the so-called renin–angiotensin (RAS) and kallikrein–kinin systems (KKS).1, 2 These systems, associated with regulation of blood pressure and several functions in the central nervous system,1 consist of a series of regulatory proteolytic steps, including conversion of angiotensinogen by renin to angiotensin I (Ang I), which is further degraded by angiotensin I-converting enzyme (ACE) to Ang II and conversion of kininogen by kallikrein to the peptide bradykinin, which in turn can be degraded by ACE.3
Because of its dual function as an angiotensin processing and kinin degrading enzyme, we therefore undertook the task to examine patients suffering from epilepsy for the occurrence of the common ACE insertion (I)/deletion (D) polymorphism, where the introduction of a 287-bp Alu-repeat sequence into intron 16 of the ACE gene has been shown to induce lower ACE plasma activities in the carriers of this mutation.4, 5
Our surprising finding in this report that a majority of patients suffering from severe forms of epilepsy belong to the DD genotype, led us to postulate that distinct activities of this enzyme (levels of angiotensin/kinins) might be involved in TLE. Aiming to corroborate these results, we then tested directly the hypothesis that polypharmacological effects of current available drugs could have effects mediated by ACE inhibition, leading to the finding that carbamazepine, one of the most commonly used drugs in the treatment of epilepsy and psychiatric disorders is a direct inhibitor of ACE.
Methods
Genotyping
The ACE I/D polymorphism was genotyped as described6 in 72 patients with epilepsy who underwent surgery and 368 controls. All subjects abstained from alcohol and caffeine intake for at least 24 h before the blood tests. Smokers and subjects who had received antihypertensive drugs were excluded from the control group. Each subject gave informed consent before collection of DNA at the beginning of the experimental procedure. The genotypes of all experimental groups are shown in Supplementary Table 1.
Surgical procedures
Surgical specimens from patients with intractable epilepsy were submitted to standard corticoamygdalohippocampectomy at the São Paulo Hospital (Epilepsy Research and Treatment Unit, UNIPETE, São Paulo, Brazil) for seizure control. All cases showing neoplasm, vascular malformations, post-traumatic and ischemic lesion in preoperative magnetic resonance imaging (MRI) were excluded. Selected patients (n=9) had detailed anamnesis, video electroencephalogram recordings, and MRI studies. Antiepileptic drugs used by these patients for seizure control included: carbamazepine, phenobarbital, valproic acid and phenytoin. Surgery was performed at least 48 h after the last seizure. The outcome of seizures after epilepsy surgery was classified according to Engel's four categories.7 Control tissues were obtained from autopsies (less than 5 h post mortem) (n=9) from brains showing no evidence of pathology based on a gross classification after routine histological examination, and were obtained from autopsies carried out by a pathologist trained especially for this purpose from the Anatomical Pathology Department (INCOR, FMUSP). By using this procedure, similar hippocampal areas from epileptic patients and autopsied subjects could be compared. Hippocampi were dissected, frozen in liquid nitrogen and stored at −80 °C. All experiments were performed under approval from the Institutional Ethics Committee of the Federal University of São Paulo (UNIFESP).
ACE activity assays
Somatic ACE activity from human hippocampi was assayed using a fluorescence resonance energy transfer substrate containing ortho-aminobenzoic (Abz) and dinitrophenyl (Dnp) as fluorescence donor/acceptor pair, namely Abz-FRK(Dnp)P-OH, as described.8 ACE specific activity for each tissue was calculated by division of velocity values in μ min−1 by the respective protein concentrations, as determined by the Bradford method (Bio-Rad Laboratories, Hercules, CA, USA). Activity of purified, or plasma ACE (control n=127; TLE n=43) was measured in the same way but normalized either by the enzyme amount or plasma volume, respectively. In the cellular assays, Chinese hamster ovary cells (CHO) were transfected with human ACE and its activity evaluated either with the fluorescence resonance energy transfer substrate Abz–FRK(Dnp)P–OH, as described,9 or with the endogenous substrate Ang I as assayed by high-performance liquid chromatography (HPLC). Measurements were done in triplicate and ACE activity was reported as μ of Abz-FRK(Dnp)P-OH hydrolyzed per minute of substrate hydrolyzed after 6 min of incubation in the presence of different concentrations of carbamazepine. For the determination of the inhibitory effect of carbamazepine on ACE hydrolytic activity with Ang I, samples were preincubated for 2 h with different concentrations of carbamazepine. Samples were then incubated in the same buffer with 10 μ of Ang I (Sigma-Aldrich, St Louis, MO, USA), in the presence or absence of 2.5 μ of lisinopril. Supernatant aliquots (400 μl) were collected after 0, 2, 5, 15 and 30 min, and the reaction was stopped by the addition of 2 μl 10% trifluoroacetic acid (TFA). The conversion of Ang I to Ang II was determined by HPLC analysis using a Vydac C18 (4.6 × 150 mm) column equilibrated with solvent A (0.1% TFA/H2O) and eluted with solvent B (0.1% TFA; 60% acetonitrile/H2O) with a 30–45% acetonitrile gradient of solvent B for 15 min, with a flow of 1.5 ml min−1 and detection at 220 nm wavelength. Some peaks were collected and analyzed in an automatic amino-acid sequencer to confirm their sequence (data not shown). Measurements were made in duplicate and the values of ACE activity were shown as percentage of basal Ang I degradation per minute per milligram of total protein (%min−1mg−1).
Kallikrein assay
Assays were performed using purified enzyme (0.57 μg ml−1; Enzyme Research Laboratories, South Bend, IN, USA) in 50 m Tris-HCL buffer (pH 7.4) using the fluorogenic substrate Z-Phe-Arg-AMC as described.6
Animal model
C57Bl/6 mice were obtained from the Center for the Development of Experimental Models for Medicine and Biology (CEDEME) at the Federal University of São Paulo and were kept at 22 °C in a light–dark cycle (12 in 12 h), with ad libitum supply of water and standard rodent chow. Carbamazepine was administered in mice by gavage, at a dose of 150 μg kg−1. After 60 min, animals were killed by cervical dislocation for blood collection; plasma was extracted by centrifugation and stored at –80°C. All experiments were conducted in accordance with the NIH guide for care of laboratory animals and their uses (Institute of Laboratory Animal Resources, National Academy Press, Washington DC, 1996) after approval by the Ethics Committee in Research of UNIFESP.
Statistics
All data are given as mean±s.e.m. Statistical evaluations were performed using Student's t-test for unpaired data or one-way analysis of variance followed by the Tukey's post-hoc test to identify significant data points. Genotype frequencies observed in our cohort were in Hardy–Weinberg equilibrium. Results were considered significant with P<0.05.
Results and Discussion
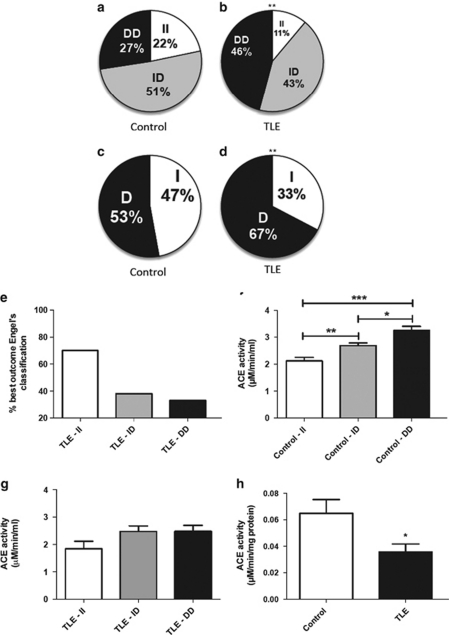
Owing to the association between ACE and epilepsy, we examined male patients suffering from refractory TLE for the occurrence of the common ACE insertion (I)/deletion (D) polymorphism, which is known to induce lower ACE plasma activities in II subjects.4 Surprisingly, we found a significant higher number of patients suffering from TLE with the DD genotype (46%) when compared with the normal subjects (27% Figures 1a–d). Concomitantly, TLE patients of both sexes with the II genotype, that express less ACE, showed a significantly better recovery rate after corticoamygdalohippocampectomies (Figure 1e). As the D form leads to higher expression levels of ACE, we expected to be able to measure higher ACE activities in the plasma of male patients suffering from TLE. ACE activity was indeed significantly elevated in subjects with the DD genotype of the control group. However, we could only detect a non-significant trend to higher ACE activities in patients suffering from TLE with the DD genotype (Figures 1f and g). Furthermore, when we examined ACE activity in the hippocampus from seizure-control corticoamygdalohippocampectomies, we detected a twofold lower ACE activity in these patients (Figure 1h).
Figure 1.
ACE activity and TLE. (a, b) Distributions of the I/D ACE polymorphism in the control and TLE group (control n=368; TLE n=72). (c, d) Allele frequencies. (e) Percentage of class 1A (best) outcome (Engel's classification7) depending on genotype. (f, g) Mean (±s.e.m.) soluble ACE activity according to I/D distribution in the control group (n=127) and in patients with TLE (n=43). (h) Hippocampal tissue ACE activity. *P< 0.05, **P<0.01, ***P<0.001.
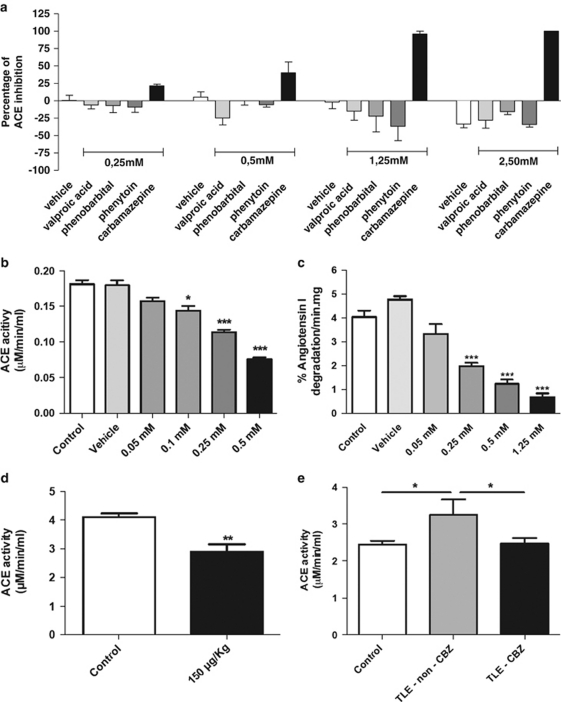
As these results seemed contradictory, we therefore hypothesized that if higher ACE expression is casually involved in the pathogenesis of TLE, perhaps its treatment could be linked to a reduction of ACE activity. Consequently, we decided to screen biochemically for the effects on ACE activity of different drugs commonly used to treat epilepsy. All the drugs tested showed insignificant changes in ACE activity, except carbamazepine, which significantly inhibited ACE activity in a dose-dependent manner (Figure 2a). Carbamazepine is normally used to treat TLE in doses of 400–1200 mg per day,9 where the minimal dose (400 mg per day) is equivalent to a concentration of 0.34 m in the blood. At this level, carbamazepine was able to inhibit ∼25% the plasma activity of human ACE (Figure 2a).
Figure 2.
Inhibition of ACE by carbamazepine. (a) Inhibitory profile of different drugs used in the treatment of epilepsy. Carbamazepine was the only antiepileptic drug able to inhibit plasmatic ACE activity. (b) Enzymatic activity of purified ACE. Mean (±s.e.m.) (*P<0.05, ***P<0.001). As a non-specificity control, carbamazepine had no inhibitory effect on either human plasmatic or purified kallikrein (Supplementary Figure 2). (c) HPLC assay demonstrating carbamazepine inhibition of Ang I degradation by ACE (***P< 0.001). (d) Mean (±s.e.m.) plasmatic ACE activity is decreased 60 min after carbamazepine administration by gavage (150 μg kg−1) in mice (**P<0.01). (e) Mean (±s.e.m.) plasmatic ACE activity of TLE patients treated with carbamazepine (n=35) resembles that of the control group (n=55), whereas non-carbamazepine-treated TLE patients (n=15) have significantly higher ACE activities (*P<0.05).
To exclude artifacts from other proteases present in blood plasma, we repeated this experiment using purified ACE corroborating our results (Figure 2b). Inhibition of ACE by carbamazepine was additionally confirmed in a dose-dependent manner using HPLC to identify direct degradation of the native Ang I peptide by the enzyme produced in ACE-transfected CHO. Carbamazepine was able to inhibit ∼75% of ACE at the cellular level (Figure 2c, Supplementary Figure 1). Taken together all these independent experiments indicate in vitro direct inhibition of ACE by carbamazepine at concentrations similar to those used by TLE patients. To analyze the action of carbamazepine in vivo, we fed mice with a dose of 150 μg per kg of carbamazepine. The mice were killed after 60 min, and their serum analyzed for ACE activity. Again in this experiment we could confirm ∼25% inhibition of ACE activity by carbamazepine (Figure 2d). Finally, direct inhibition of ACE by carbamazepine was additionally confirmed by comparing treated with non-carbamazepine-treated TLE patients. In this experiment, treatment with carbamazepine led to a similar ACE activity as in the control group, whereas non-carbamazepine-treated TLE patients had a higher ACE activity (Figure 2e). However, we did not observe blood pressure differences between the treated TLE and non-treated TLE groups (Supplementary Table 2).
All components of the RAS and KKS have been found in the brain10 modulating functions such as stress, anxiety, learning and memory acquisition.11 Under pathological conditions the RAS promotes inflammatory responses increasing neural excitability,12 glial scar formation and transformation of a normal neural network into a seizure-generating network, thus providing a basis for a linkage between angiotensin signaling and epilepsy.13 Our results provide further evidence for this hypothesis, as we find a higher proportion of TLE patients with the DD ACE polymorphism phenotype, which leads to higher ACE activity levels of the enzyme. Furthermore we surprisingly find that carbamazepine is an inhibitor of ACE in the concentration range used to treat patients.
It has been recently reported that TLE patients show increased expression of the Ang II AT1 and AT2 receptors1 as well as the kinin B1 and B2 receptors in the hippocampus2 and that captopril, a classical ACE inhibitor, enhances the anticonvulsant activity of carbamazepine.14 These results indeed strengthen the case for a link between the RAS and KKS and the pathogenesis of epilepsy. Furthermore, as carbamazepine is thought to display anticonvulsant activity due to its inhibition of sodium channels,9 our results enrich once again the list of known drugs with polypharmacological effects.
Acknowledgments
We acknowledge financial support from INNT, CNPq, CAPES CInAPCe and FAPESP.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Arganaraz GA, Konno AC, Perosa SR, Santiago JF, Boim MA, Vidotti DB, et al. The renin-angiotensin system is upregulated in the cortex and hippocampus of patients with temporal lobe epilepsy related to mesial temporal sclerosis. Epilepsia. 2008;49:1348–1357. doi: 10.1111/j.1528-1167.2008.01581.x. [DOI] [PubMed] [Google Scholar]
- Perosa SR, Arganaraz GA, Goto EM, Costa LG, Konno AC, Varella PP, et al. Kinin B1 and B2 receptors are overexpressed in the hippocampus of humans with temporal lobe epilepsy. Hippocampus. 2007;17:26–33. doi: 10.1002/hipo.20239. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Hagaman JR, Kim HS, Smithies O. Minireview: computer simulations of blood pressure regulation by the renin-angiotensin system. Endocrinology. 2003;144:2184–2190. doi: 10.1210/en.2002-221045. [DOI] [PubMed] [Google Scholar]
- Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danser AH, Schalekamp MA, Bax WA, van den Brink AM, Saxena PR, Riegger GA, et al. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- Almeida SS, Barros CC, Moraes MR, Russo FJ, Haro AS, Rosa TS, et al. Plasma kallikrein and angiotensin I-converting enzyme N- and C-terminal domain activities are modulated by the insertion/deletion polymorphism. Neuropeptides. 2010;44:139–143. doi: 10.1016/j.npep.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Carmona AK, Schwager SL, Juliano MA, Juliano L, Sturrock ED. A continuous fluorescence resonance energy transfer angiotensin I-converting enzyme assay. Nat Protoc. 2006;1:1971–1976. doi: 10.1038/nprot.2006.306. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Paul M, Bader M, Steckelings UM, Voigtlander T, Ganten D. The renin-angiotensin system in the brain. Localization and functional significance. Arzneimittelforschung. 1993;43:207–213. [PubMed] [Google Scholar]
- Wright JW, Harding JW. The brain angiotensin system and extracellular matrix molecules in neural plasticity, learning, and memory. Prog Neurobiol. 2004;72:263–293. doi: 10.1016/j.pneurobio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Das UN. Is angiotensin-II an endogenous pro-inflammatory molecule. Med Sci Monit. 2005;11:RA155–RA162. [PubMed] [Google Scholar]
- Lukawski K, Jakubus T, Raszewski G, Czuczwar SJ. Captopril potentiates the anticonvulsant activity of carbamazepine and lamotrigine in the mouse maximal electroshock seizure model. J Neural Transm. 2010;117:1161–1166. doi: 10.1007/s00702-010-0455-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.