Abstract
Aberrant expression of the presynaptic serotonin 1A receptor (5-HT1A-R) because of a polymorphism in the 5-HT1A-R gene is associated with severe depression in human, whereas its absence up to postnatal day 21 (P21) in the forebrain of mice results in heightened anxiety in adulthood. These observations collectively indicate that the 5-HT1A-R has a crucial role in brain development. To understand the mechanistic underpinnings of this phenomenon, we used organotypic slice cultures of hippocampi from C57BL6 mice (C57) at P15, which coincides with the peak of neonatal synaptogenesis. Stimulation of the hippocampal 5-HT1A-R caused a dramatic increase in PSD95 expression and dendritic spine and synapse formation through sequential activation of the mitogen-activated protein kinase isozymes Erk1/2 and protein kinase C (PKC). Intrahippocampal infusion of 5-HT1A-R agonists and signaling inhibitors at P15 revealed that the same pathway through PKCα augments PSD95 expression and synaptogenesis in vivo in 24 h in both C57 as well as Swiss Webster mice. Furthermore, intrahippocampal infusion of the antidepressant fluoxetine, a serotonin reuptake inhibitor, also augmented PSD95 expression and synaptogenesis through the same pathway. This increased synaptogenesis was observed even 5 days after treatment. Finally, compared with the wild type, the 5-HT1A-R(−/−) mice harbor significantly less synapses in the hippocampus, but infusion of the PKC-stimulator and Alzheimer drug bryostatin into the 5-HT1A-R(−/−) mice to bypass the non-existent 5-HT1A-R boosted PSD95 expression and synaptogenesis. The elucidated signaling cascade explains how 5-HT1A-R regulates hippocampal sculpting and function, which may determine the affective phenotype of an adult.
Keywords: hippocampus, 5-HT1A receptor, neonatal, PKCalpha, synaptogenesis
Introduction
Signaling activity mediated by the brain serotonin 1A receptor (5–HT1A-R) has been implicated in a large number of behavioral abnormalities. Both decreased as well as increased 5–HT1A-R signaling in the brain have been linked to a number of affective disorders. In post-mortem human studies, increased 5–HT1A-R agonist binding has been observed in the frontal cortex of schizophrenia patients.1 Positron emission tomography have indicated above-normal levels of serotonin in the brain of adolescent autistic children,2 which may elicit elevated 5–HT1A-R signaling. Similarly, in mouse studies, an increase in brain serotonin because of monoamine oxidase A-deficiency during the critical period of development resulted in the lack of barrels in the somatosensory cortex.3 Conversely, other studies showed that a systemic absence of the 5-HT1A receptor was associated with elevated levels of anxiety.4, 5, 6 More recent experiments have shown that elimination of the presynaptic 5-HT1A auto receptors but not the heteroreceptors from P14 is sufficient for the development of anxiety phenotype in adulthood.7 The same report submits that elimination of the 5-HT1A heteroreceptors (but not the autoreceptors) starting from early brain development elicits increased behavioral despair, which is believed to be the mouse equivalent of depression. Further emphasizing the importance of brain 5-HT1A-R levels, an earlier report showed that a C-1019G polymorphism in the promoter of the 5-HT1A receptor gene causes de-repressed expression of the 5-HT1A autoreceptor selectively in the serotonergic raphé neurons and suggest a downregulation of the same receptor in the postsynaptic neurons.8 This is associated with severe depression and suicide.9 Similarly, attenuated 5-HT1A-R expression has been reported in the cortex of suicide victims.10 Finally, post-mortem analysis of human brain samples revealed that reduced 5-HT1A-R binding in the temporal cortex correlates with aggressive behavior in Alzheimer patients.11
Thus, prior studies strongly indicate that an optimal level of 5-HT1A-R signaling is required during brain development for normal affect in adulthood. However, the biochemical cascades, which are initiated by serotonin binding to the 5-HT1A-R and the identity of downstream signaling proteins that help in the formation of brain structures and circuitry during neonatal brain development have remained unclear. This study focuses on the hippocampus, which harbors high levels of the 5-HT1A heteroreceptors, to address four fundamental issues: (i) If increased 5-HT1A-R signaling affects neonatal synaptogenesis; (ii) What signaling pathway is involved in this process? (iii) Does the absence of this cascade in the 5-HT1A-R(−/−) mice affect synaptogenesis? (iv) Could we normalize synaptogenesis by stimulating a downstream member of this pathway in the 5-HT1A-R(−/−) mice?
Our earlier studies in mouse brain slices demonstrated a 5-HT1A-R-evoked increase in field excitatory postsynaptic potential (fEPSP) in the Schaffer Collateral pathway of the hippocampus at P15.12 This report also showed that a 5-HT1A-R-linked mitogen-activated protein kinase pathway, linked to protein kinase C (PKC), was involved in the boosted synaptic activity. The current study addresses the possibility that this pathway is operant in vivo in the P15 hippocampus and it regulates synaptogenesis, which is known to be at its peak in the cortex at P15.13
Results presented here establish the importance of a synaptogenic pathway involving 5-HT1A-R, Erk and PKCα in the neonatal hippocampus. Hyper-normal levels of cortical serotonin is believed to occur in autism 2 and also with the use of selective serotonin reuptake inhibitors during adolescence. On the basis of our results, such a hyperserotonemic state could elicit aberrant hippocampal development via unwanted synaptogenesis through the same signaling pathway, thereby causing local overconnectivity, which is believed to occur in autism.14
Materials and methods
Reagents
Animals
C57BL6 5-HT1A-R(+/−) mice were obtained as a kind gift from Dr Laurence Tecott 4 and then bred to obtain 5-HT1A-R(−/−) mice. Genotyping was performed by PCR using the following primers: Wild type: Fwd 5′-ctgctcatgctggtcctctatg-3′, Rvs 5′-taggaggtagctcctgattcgc-3′ (product: 323 bp); KO: Fwd 5′-caccttgctcctgccgagaaa-3′, Rvs 5′-agaaggcgatagaaggcgatg-3′ (product: 464 bp). Swiss Webster (SW) 5-HT1A-R(−/−) mice were obtained from Dr Toni Shippenberg.15 Mice were housed in the College of Staten Island Animal Care Facility and handled following a protocol approved by the CSI Institutional Animal Care Committee.
Hippocampal slice culture
Details of the procedure for hippocampal slice culture have been reported earlier and also included in the Supplementary Methods.12
Western blotting
Details of the western-blotting procedure have been included in the Supplementary Methods.
Intra-hippocampal injections
(See Supplementary Methods for details). Injections were made at stereotaxic coordinates corresponding to Bregma: anterioposterior=−1.8 mm, mediolateral=−1.5 mm, dorsoventral=−1.8 mm. This corresponds to a site in the dorsal hippocampus in the apical dendritic zones of the CA1 region near the hippocampal fissure.16 A 10-μl Hamilton syringe was used to deliver the drugs into the hippocampi of the pups17 at the rate of 1 μl per minute using a stereotaxic set-up (KDS Model 310 plus infusion-withdrawal syringe pump).
Drug concentrations and infusion strategy
(See Supplementary Methods for details). Drug concentrations were made for the observed hippocampal volume of 10 μl at P15. Inhibitors were infused 30 min before infusing 8-OH-DPAT or fluoxetine.
Immunohistochemistry
(See Supplementary Methods for details). Hippocampal sections were blocked in (0.1% Triton X-100, 10% serum from the animal used to raise the secondary antibody-in 1 × PBS) for overnight at 4 °C. This was followed by treatment with primary antibody in antibody solution (0.1% Triton X-100, 2% serum-in 1 × PBS) for 48 h at 4 °C and then with fluorescent secondary antibody covalently linked to Alexa Fluor 488 (Green) or Alexa Fluor 568 (Red) for 24 h.
Confocal microscopy of the immunostained slices, fluorescence quantification, counting of spine numbers and statistical analysis
(See Supplementary Methods for details). All the images were acquired with the frame average of four using a Nikon C1-LU3 laser-scanning confocal system (Nikon Instruments Inc., 1300 Walt Whitman Road, Melville, NY, USA). The Nikon EZ-C1-system software was used to determine the total thickness of each slice after adjusting channels to obtain pictures from each exciting wavelength separately. In addition to images taken at × 4 and × 20, Z-stacks were also acquired at × 40 and such volume-rendered representative images were used in the figures shown in this report. All the values were then converted to percent ‘Carrier-treated'. Statistical analysis was carried out using ANOVA with Bonferroni post hoc test.
Electron microscopic studies
Animals were anesthetized with the ketamine+xylazine mixture according to the suggested dosage and were perfused intra-cardially with 2.5% glutaraldehyde+4% paraformaldehyde for 15 min. Brains were removed and were post fixed in the same perfusion solution overnight. Hippocampi were removed and 1-mm thick sections were cut. After washing with 0.1 PBS for 30 min (3 × ) brain sections were post-fixed in 1% osmium tetra-oxide for 90 min.18 Sections were rinsed again with 0.1 PBS for 30 min and dehydrated with graded ethanol (50%, 70%, 85%, 95%, 2 × 100%) for 10 min each step, infiltrated with propylene oxide and plastic Spurr medium, and finally embedded with pure Spurr medium19 in BEEM capsules and polymerized in oven at 70 °C overnight. Ultrathin sections (70–100 nm) were placed on uncoated 200 mesh copper grids, stained with saturated uranyl acetate in 50% ethanol for 2 min, rinsed with 0.22 μ Millipore-filtered distilled water for 2 min to obtain clean sections without any trace of uranyl acetate residue, stained with Reynolds's lead citrate for 90 s, and rinsed with Millipore-filtered distilled water for 90 s.20 Sections were examined and imaged with a Hitachi 7500 electron microscopy operated at 80 kV. Quantification was achieved using about 10 sections per mouse hippocampus and three mice were used for each treatment set.
Results
The post synaptic density (PSD) protein of 95 Da, PSD95—a postsynaptic membrane-associated protein with a guanylate kinase-like domain without any kinase activity—is an established postsynaptic marker of spine and synapse maturation.21, 22, 23, 24 In neuronal signaling, it is reported to exert a major influence on synaptic strength and plasticity by recruiting AMPA receptors to the postsynaptic membrane.22, 23 On the basis of this information, we used PSD95 expression and electron microscopy to demonstrate 5-HT1A-R-mediated synaptogenesis in the P15 hippocampus and also elucidate the signaling cascade involved in this process.
Stimulation of the 5-HT1A-R in organotypic cultures of P15 hippocampal slices augments PSD95 expression, spine formation and synaptogenesis
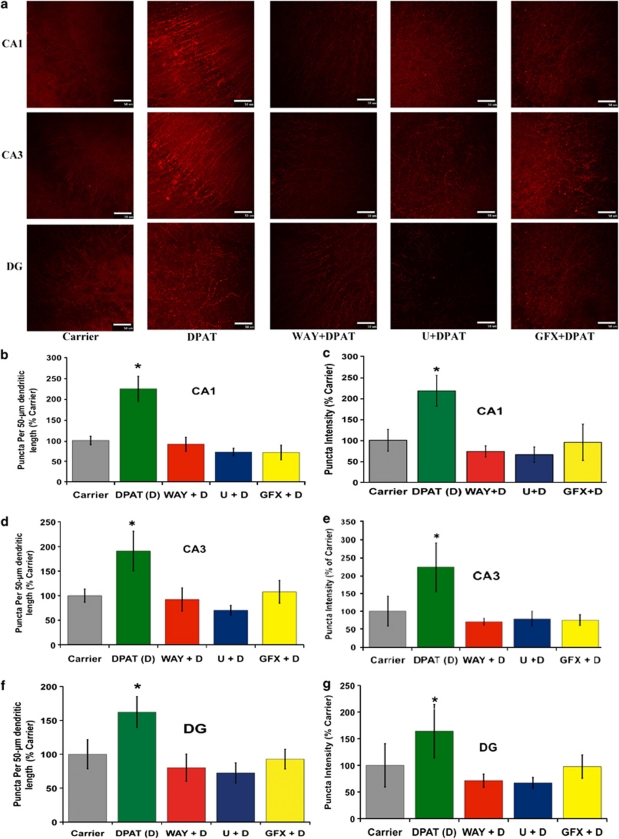
A dramatic 5-HT1A-R-evoked increase in PSD95 expression was evident from immunohistochemistry (Figure 1a) and it was inhibited in presence of WAY100635 and the pharmacological inhibitors of MEK (U0126, 10 μ) and PKC (bisindolylmaleimide I or GFX, 2 μ), respectively (Figures 1a–d). PSD95 was shown as punctuate staining in the spines. The number of puncta per unit length of the dendritic spines and puncta intensity representing the number of PSD95 molecules per spine showed a striking increase in presence of the 5-HT1A-R agonist 8-OH-DPAT (DPAT). This DPAT-evoked increase in spine formation was eliminated in presence of WAY, U0126 and GFX, thereby confirming the involvement of MEK → Erk1/2 signaling and PKC in the 5-HT1A-R-mediated induction of PSD95.
Figure 1.
Increased PSD95 in dendritic spines upon 5-HT1A-R activation in cultured hippocampal slices: involvement of Erk1/2 and PKC. Organotypic cultures of P15 hippocampal slices at 6DIV were treated with DPAT (100 n) (abbreviated as D in the graphs) in the absence and presence of WAY100635 (4 μ) (WAY), U0126 (10 μ) (U), or GFX (2 μ) for 16 h (Scale bar: 50 μ). The slices were fixed and immunostained to detect PSD95 expression in dendritic spines. DPAT treatment caused a significant increase in both PSD95 expression (puncta intensity, c, e, and g) as well as spine number per unit length (b, d, and f). This increase in PSD95 and spine density was eliminated in the presence of WAY100635, U0126 and GFX (a-g). Quantification from three experiments each with triplicate slices revealed significance (b, d, and f: *P<0.001 DPAT versus all other groups); (c, e, and g: *P<0.001 DPAT versus all other groups; n=3).
We also monitored the influence of 5-HT1A-R signaling on synaptogenesis in organotypic cultures of hippocampal slices by electron microscopy. The cultured P15 hippocampal slices at 6DIV were treated with the 5-HT1A-R agonist 8-OH-DPAT (DPAT) or carrier to show that the DPAT-treated slices harbored a significantly higher number of excitatory synapses (P<0.0001), which were marked by the presence of post-synaptic densities (Supplementary Figures 1a and b). Simultaneously, we used western blotting to monitor the influence of 5-HT1A-R signaling on PSD-95 expression in cultured hippocampal slices from P15 mice. We observed a significant increase in PSD95 expression in the presence of DPAT (100 n). The PSD95 levels reached a peak after 16 h of DPAT treatment (unpublished data) and the induction was abrogated in the presence of the 5-HT1A-R antagonist WAY100635 (WAY) (4 μ), thus confirming the involvement of the 5-HT1A-R in the induction of PSD95 (Supplementary Figure 1c).
Intra-hippocampal infusion of DPAT and inhibitors into P15 mice elicit PSD95 induction in vivo through the 5-HT1A-R, Erk1/2, PKCα pathway
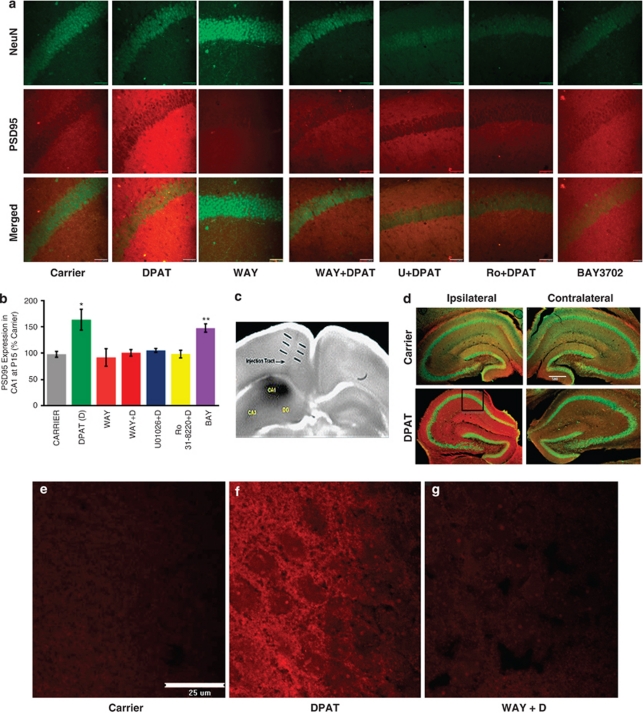
Our earlier studies in the hippocampal HN2-5 cells and cultured hippocampal slices indicated that a 5-HT1A-R → → ERK → PKCα pathway has an important role in neuroprotection as well as increased synaptic activity.12, 25 In order to confirm that the same 5-HT1A-R → → ERK → PKCα pathway also functions in vivo, we performed intra-hippocampal injections26 of DPAT with or without the pharmacological inhibitors into the right hippocampus of P15 C57BL6 muse pups (Figures 2c and d). After 24 h, the pups were subjected to intracardiac perfusion of paraformaldehyde followed by isolation of the ipsilateral (right) and contralateral hippocampi for sectioning into 30-μ slices. The sections were processed for immunohistochemistry. The red staining for PSD95 was markedly higher in the ipsilateral hippocampus from the DPAT-injected brains than in the contralateral hippocampus or the hippocampi from carrier-treated brains (Figure 2d). Zooming in on a CA1 region (indicated by the square area), we found that this increase in the PSD95 staining was absent or sharply diminished in slices that received WAY100635, U0126 or Ro 31-8220 (a selective PKCα inhibitor ⩽10 n; IC50=5 n) in addition to DPAT18 (Figures 2a and b). This strongly indicates that the same 5-HT1A-R mediated signaling pathway as shown above in Figure 2 also boosts the expression of PSD95 in vivo.
Figure 2.
A 5-HT1A-R-mediated signaling pathway involving Erk1/2 and PKCα causes heightened expression of PSD95 in P15 mouse hippocampus. (a) A DPAT (100 n)-evoked induction in PSD95 expression in 24 h is eliminated in the presence of WAY100635 (4 μ) (WAY), U0126 (10 μ) (U), and the selective PKCα inhibitor Ro 31-8220 at 10 n (Ro). A second 5-HT1A-R agonist BAY3702 (100 n) also causes induced PSD95 expression (Scale bar: 50 μ). (b) Densitometric quantification from three infusions (three mice and quadruplicate sections from each mouse) shows a significant induction of PSD95 following infusion of DPAT or BAY3702 (BAY) (*P<0.001 DPAT versus all other groups except BAY3702; **P=0.1345 DPAT versus BAY3702) (n=3). (c) The injection track and the site of injected drugs in the dorsal hippocampus marked by Coommassie Blue. (d) The ipsilateral hippocampus shows DPAT-evoked induction of PSD95 (red), whereas the contralateral hippocampus does not. These images were acquired at × 4 magnification (Scale: 500 μ). The square area in the CA1 region of the ipsilateral hippocampus was imaged at × 40 and then used for the comparison of histochemical staining presented in panel a. (e–g) High-magnification images acquired at × 100 show strong, punctate, PSD95 immunoreactivity in numerous dendrites and spines in the CA1 region of a DPAT-infused hippocampus.
It should be noted that a P15 hippocampal slice cultured 6 days in vitro (6DIV) maintains its overall structure but spreads out significantly on the substratum (membrane). This allows the individual PSD95-immunopositive dendrites and spines to be imaged clearly (Figure 1). In contrast, the cryosections of the freshly isolated hippocampi from in vivo-treated brains harbor densely packed dendrites, thus yielding strong PSD95 immunostaining in the entire hippocampus (Figure 2). High-magnification images acquired at × 100 show the presence of punctate PSD95 immunostaining on the dendrites and spines especially following 8-OH-DPAT treatment (Figures 2e–g).
Two-dimensional cultures of hippocampal neurons form synaptic connections in the horizontal plane, which allows for double staining of pre- and postsynaptic terminals for specific markers such as synaptophysin (pre-synaptic) and PSD95 (postsynaptic) and confocal imaging of both in the same plane. In sharp contrast, hippocampal tissue in the 6DIV cultures as well as the cryosections from in vivo treatment were three-dimensional units, in which simultaneous imaging of pre- and postsynaptic terminals was difficult to achieve by confocal microscopy. Therefore, in addition to demonstrating the induction of the postsynaptic protein PSD95, we have performed electron microscopy to image and quantify the hippocampal synapses.
Increased synaptogenesis via PKCα downstream of 5-HT1A-R in C57BL6 mouse hippocampus
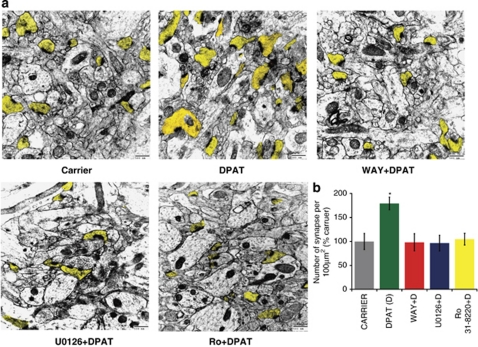
In order to determine the role of 5-HT1A-R mediated pathway in synaptogenesis in vivo we performed electron microscopic analysis on hippocampi obtained from brains that were infused intra-hippocampally with DPAT (final intrahippocampal concentration of 100 n) in the absence or presence of different pharmacological inhibitors. We observed that DPAT treatment caused a significant increase in the number of excitatory synapses in the hippocampal CA1 region. This increase in synaptogenesis was abolished in presence of any of the inhibitors: U0126, Ro 31-822018 and WAY. These results confirm that 5-HT1A-R-mediated signaling via Erk and PKCα causes induced synaptogenesis in vivo in P15 mouse hippocampus (Figure 3).
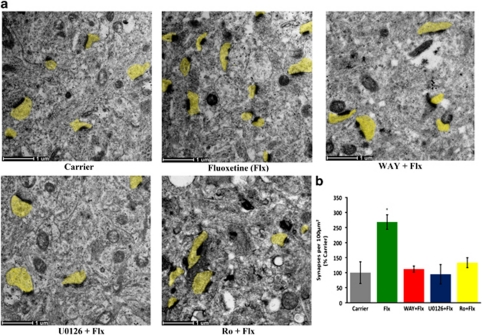
Figure 3.
The 5-HT1A-R, Erk, PKCα signaling cascade induces synaptogenesis in the P15 hippocampus. (a) Electron microscopy revealed a significant increase in synapse number in the hippocampal CA1 region of the DPAT-infused mice. This DPAT-stimulation of synaptogenesis was eliminated in the presence of WAY100635 (4 μ), U0126 (10 μ) and Ro 31-8820 (Ro) (Scale bar: 500 nm; post-synaptic terminals highlighted in yellow). (b) Quantification from three mice with eight sections per mouse revealed a significant effect of 5-HT1A-R signaling, which was eliminated in the presence of U0126 and Ro 31-8820 (*P<0.001 for DPAT versus all other groups; n=3).
Intrahippocampal infusion of fluoxetine causes 5-HT1A-R-, Erk1/2-, and PKCα-dependent induction of PSD95
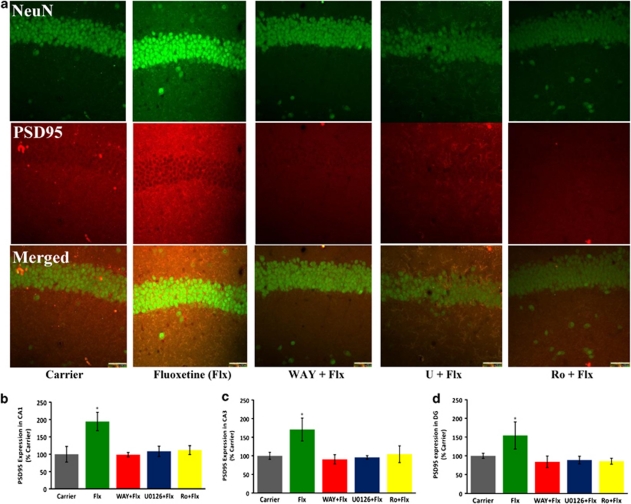
The optimum concentration of 5-HT1A agonist 8-OH-DPAT (100 n) for the activation of Erk1/2 had been determined earlier from in vitro experiments using organotypic cultures of P15 hippocampal slices.12 Although the same intrahippocampal concentration of 8-OH-DPAT allowed us to confirm that a 5-HT1A-R-mediated signaling through Erk1/2 and PKCα causes PSD95 induction and synaptogenesis, the physiological relevance of this phenomenon could be elucidated only through an increase in intrasynaptic serotonin concentration above the basal levels (<1 n) in the P15 mouse hippocampus.15, 27 This has been typically achieved by administering fluoxetine (Prozac), which is an inhibitor of the serotonin reuptake protein SERT. Albeit the same effect from systemic infusion in adult mice,15, 27 direct intracortical infusion of a fluoxetine analog also causes a 3-5-fold increase in extracellular serotonin within 60 min.28 We investigated whether such augmented serotonin levels at the hippocampal synapses would elicit the same 5-HT1A-R, Erk and PKCα-mediated synaptogenic signaling in P15 mice. As shown in Figures 4 and 5, intrahippocampal infusion of fluoxetine into P15 mice caused a significant increase in PSD95 expression and synaptogenesis and this effect was eliminated upon inhibition of the Erk activator MEK with U0126 and PKCα with Ro 31-8220.
Figure 4.
Infusion of fluoxetine into P15 mouse hippocampus causes induction of PSD95 via 5-HT1A-R, Erk1/2, and PKCα. (a) Intrahippocampal infusion of fluoxetine (Flx) caused a significant increase in PSD95 expression in all regions of the hippocampus, but confocal images from CA1 are shown (Scale bar: 50 μ). (b–d) Quantification from three mice performed with quadruplicate sections per mouse showed a significant increase in PSD95 expression in the presence of Flx, which was eliminated in the presence of WAY100635, U0126 and Ro 31-8820 (P<0.0001 for Flx versus all other groups for each hippocampal region; n=3).
Figure 5.
Intra-hippocampal infusion of fluoxetine into P15 hippocampus causes induction of synaptogenesis through 5-HT1A-R, Erk1/2 and PKCα. (a) A significant increase in synapses was observed in the CA1 region of the Flx-infused hippocampi. (b) Quantification from three mice performed with eight sections per mouse revealed a significant increase the number of synapses in the presence of Flx, which was eliminated in the presence of WAY, U0126 and Ro 31-8820 (P<0.0001 for Flx versus all other groups; n=3).
The hierarchy of signaling molecules in the 5-HT1A-R → → Erk1/2 → PKCα pathway in C57BL6 mouse hippocampus
The widespread induction of PSD95 over the entire hippocampus (Figure 3d) enabled us to perform western-blot analysis on whole hippocampal lysates to study the possible link among 5-HT1A-R, Erk and PKCα. We used T638 phosphorylation of PKCα and dual phosphorylation of T202 and Y204 on Erk1/2 as a measure of stimulation of these kinases. As shown in Supplementary Figures 2a and b, short-term stimulation of the hippocampal 5-HT1A-R by intrahippocampal infusion of 8-OH-DPAT (final concentration of 100 n) in the absence and presence of inhibitors followed by killing of the treated mice after 60 min, removal of hippocampi, and lysis in protease inhibitor-containing buffers yielded protein samples that could be analyzed by western blotting for P-PKCα and P-Erk. We observed a significant induction of P-PKCα in the DPAT-infused hippocampi, which was eliminated in the presence of WAY, U0126 and Ro 31-8220 (Supplementary Figure 2a). In contrast, 5-HT1A-R-mediated induction of P-Erk was eliminated in the presence of WAY and U0126 but not Ro 31-8220 (Supplementary Figure 2b). These two data sets place Erk above PKCα in a 5-HT1A-R signaling pathway the long-term effect of which would be increased synaptogenesis (Supplementary Figure 2c).
Downstream involvement of PKCα was utilized to induce PSD95 and synaptogenesis in C57BL6 5-HT1A-R(−/−) mice
To further confirm the role of 5-HT1A-R-mediated signaling pathway in the expression of PSD95, we used 5-HT1A-R(−/−) C57BL6 mice at P15. These mice were injected with carrier, DPAT and bryostatin. Bryostatin is a well-known memory-enhancing drug, which selectively activates PKC.18 We observed that PSD95 expression was significantly higher (P<0.0001) in the bryostatin-treated hippocampi than the carrier or DPAT-treated ones. This enhancement was observed in the CA1 region of the hippocampus (Figures 6a and b) as well as CA3 and DG regions of the hippocampus (data not shown here). Simultaneous electron microscopy analysis revealed that the bryostatin-treated hippocampi harbored significantly more CA1 excitatory synapses (Figures 6c–f).
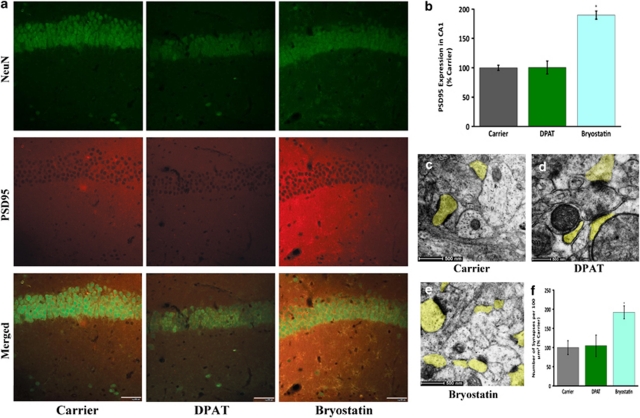
Figure 6.
Bryostatin treatment of P15 5-HT1A-R (−/−) C57BL6 mice leads to heightened expression of PSD95 and boosted synaptogenesis. (a) In the 5-HT1A-R-deficient mice, PSD95 staining is increased in the CA1 region in bryostatin-, but not DPAT-infused mice (Scale bar 50 μ). (b) Quantification of volume-rendered images from three experiments with triplicate sections per treatment revealed a significant increase in PSD95 in the presence of bryostatin (P<0.0001 bryostatin versus all other groups; n=3). (c–f) Electron microscopy revealed a simultaneous increase in the number of synapses in the bryostatin-treated animals (Scale bar: 500 nm) (f, P<0.0001 bryostatin versus all other groups; n=3).
In vivo analysis in SW mice at P15 show the involvement of PKCα in the induction of PSD95
To address the question whether this 5-HT1A-R- and PKCα-mediated signaling was strain-independent, we performed parallel experiment with wild-type SW mice with intact 5-HT1A-Rs. Inhibition of the downstream effector PKCα with Ro 31-8220 again caused elimination of the 5-HT1A-R-mediated induction of PSD95 (Supplementary Figures 3a and b). In order to confirm the involvement of PKCα in the increased hippocampal expression of PSD95 at P15 in this mouse strain, we used SW 5-HT1A-R (−/−) mice and stimulated the downstream molecule, PKCα, by injecting the suggested dose of bryostatin18 intra-hippocampally. As expected, DPAT treatment elicited no increase in PSD95 staining in these 5-HT1A-R-deficient mice, but bryostatin-treatment resulted in a significantly higher level of PSD95 expression (Supplementary Figures 3c and d). This re-confirms that the 5-HT1A-R-mediated signaling pathway leads to higher expression of PSD95 in the hippocampus of P15 SW mice.
Absence of the 5-HT1A-R results in a decreased density of excitatory synapses in the hippocampus of P15 mice
On the basis of our observation that 5-HT1A-R signaling in the hippocampus was critical in synaptogenesis, an obvious question remains, ‘Is the hippocampal synaptic density significantly lower in the 5-HT1A-R(−/−) mice?' As shown in Supplementary Figures 4a, b, and c, the 5-HT1A-R(−/−) mice harbor a significantly lower number of excitatory synapses in the CA1 region (P<0.0001). As explained in the discussion, this could have a detrimental effect on hippocampal maturation, which in turn could account for the behavioral abnormalities observed by others in these mice.4, 5, 6, 7
The induced synaptogenesis was persistent even 5 days after infusion of 8-OH-DPAT or Fluoxetine
A pertinent argument is that the observed increase in synaptogenesis could be only transient and therefore of insignificant consequence. To address this important point, we performed electron microscopy 5 days after carrier, 8-OH-DPAT or fluoxetine treatment. As shown in Supplementary Figure 5, the boosted density of excitatory synapses observed in the CA1 region after 24 h persisted even 5 days after DPAT or Flx treatment at P15. Intriguingly, systemic infusion (intraperitoneal) of fluoxetine from P15, both once as well as chronic (daily) for 5 days, caused an inhibition of the number of CA1 synapses compared with the carrier-infused controls (Supplementary Figure 6).
Discussion
Mice open their eyes between P12 and P14 and closely thereafter (P15) is a stage of heightened synaptogenesis.29 Our report shows that 5-HT1A-R signaling has a crucial role at this stage by triggering PSD95 expression and formation of excitatory synapses in the hippocampus at P15.30 A deficiency in 5-HT1A-R expression is associated with a significant decrease in these synapses (Supplementary Figure 4) and the 5-HT1A-R-linked increase in excitatory synapses in the wild-type hippocampal CA1 region persists for at least 5 days (Supplementary Figure 5). By elucidating a 5-HT1A signaling pathway linked to synaptogenesis, we also submit a possible strategy of combating 5-HT1A-R deficiency via downstream stimulation of PKCα with the memory-enhancing drug bryostatin (Figure 6 and Supplementary Figure 3).18 The same 5-HT1A-R-mediated PSD95 induction and synaptogenesis is observed in the presence of prozac (Flx) (Figures 4 and 5), which causes an increase in extracellular serotonin. This raises the possibility that a local increase in serotonin during inappropriate stages may result in unwanted overconnectivity in cortical centers. By applying intra-hippocampal infusion of drugs, we have avoided all confounds ensuing from systemic application of agonists or Flx, which could cause differential activation of somatodendritic and hetero 5-HT1A-R molecules. Furthermore, our experiments involved infusion of pharmacological agents into the right hippocampus, which left the contralateral hippocampus essentially unaffected, thereby confirming that the 5-HT1A-R agonists produce a specific effect in the ipsilateral hippocampus (Figure 2d).
Confirming our concern about confounds ensuing from systeming administration of Flx, intraperitoneal infusion of Flx into P15 mice caused an inhibition in the number of CA1 synapses compared with that observed in carrier-infused mice (Supplementary Figure 6). It should be noted that the raphé neurons, which actually synthesize 5-HT and express 5-HT1A receptors and SERT, develop much earlier than the hippocampal neurons. Therefore, it is quite likely that peripherally administered fluoxetine first reaches brain-stem raphé neurons, blocks the local SERT molecules, causing a local increase in extracellular 5-HT, which in turn binds to the ample somatodendritic 5-HT1A receptors to cause an inhibition of 5-HT firing at the hippocampal terminals. In contrast, when systemic fluoxetine molecules eventually diffuse to the relatively less-developed hippocampal region, their effect on the still-developing synapses with the maturing postsynaptic hippocampal neurons is likely to be far less than that in the raphé region. This out-of-balance high Flx-evoked 5-HT activity in the raphé region is expected to cause a decrease in net 5-HT firing at the hippocampal synapses, which could explain the apparent decrease in the number of CA1 synapses after systemic Flx treatment of P15 mice (Supplementary Figure 6). This should not be compared with the effect of systemic administration of Flx in adult animals wherein both the raphé as well as the hippocampal regions are fully developed with the full repertoire of SERT molecules and 5-HT receptors.
The effects of the inhibitors of the Erk pathway (U0126) and PKCα (Ro 31-8220) on 5-HT1A-R-linked PSD95 induction and synaptogenesis were mutually occlusive, which indicated that these two signaling molecules functioned in the same pathway. Short-term DPAT treatment (60 min) followed by western blotting revealed that the activation of PKCα was sensitive to U0126, which placed PKCα downstream of Erk (Supplementary Figures 2b and c). This inference was consistent with our earlier observation that in hippocampal neuron-derived HN2 cells, Erk physically bound to and phosphorylated PKCα at T638, which was crucial for the activation of PKCα.31 Earlier studies have also shown that 5-HT1A-R-mediated activation of PLCß occurs in a hippocampal neuron-derived cell line HN2-525, 32 and many other neuronal cell lines through Gißγ.33, 34 This pathway could be responsible for IP3-mediated calcium release from the endoplasmic reticulum, thereby causing stimulation of the Ras → Raf-1 → MEK → Erk1/2 pathway via Ca2+-sensitive signaling proteins like Pyk2 and Src.35, 36, 37, 38
The 5-HT1A-R agonist 8-OH-DPAT also stimulates the adenylyl cyclase-coupled 5-HT7 receptor causing an increase in intracellular cAMP levels. This would cause stimulation of protein kinase A, which is known to stimulate the Ras homolog Rap-1 through phosphorylation. Stimulated Rap-1 binds to and activates B-Raf that phosphorylates MEK to stimulate the Erk pathway.39 The concentration of the 5-HT7-R in the adult cortex is much lower than that of the 5-HT1A-R. Nonetheless, based on 5-HT7-R-mediated membrane depolarization, the cortical expression of the two receptors at P15 appears to be comparable.40 Although 8-OH-DPAT binds much more avidly to 5-HT1A-R (KD∼1 n) than to 5-HT7-R (KD=50 n),41, 42 the relative abundance of the two 8-OH-DPAT-binding receptors at P15 evokes the prospect that in the 5-HT1A-R (−/−) mice at P15, 8-OH-DPAT may cause 5-HT7-R-mediated activation of Erk, thereby stimulating PKCα, which would then elicit induced expression of PSD95. Contrary to this possibility, we observed no 8-OH-DPAT-evoked increase in PSD95 expression in 5-HT1A-R(−/−) mice of either C57BL6 or SW background. Although volume-renderings of some z-stack images showed a slight increase (Supplementary Figure 3; SW 5-HT1A-R−/−) or a slight decrease (Figure 6) in PSD95 immunostaining in the 8-OH-DPAT-treated hippocampi, these changes were not significant among the three sets of mice and large number hippocampal slices used for statistical analysis. In sharp contrast, in both strains of mice, bryostatin caused a dramatic and significant increase in PSD95 expression in the CA1 region. Thus, 5-HT7-R-mediated signaling is unlikely to have any significant effect on PSD95 expression in the P15 hippocampus.
Prior studies indicate that multiple subunits of the GABAA receptor are phosphorylated by Ca2+-dependent conventional PKCs,43, 44, 45, 46 thereby causing inhibition of GABAA signaling and internalization of the receptor.47 Thus, the activation of PKCα (a conventional Ca2+-dependent PKC) via 5-HT1A-R-mediated signaling is expected to cause an inhibition of GABAA receptor function in the target pyramidal CA1 neurons of the SC pathway. Such reduction of inhibitory signals would lead to an increase in the net excitatory signals in the pyramidal neurons of the CA1 area (fEPSP), which could cause increased synaptic strength, induction in PSD95, and augmented synaptogenesis as observed in the hippocampus after 24-h of in vivo 8-OH-DPAT treatment.
Alternative Erk and PKC-dependent mechanisms could also operate beyond the acute phase of fEPSP (1 h), causing an induction in PSD95. Prior studies by Elkobi et al. have demonstrated Erk-dependent PSD95 induction in the gustatory cortex as an essential step in taste learning;48 however, the mechanistic link between Erk activity and PSD95 induction has not been elucidated. Our results strongly indicate that Erk-dependent induction of PSD95 in the hippocampus involves PKCα. Among molecules that may link PKC to the induction of PSD95, neuroligin-1 (Nrg), which elicits induced expression of PSD95,49 is a likely candidate. The intracellular domain of Nrg is shed in a PKC-dependent manner,50 following which the released Nrg fragment complexes with the transcription factor Eos to bind to an enhancer element in the PSD95 promoter to elicit induced expression of PSD95.49 Further investigation will test this possibility.
Performed in mice of 129S6/SvEvTac;C57BL/6;CBA mixed background, pharmacological inhibition of 5-HT1A-R from P13-34 has been shown to phenocopy the effect of neonatal absence of this receptor, thereby linking 5-HT1A-R-mediated CaMIIα stimulation to the maintenance of normal anxiety levels in adulthood.51 Our analyses, performed in mice of different backgrounds (C57BL6 and SW), submit Erk1/2 and PKCα as possible substrates for affective disorders, linked to aberrant 5-HT1A-R expression/signaling. Intriguingly, in dissociated cell cultures, Ferreira et al. report the opposite finding that 5-HT1A-R signaling causes suppression of growth and sprouting of oblique dendrites.52 However, at 3–5 postnatal weeks, the mice used in these studies were older than the P15 mice used here. More consistent with our observation, Chen et al. have demonstrated 5-HT1A-R-mediated mobilization of mitochondria,53 which may stimulate synaptogenesis.54 However, the specific stage of P15 has not been the focus of these studies, which may contribute to the mechanistic difference (involving Akt-GSK3b) observed by Chen et al.53
In mouse and rat pups, the event of eye opening at P12-14 is marked by a dramatic increase in PSD95 expression and redistribution into synapases.29 Thus, as mentioned in the introduction, P15 is at the epicenter of a major synaptogenic event,13 and a significant part of it is visual activity-driven. By elucidating 5-HT1A-R, Erk, and PKCα-mediated PSD95 induction and synaptogenesis and also demonstrating that the absence of the 5-HT1A-R in the 5-HT1A-R (−/−) mice is associated with a decrease in synapse density in the CA1 region at P15, we have strengthened the possibility that the hippocampal 5-HT1A-R has the essential role of orchestrating synaptogenesis. Our study also suggests that sluggish hippocampal development because of impaired 5-HT1A-R, Erk, PKCα signaling could be remedied by the use of the PKC-activating drug bryostatin.
Our experiments have used ketamine for anesthesia and a recent study has demonstrated ketamine regulates hippocampal synapse formation.55 As both control and 5-HT1A agonist-treated mice used in our experiments were anesthetized using the same concentration of ketamine, it is unlikely that a different anesthetic would alter our conclusion. However, it will be important to analyze how anesthetics regulate synaptogenesis at P15.
Normal brain development requires that early postnatal synaptogenesis be followed by a phase of synaptic pruning for the refinement of neuronal connections. Based on our results from inra-hippocampal infusion of Flx, we initially conjectured that the use of prozac during the period of synaptic pruning could impair brain development by triggering unwanted synaptogenesis through the same 5-HT1A-R pathway. Intriguingly, systemic administration of Flx in P15 mice actually caused a significant decrease in the number of excitatory synapses in the CA1 region (Supplementary Figure 6). Therefore, administration of prozac may impair neonatal brain development by inhibiting synaptogenesis.
Our strategy of increasing local extracellular 5-HT by intrahippocampal administration of Flx may replicate the state of increased cortical serotonin, which has been reported in juvenile autistic subjects.2 The resulting synaptogenesis through 5-HT1A-R signaling may precipitate short-range hyper-connectivity (for example, within the hippocampus), which is an endophenotype of autism.14
The half-life of DPAT in the body and the brain has been reported as 143 min and 26 min, respectively.56, 57 Therefore, the long-term synaptogenic effect observed after 5 days is likely to be initiated through an DPAT or 5-HT-evoked change in gene expression. This suggests the existence of a direct relationship between the basal profiles of 5-HT1A-R expression and signaling and synapse formation during early postnatal hippocampal development. Our observation of a lower level of excitatory synaptic connections in the hippocampal CA1 region in the 5-HT1A-R(−/−) mice strongly supports this postulate (Supplementary Figure 4). In conclusion, our findings elucidate a 5-HT1A-R-linked synaptogenic pathway involving Erk1/2 and PKCα in P15 mouse hippocampus, which may have a pivotal role in early postnatal brain development.
Acknowledgments
We are grateful to Dr George Merz for his meticulous training of AM. Fellowship support from the CDNDD (OMRD) for AM is gratefully acknowledged here. This project was supported by the NIMH Grant R01 MH071376 and a bridge Grant from The Office of Vice Chancellor, City University of New York.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu H-M, Brennan TJ, Danao JA, Bajwa P, Parsons LH, et al. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J Neurosci. 2006;26:1864–1871. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-Hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung SC, Adlersberg M, Arango V, Mann JJ, Tamir H, Liu KP. Attenuated 5-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase Akt and mitogen-activated protein kinase. J Neurochem. 2003;87:182–194. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Esiri MM, Keene J, Hope T, et al. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003;974:82–87. doi: 10.1016/s0006-8993(03)02554-x. [DOI] [PubMed] [Google Scholar]
- Mehta M, Ahmed Z, Fernando SS, Cano-Sanchez P, Adayev T, Ziemnicka D, et al. Plasticity of 5-HT1A receptor-mediated signaling during early postnatal brain development. J Neurochem. 2007;101:918–928. doi: 10.1111/j.1471-4159.2007.04448.x. [DOI] [PubMed] [Google Scholar]
- Benitez-Diaz P, Miranda-Contreras L, Mendoza-Briceno RV, Pena-Contreras Z, Palacios-Pru E. Prenatal and postnatal contents of amino acid neurotransmitters in mouse parietal cortex. Dev Neurosci. 2003;25:366–374. doi: 10.1159/000073514. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Etienne S, Benjamin D, Toth M, Shippenberg T. Differential effects of 5-HT1A receptor deletion upon basal and fluoxetine-evoked 5-HT concentrations as revealed by in vivo microdialysis. Brain Res. 2001;902:11–17. doi: 10.1016/s0006-8993(01)02271-5. [DOI] [PubMed] [Google Scholar]
- Le Duigou C, Wittner L, Danglot L, Miles R. Effects of focal injection of kainic acid into the mouse hippocampus in vitro and ex vivo. J Physiol. 2005;569:833–847. doi: 10.1113/jphysiol.2005.094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ.The Mouse Brain in Stereotaxic Coordinates2nd ednAcademic Press: New York; 2001 [Google Scholar]
- Hongpaisan J, Alkon DL. A structural basis for enhancement of long-term associative memory in single dendritic spines regulated by PKC. Proc Nat Acad Sci. 2007;104:19571–19576. doi: 10.1073/pnas.0709311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Wen GY, Yang SY, Kaczmarski W, He XY, Pappas KS. Presence of hydroxysteroid dehydrogenase type 10 in amyloid plaques (APs) of Hsiao′s APP-Sw transgenic mouse brains, but absence in APs of Alzheimer′s disease brains. Brain Res. 2002;954:115–122. doi: 10.1016/s0006-8993(02)03354-1. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci USA. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- Adayev T, Ray I, Sondhi R, Sobocki T, Banerjee P. The G protein-coupled 5-HT1A receptor causes suppression of caspase-3 through MAPK and protein kinase Ca. Biochim Biophys Acta. 2003;1640:85–96. doi: 10.1016/s0167-4889(03)00023-5. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Watanabe S. Effects of intrahippocampal injections of N-methyl-D-aspartate receptor antagonists and scopolamine on working and reference memory assessed in rats by a three-panel runway task. J Pharmacol Exp Ther. 1992;263:943–950. [PubMed] [Google Scholar]
- Malagie I, David DJ, Jolliet P, Hen R, Bourin M, Gardier AM. Improved efficacy of fluoxetine in increasing hippocampal 5-hydroxytryptamine outflow in 5-HT1B receptor knock-out mice. Eur J Pharmacol. 2002;443:99–104. doi: 10.1016/s0014-2999(02)01604-7. [DOI] [PubMed] [Google Scholar]
- de Groote L, Olivier B, Westenberg JGM. Extracellular serotonin in the prefrontal cortex is limited through terminal 5-HT1B autoreceptors: a microdialysis study in knockout mice. Psychopharmacology. 2001;162:419–424. doi: 10.1007/s00213-002-1117-z. [DOI] [PubMed] [Google Scholar]
- Yoshii A, Sheng MH, Constantine-Paton M. Eye opening induces a rapid dendritic localization of PSD95 in central visual neurons. Proc Natl Acad Sci U S A. 2003;100:1334–1339. doi: 10.1073/pnas.0335785100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Fiore G. Postsynaptic density scaffolding proteins at excitatory synapse and disorders of synaptic plasticity: implications for human behavior pathologies. Int Rev Neurobiol. 2004;59:221–254. doi: 10.1016/S0074-7742(04)59009-8. [DOI] [PubMed] [Google Scholar]
- Debata PR, Ranasinghe B, Berliner A, Curcio GM, Tantry SJ, Banerjee P. Erk1/2-dependent phosphorylation of PKCalpha at threonine 638 in hippocampal 5-HT1A receptor signaling. Biochem Biophys Res Commun. 2010;397:401–406. doi: 10.1016/j.bbrc.2010.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adayev T, El-Sherif Y, Barua M, Banerjee P. Agonist stimulation of the serotonin 1A receptor causes supression of anoxia-induced apoptosis via mitogen-activated protein kinase in neuronal HN2-5 cells. J Neurochem. 1999;72:1489–1496. doi: 10.1046/j.1471-4159.1999.721489.x. [DOI] [PubMed] [Google Scholar]
- Albert PR, Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- Kushwaha N, Albert N. Coupling of 5-HT1A autoreceptors to inhibition of mitogen-activated protein kinase activation via G beta gamma subunit signaling. Eur J Neurosci. 2005;21:721–732. doi: 10.1111/j.1460-9568.2005.03904.x. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJS. Extracellular-signaling-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Adayev T, Ranasinghe B, Banerjee P. Transmembrane signaling in the brain by serotonin, a key regulator of physiology and emotion. Biosci Rep. 2005;25:363–385. doi: 10.1007/s10540-005-2896-3. [DOI] [PubMed] [Google Scholar]
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and Calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson TB, Xu B-E, Karandikar M, Berman K, et al. Mitogen-Activated Protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Morozov A, Muzzio IA, Bourtchouladze R, Van-Strien N, Lapidus K, Yin DQ, et al. Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning, and memory. Neuron. 2003;39:309–325. doi: 10.1016/s0896-6273(03)00404-5. [DOI] [PubMed] [Google Scholar]
- Béique J-C, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, et al. Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-Hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MD, Mestikawy SE, Emerit MB, Pichat L, Hamon M, Gozlan H. [3H]8-Hydroxy-2-(Di-n-Propylamino)tetralin binding to pre-and postsynaptic 5-hydroxytryptamine sites in various regions of the rat brain. J Neurochem. 1985;44:1685–1696. doi: 10.1111/j.1471-4159.1985.tb07155.x. [DOI] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang J-M, et al. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci U S A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidenheimer NJ, McQuilkin SJ, Hahner LD, Whiting P, Harris RA. Activation of protein kinase C selectively inhibits the gamma-aminobutyric acidA receptor: role of desensitization. Mol Pharmacol. 1992;41:1116–1123. [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, et al. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem. 2000;275:38856–38862. doi: 10.1074/jbc.M004910200. [DOI] [PubMed] [Google Scholar]
- Leidenheimer NJ, Chapell R. Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABAA receptors. Mol Brain Res. 1997;52:173–181. doi: 10.1016/s0169-328x(97)00255-6. [DOI] [PubMed] [Google Scholar]
- Kumar S, Khisti R, Morrow A. Regulation of native GABAA receptors by PKC and protein phosphatase activity. Psychopharmacology. 2005;183:241–247. doi: 10.1007/s00213-005-0161-x. [DOI] [PubMed] [Google Scholar]
- Chapell R, Bueno OF, Alvarez-Hernandez X, Robinson LC, Leidenheimer NJ. Activation of protein kinase C induces γ-aminobutyric acid type a receptor internalization in xenopus oocytes. J Biol Chem. 1998;273:32595–32601. doi: 10.1074/jbc.273.49.32595. [DOI] [PubMed] [Google Scholar]
- Elkobi A, Ehrlich I, Belelovsky K, Barki-Harrington L, Rosenblum K. ERK-dependent PSD-95 induction in the gustatory cortex is necessary for taste learning, but not retrieval. Nat Neurosci. 2008;11:1149–1151. doi: 10.1038/nn.2190. [DOI] [PubMed] [Google Scholar]
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim T-W, et al. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- Lo Iacono L, Gross C. Alpha-Ca2+/Ca;modulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira TA, Lo Iacono L, Gross C. Serotonin 1A receptor modulates actin dynamics and restricts dendritic growth in hippocampal neurons. Eur J Neurosci. 2010;32:18–26. doi: 10.1111/j.1460-9568.2010.07283.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Owens GC, Crossin KL, Edelman DB. Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol Cell Neurosci. 2007;36:472–483. doi: 10.1016/j.mcn.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Chen S, Owens GC, Makarenkova H, Edelman DB. HDAC6 regulates mitochondrial transport in hippocampal neurons. PLoS One. 2010;5:e10848. doi: 10.1371/journal.pone.0010848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuideveld KP, Treijtel N, Maas HJ, Gubbens-Stibbe JM, Peletier LA, van der Graaf PH, et al. Competitive interaction model predicts the effect of WAY-100,635 on the time course of R-()-8-Hydroxy-2-(di-npropylamino) tetralin-induced hypothermia. J Pharmacol ExperTherapeu. 2002;300:330–338. doi: 10.1124/jpet.300.1.330. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ängeby-Möller K. Effects of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on locomotor activity and rearing of mice and rats. Psychopharmacology. 1990;102:485–491. doi: 10.1007/BF02247129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.