Abstract
The recent discovery of a large latent population of precursor cells in the dentate gyrus of adult mice led us to investigate whether activation of this population is regulated by synaptic activity, thereby explaining the observation that environmental signals can affect neurogenesis. Using a variety of stimulation protocols, we found that only a long-term potentiation (LTP)-inducing protocol activated the latent precursor pool, leading to increased neurogenesis in the dentate gyrus. LTP induced by high-frequency stimulation (HFS) of the perforant pathway in vivo produced a two-fold increase in the number of neurospheres cultured from the stimulated hippocampus, compared with the unstimulated hippocampus. No increase in neurosphere number or neurogenesis was observed when the HFS failed to induce LTP. These results show that LTP can activate latent neural precursor cells in the adult mouse dentate gyrus, thereby providing a direct mechanism for regulating activity-driven neurogenesis. In the future, it may be possible to utilize such learning- or stimulation-induced neurogenesis to overcome disorders characterized by neuronal loss.
Keywords: dentate gyrus, hippocampus, latent precursors, long-term potentiation, neurogenesis, proliferation
Introduction
With the emergence of strong experimental evidence that neurogenesis has a role in some forms of hippocampal-based learning and memory,1, 2 uncovering the mechanisms that regulate this process has become a major priority. The gain of mechanistic insight is of particular importance, as the ability to activate hippocampal neurogenesis may have great novel therapeutic potential for the treatment of psychiatric and neurological conditions. Given that neurogenesis can be enhanced by a range of stimuli, including learning,1 exercise,3 environmental enrichment,4 antidepressant treatment,5 electroconvulsive seizure5 and deep brain stimulation,6, 7 the regulatory mechanism may be linked to synaptic activity. It has been shown that running enhances long-term potentiation (LTP), particularly in recently born neurons.3 Moreover, evidence now suggests that LTP per se activates neurogenesis and the survival of newborn neurons, although the mechanism by which this occurs remains unclear.8, 9, 10, 11
Recently, we discovered that K+-mediated depolarization, an in vitro correlate of synaptic activity, can activate a large pool of quiescent precursor cells in the adult hippocampus.12 This precursor pool was approximately three times larger than the cycling population and contained true stem cells, thus representing an enormous reservoir of neurogenic precursors. Although we demonstrated that this latent population could be activated using an in vivo epilepsy model, it was unclear whether these cells could be activated by electrophysiological stimulation associated with learning.
Here, we report that the induction of long-lasting LTP in vivo can activate the pool of latent precursor cells in the mouse hippocampus, thereby increasing neurogenesis. Protocols that failed to induce LTP, induced only early LTP, used low-frequency stimulation (LFS), or included a pharmacological blocker of LTP, all failed to activate neurogenesis.
Materials and methods
Surgery and perforant pathway stimulation
Experiments were conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the University of Queensland Animal Ethics Committee.
Adult male C57BL/6 mice (10-weeks old) were anesthetized with either sodium pentobarbital (injected i.p.; 60 mg kg−1), which was supplemented throughout surgery and recording as required (15 mg kg−1), or isoflurane (2.0±0.5% Attane, Bomac Pty, NSW, Australia) vaporized with oxygen (2 l min−1; Isotec 5, Mediquip, QLD, Australia). Mice were placed in a stereotaxic frame and body temperature was maintained at 37 °C.
In one hemisphere only, either a glass pipette-recording electrode filled with 1 NaCl or a Teflon-coated stainless steel wire (0.076 mm; A-M Systems, Carlsborg, WA, USA) was positioned 2 mm posterior to bregma, 1.4 mm lateral to the midline, and lowered into the hilus of the dentate gyrus. A Teflon-coated stainless steel stimulating electrode was positioned ipsilaterally 2.5 mm lateral to lambda and lowered into the perforant pathway. Electrode positioning was limited to four penetrations while maximizing the field extracellular postsynaptic potential (fEPSP) response. After generating an input/output curve (see below), the recording electrode was raised to the dentate molecular layer, to allow the recording of a fEPSP uncontaminated by the population spike. A stable 15 min baseline of evoked potentials was recorded (stimulus pulse width 50 μs, at 0.033 Hz) before tetanization (see below). All recorded signals were amplified, filtered, digitized and analyzed offline.
In the first set of experiments, mice were assigned to one of four groups: (1) the LTP(+) group, if fEPSP response was >120% of the baseline at 60 min after high-frequency stimulation (HFS); (2) the CPP (3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid) group, in which LTP was blocked with CPP (13 mg kg−1, i.p.; Tocris Cookson, Buckhurst Hill, UK) injected 1 h before HFS; (3) the LFS group that was subjected to 1 Hz LFS; or (4) the LTP(−) group, in which mice received HFS but LTP was not induced (that is, the fEPSP response was <120% at 10 min after HFS). Evoked responses were recorded for 60 min after tetanization. In a second set of experiments, mice were assigned to one of two groups: (1) the early-LTP group, if the fEPSP response was <120% of the baseline at 60 min after HFS; or (2) the late-LTP group, if the fEPSP response was >120% at 60 min after HFS. In this experiment, evoked responses were recorded for 160 min after tetanization.
The baseline stimulus intensity was set to 70% of threshold for generating a population spike in the hilus (from the input/output curve). Tetanic stimuli were delivered at an intensity that produced a hilar population spike 30% of maximum. Mice in the LTP(+) and LTP(−) groups were given one of two HFS protocols. The 250 Hz HFS protocol consisted of 50 pulses (pulse width 50 μs) at 250 Hz, repeated three times (20 s between repetitions). The 400 Hz HFS protocol consisted of six trains of six pulses (pulse width 50 μs, 100 ms inter-train interval) repeated six times (15 s between repetitions). Mice in the CPP, early-LTP and late-LTP groups all received the 400 Hz HFS protocol. The LFS group received a 1 Hz stimulation protocol that consisted of an equal number of pulses (216) to the 400 Hz HFS protocol.
Online analysis of the fEPSP slope was made in several selected regions of interest using pClamp software (Molecular Devices, Sunnyvale, CA, USA). This analysis was used to determine the number of HFS trains required to induce stable LTP. An average of two HFS protocols was required to achieve a stable LTP >120% of baseline at 10 min after HFS.
The magnitude of LTP was calculated at the end of the recording session (that is, between 50 and 60 min for the first set of experiments, and 150 and 160 min for the second set of experiments), and expressed as the percentage change from the fEPSP baseline (10 min preceding HFS). After recordings were completed, the electrodes were removed, the skin was closed with Vetbond (3 Animal Care, St Paul, MN, USA) and the animals were returned to their home cages. Post-operative care consisted of antibiotic (5 mg kg−1 enrofloxacin, Baytril, Bayer Healthcare, Pymble, Australia) and analgesic (1 mg kg−1 butorphanol tartrate, Torbugesic, Ausrichter Pty Ltd, Annandale, Australia) treatment, administered for 2 days after surgery.
Neurosphere culture
At 60 min, 2 days or 4 days after stimulation, mice were killed by cervical dislocation, their brains were removed, and the control and stimulated hippocampi were carefully dissected. Tissues were manually homogenized, then digested in 1 mg ml−1 papain (Worthington Biochemical, Lakewood, NJ, USA) and 0.25 mg ml−1 DNase I (Roche, Basel, Switzerland) in 1 ml Hank's balanced salt solution (HBSS; Thermo Fischer Scientific, Waltham, MD, USA) for 20 min at 37 °C and triturated gently twice during incubation. Tissue samples were centrifuged at 100 rcf for 5 min and the resulting pellet resuspended in 1 ml Hank's balanced salt solution. After a second centrifugation, the pellet was resuspended in 1 ml neurosphere growth medium and filtered through a 40-μm cell sieve (Falcon/BD Biosciences, Sydney, Australia). The neurosphere growth medium consisted of mouse NeuroCult NSC Basal Medium, plus mouse NeuroCult NSC Proliferation Supplements (StemCell Technologies, Vancouver, Canada) containing 2% bovine serum albumin (Sigma-Aldrich, Sydney, Australia) and 2 μg ml−1 heparin (Sigma-Aldrich). The following growth factors were also included: 20 ng ml−1 purified mouse receptor-grade epidermal growth factor (EGF) (BD Biosciences) and 10 ng ml−1 recombinant bovine fibroblast growth factor (FGF)-2 (Roche). Cells were plated at a density of half of the hippocampus per 96-well plate (Falcon/BD Biosciences) with 200 μl neurosphere medium per well. Primary hippocampal cells were incubated for 12 days at 37 °C in humidified 5% CO2 to allow neurosphere formation under high K+ (15 m KCl) or low K+ (standard culture medium) conditions.
5-bromo-2′-deoxyuridine (BrdU) protocols
BrdU (Sigma-Aldrich) was injected i.p. 4 days after stimulation (120 mg kg−1, six injections, once every 2 h).12 Mice were killed 30 min (for proliferation analysis) or 1 week (for differentiation analysis) after the last BrdU injection with an overdose of sodium pentobarbital. Animals were perfused transcardially with 4% paraformaldehyde in phosphate-buffered saline (PBS). Brains were post-fixed overnight at 4 °C, then cryoprotected in PBS with 30% sucrose. Serial coronal frozen sections (20 μm) of the hippocampus were cut on a cryostat.
Immunohistochemistry
Every sixth section throughout the hippocampus was stained with an anti-BrdU antibody to assess proliferation, or anti-BrdU and anti-doublecortin (DCX) antibodies to assess differentiation. Sections were denatured in 2 HCl for 30 min at 37 °C, washed three times in PBS, and then blocked in PBS containing 10% normal goat serum. The following primary antibodies were used: mouse anti-BrdU conjugated with biotin (1:500; Invitrogen, Melbourne, Australia) and rabbit anti-DCX (1:1000, Sapphire Bioscience, Sydney, Australia). Sections were incubated overnight at 4 °C in primary antibody, washed with PBS, then incubated at room temperature for 2 h with secondary antibody, either streptavidin-Cy3 (1:500, GE Healthcare Bio-Science, Sydney, Australia) or goat anti-rabbit Alexa-488 (1:2000, Invitrogen). Sections were washed three times then coverslipped with fluorescence-mounting medium (Dako, Carpinteria, CA, USA).
Image analyses
A Zeiss Axio Imager (Zeiss, Goettingen, Germany) was used to collect pictures of every sixth section with an Apotome optical sectioning device ( × 20 objective; Zeiss, Goettingen, Germany). Stereological quantification involved measuring the perimeter of the subgranular zone of the dentate gyrus and counting BrdU-positive and BrdU/DCX double-positive cells along the entire z axis (20 μm), to enable the density of BrdU-positive or BrdU/DCX double-positive cells to be calculated. False double-positive cells caused by overlay signal from different cells were excluded by rotating in orthogonal planes.
Statistical analysis
LTP was expressed as the percentage change from the fEPSP baseline. The early-LTP and late-LTP groups were statistically compared using Student's unpaired t-test. For each LTP protocol, the number of neurospheres obtained from each mouse was calculated as a ratio (stimulated hemisphere compared with unstimulated hemisphere), and the group data was evaluated statistically using the Wilcoxon matched-pairs signed rank test. To compare neurosphere numbers with LTP protocols, Student's unpaired t-test with Welch's correction was used. Pearson's r correlation was used to assess the relationship between the magnitude of LTP and degree of precursor activation. To determine the number of BrdU and BrdU/DCX double-positive cells, the number of labeled cells observed in each mouse was evaluated using Student's paired t-test (stimulated hemisphere and unstimulated hemisphere). Comparisons between groups were performed using Student's unpaired t-test. Statistical significance was defined as P<0.05.
Results
We examined whether synaptic activity associated with learning and memory can stimulate latent hippocampal precursor cells to undergo neurogenesis. HFS was applied to the perforant pathway in vivo to induce LTP (LTP(+) group). In some animals, this protocol failed to induce LTP, allowing for a no-LTP control (LTP(−) group) to be examined. To assess the effect of afferent stimulation per se on hippocampal precursor activation, a non-LTP-inducing 1 Hz LFS protocol was also used (LFS group). To examine the involvement of N-Methyl--aspartic acid (NMDA) receptors, the antagonist CPP was administered to animals 60 min before HFS (CPP group).
Induction of LTP was required to activate latent precursors in the mouse dentate gyrus
To establish an optimal HFS protocol in mice, we stimulated the perforant pathway with brief trains of HFS at 250 Hz or 400 Hz. Stable LTP was induced in 86% of the mice stimulated at 400 Hz and 40% of those stimulated at 250 Hz. At 60 min after HFS, the mean fEPSP in the LTP(+) group was 149.0±6.8% (mean±s.e.m.; n=11) of the baseline (Supplementary Figure S1). The LTP(−) group of mice showed a mean fEPSP of 95.0±2.7% at 10 min after HFS (n=4), and 97.7±1.7% at 60 min after HFS. In the presence of CPP, HFS produced no LTP, with the mean fEPSP 60 min after HFS reduced to 106.1±5.8% (n=4), whereas delivery of LFS (1 Hz) to the perforant pathway also failed to induce LTP, resulting in a mean fEPSP of 95.1±3.8% (n=6).
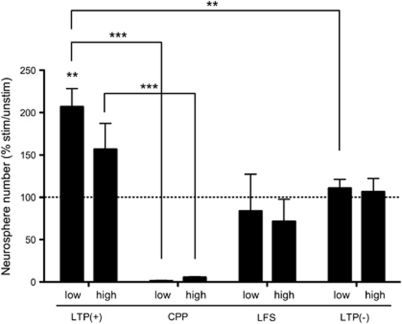
To investigate whether these stimulation protocols resulted in precursor activation, we assayed for hippocampal neurosphere formation under low and high K+ culture conditions. This was done to determine both the magnitude of activation and the proportion of the latent pool activated relative to the maximum response seen in high K+. We first explored the extent of precursor activation 48 h after HFS, as we had previously found that 24–48 h of K+ depolarization in vitro was required for the activation of latent precursor cells in the mouse dentate gyrus.12 In the low K+ assay, we found an approximate two-fold increase in the number of neurospheres generated in LTP-stimulated hippocampi, compared with unstimulated control hippocampi (Figure 1; P=0.002, n=11, Wilcoxon matched-pairs signed rank test). No significant change in neurosphere number was observed in the CPP, LFS or LTP(−) groups relative to the unstimulated hemisphere (Wilcoxon matched-pairs signed rank test). A significantly greater number of neurospheres were formed from the stimulated hippocampus of the LTP(+) group compared with the stimulation control (stimulated hippocampus of the LTP(−) group; Figure 1; P=0.0016, Student's t-test with Welch's correction). However, when LTP was blocked with CPP (n=4), there was a significant decrease in neurosphere number compared with the LTP(+) group (P<0.0001, Student's t-test with Welch's correction) and the LTP(−) group (P=0.0018, Student's t-test with Welch's correction). In the presence of depolarizing levels of K+, a slight increase in the number of neurospheres cultured from the stimulated hippocampus of the LTP(+) group was observed, when compared with the unstimulated hippocampus, although this result was not statistically significant (Figure 1; P=0.0753, n=11, Wilcoxon matched-pairs signed rank test). Again, LFS and LTP(−) produced no change, whereas the CPP group showed a marked suppression of precursors activated by K+ compared with the LTP(+) group in high K+ (Figure 1; P=0.0006, Student's t-test with Welch's correction) and the LTP(−) group in high K+ (P=0.0078, Student's t-test with Welch's correction).
Figure 1.
LTP activates hippocampal precursors in vitro. To investigate whether LTP enhances proliferation of neural precursors, mice were assayed for neurosphere formation 2 days after stimulation (n=11, 4, 6 and 4, for the LTP(+), CPP, LFS and LTP(−) groups, respectively). Under normal K+ conditions, only the LTP(+) group revealed a significant increase in neurosphere number from the stimulated hemisphere relative to control hemisphere (P=0.002, Wilcoxon matched-pairs signed rank test). Furthermore, a significantly greater number of neurospheres were generated from stimulated hippocampi of the LTP(+) group compared with the simulated hippocampi of the CPP group (P<0.0001, Student's t-test with Welch's correction) and LTP(−) group (P=0.0016, Student's t-test with Welch's correction). When CPP was administered to block the NMDA receptor, neurosphere formation was significantly reduced compared with the LTP(+) group (low K+: P<0.0001, high K+: P=0.0006, Student's t-test with Welch's correction) and LTP(−) group (low K+: P=0.0018, high K+: P=0.0078, Student's t-test with Welch's correction). Error bars indicate s.e.m., low: low K+, high: high K+. **P<0.01, ***P<0.001. Abbreviations: CPP, 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid; LTP, long-term potentiation; NMDA, N-Methyl--aspartic acid.
Although there was a trend suggesting that together, the induction of LTP and depolarizing levels of K+ could give rise to more neurospheres than depolarizing K+ alone, we sought to investigate this further. To determine the extent to which LTP activated the quiescent hippocampal precursor population, we compared the number of neurospheres generated in low K+ culturing conditions following LTP with the number generated from the unstimulated hemisphere cultured in high K+. We found a similar number of neurospheres could be generated with LTP as with high K+ depolarizing conditions alone (data not shown; 99.3±17.8% of the unstimulated high K+ condition), indicating that the induction of LTP can activate neural precursor cells to a similar extent as depolarizing levels of K+.
To investigate the time course of precursor activation following the successful induction of LTP, we assayed hippocampi 60 min and 4 days after HFS. At 60 min following the delivery of HFS, the mean fEPSP change was 174.5±21.0% (n=6) of the baseline (Supplementary Figure S2A). When hippocampi were dissociated and cultured 60 min after HFS, we observed an increase in the number of spheres cultured in both low and depolarizing levels of K+; however, these trends were not statistically significant (Supplementary Figure S2B; P>0.05, n=3, Wilcoxon matched-pairs signed rank test). Similarly, when hippocampi were assayed 4 days after HFS, there were no differences in neurosphere number between the stimulated and unstimulated hippocampi, for either of the two K+ conditions (Supplementary Figure S2B; P>0.05, n=3, Wilcoxon matched-pairs signed rank test).
Induction of late-LTP, but not early-LTP, is required to activate precursor cells
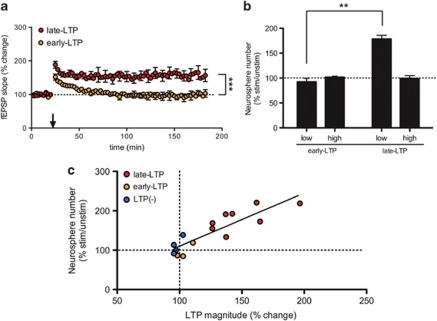
To begin teasing apart the mechanisms that underlie the activation of latent precursor cells in the mouse dentate gyrus following the induction of LTP, we took advantage of the phasic nature of LTP by inducing either early- or late-phase LTP (Figure 2a). Mice were assigned to the early- or late-LTP group according to the fEPSP outcome following 400 Hz HFS (see Methods). In the early-LTP group, the mean fEPSP response was 139.0±5.8% at 10 min after HFS (n=3), but it subsequently decayed back to baseline (104.0±3.7% at 60 min after HFS, and 101.4±10.4% at 160 min after HFS). In the late-LTP group, the fEPSP response was 171.3±6.4% at 10 min after HFS (n=4) and remained potentiated for the duration of the experiment (153.4±16.9% at 60 min after HFS, and 163.1±17.4% at 160 min after HFS). At the end of recording (160 min), the magnitude of potentiation in the late-LTP group was significantly different from the early-LTP group (Figure 2a; P<0.0001, Student's t-test).
Figure 2.
Late-LTP but not early-LTP activates hippocampal precursors in vitro. To narrow down the mechanisms of LTP that underlie the activation of precursors, we investigated the ability of early-LTP (n=3) and late-LTP (n=3) to activate precursors using the neurosphere assay, 2 days after stimulation. (a) Data showing the mean fEPSP slope (±s.e.m.) as a percentage of baseline. Mice assigned to early-LTP group showed a 139.0±5.8% increase in the fEPSP response 10 min after HFS. However, this potentiation did not persist, and decayed to baseline within 60 min (104.0±3.7% at 60 min after HFS, and 101.4±10.4% at 160 min after HFS). The late-LTP group showed robust LTP at all time points analyzed (171.3±6.4%, 153.4±16.9% and 163.1±17.4%, respectively). At the end of recording, the magnitude of potentiation in the late-LTP group was significantly greater than the early-LTP group (P<0.0001, Student's t-test). (b) Following the induction of late-LTP, a significant increase in the number of neurospheres was observed in the low K+ condition, compared with the early-LTP group (P=0.0051, Student's t-test with Welch's correction). (c) The magnitude of LTP, calculated 60 min after HFS, showed a significant positive correlation (r=0.879, n=15, P<0.0001, Pearson's correlation) with the number of neurospheres grown in vitro, after hippocampi were dissected 2 days after HFS. Arrow indicates HFS, error bars indicate s.e.m., low: low K+, high: high K+. **P<0.01, ***P<0.001. Abbreviations: fEPSP, field extracellular postsynaptic potential; HFS, high-frequency stimulation; LTP, long-term potentiation.
We then processed the hippocampi for the neurosphere assay to investigate whether the induction of short-lasting early-LTP is sufficient to activate precursor cells in the dentate gyrus, or whether the mechanisms underlying the maintenance phase of late-LTP are required for precursor cell activation. When LTP did not persist beyond 60 min (early-LTP group), we observed no significant difference in neurosphere number between the stimulated and unstimulated hippocampi, in both the low and high K+ culturing conditions (Figure 2b; P>0.05, n=3, Wilcoxon matched-pairs signed rank test). When LTP was maintained for at least 160 min after tetanus (late-LTP group), we observed a 1.8-fold increase in the number of neurospheres cultured in low K+ media, relative to the unstimulated hippocampus; however, this difference did not reach significance (Figure 2b; P>0.05, n=4, Wilcoxon matched-pairs signed rank test). Nevertheless, the induction of late-LTP resulted in a significantly greater number of neurospheres cultured in low K+ than did the induction of early-LTP (Figure 2b; P=0.0051, Student's t-test with Welch's correction).
To further confirm that the induction of LTP is associated with precursor cell activation, we looked at the relationship between the magnitude of LTP at 60 min after HFS and the number of neurospheres grown in vitro, when hippocampi were isolated 48 h after HFS (mice from the LTP(+), LTP(−), early-LTP and late-LTP groups were included in the analysis, n=4, 4, 3 and 4, respectively). We found a striking correlation between the magnitude of LTP and extent of precursor cell activation (Figure 2c; r = 0.879, P<0.0001, n=15, Pearson's correlation), indicating that activation of precursors is a graded phenomenon and is related to the strength of LTP induced.
LTP enhanced proliferation and neurogenesis in the dentate gyrus
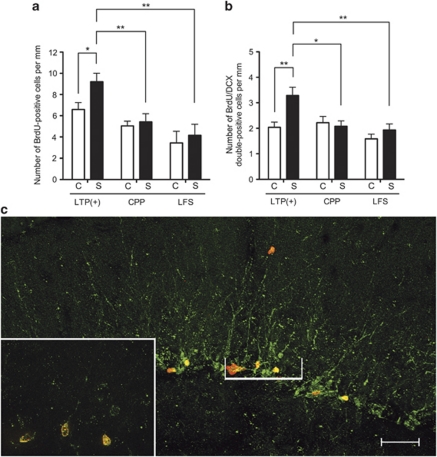
To examine whether the observed significant increase in precursor number as assessed in vitro was reflected in situ by an increase in proliferation and production of neurons in the dentate gyrus, BrdU was injected i.p. 4 days after perforant pathway stimulation. The mice were then killed either 30 min (to assess proliferation) or 1 week (to assess neuronal production) after the last BrdU injection. The stimulated hippocampus from the LTP(+) group contained a significantly higher number of BrdU-positive cells than the opposite control hemisphere (Figure 3a; stimulated hemisphere 9.2±0.8 cells mm−1 of dentate gyrus, control hemisphere 6.6±0.6 cells mm−1; P=0.038, n=5, Student's paired t-test). Furthermore, the LTP(+) group showed a significantly greater number of BrdU-positive cells than the CPP (Figure 3a; P=0.0096, n=5, Student's unpaired t-test) and LFS (P=0.028, n=5, Student's unpaired t-test) groups.
Figure 3.
Proliferation and differentiation was significantly enhanced in the LTP(+)-stimulated hippocampus. The effect of LTP induction on precursor cell proliferation and differentiation was assessed by BrdU incorporation. BrdU was administered 4 days after LTP induction, and mice were killed 30 min (proliferation) or 1 week (differentiation) after the last BrdU injection. (a) The number of BrdU-positive cells per length of the dentate gyrus (mm) was significantly enhanced in stimulated hippocampi of the LTP(+) group, relative to the unstimulated hemisphere (P=0.0381, n=5, Student's paired t-test). Furthermore, there were a greater number of BrdU-positive cells following LTP induction, compared with the CPP group (P=0.0096, n=5, Student's unpaired t-test) and LFS group (P=0.0028, n=6, Student's unpaired t-test). (b) Following LTP induction, the number of BrdU/DCX double-positive cells per perimeter length of the dentate gyrus (mm) was significantly enhanced in the stimulated hippocampus compared with the control hippocampus (P=0.0053, n=6, Student's paired t-test). BrdU/DCX-double labeling also revealed a significantly greater number of neurons in the stimulated hippocampi of the LTP(+) group compared with the stimulated hippocampi of the CPP (P=0.0121, n=6, Student's unpaired t-test) and the LFS (P=0.0084, n=6, Student's unpaired t-test) groups. (c) Representative maximum intensity projection image of BrdU (red) and DCX (green) immunostaining reconstructed from 40 optical sections of the LTP-stimulated hippocampus of the LTP(+) group. Inset shows a higher magnification, orthogonal view image of the indicated region in a single optical section, to illustrate that individual cells were double labeled. Scale bar: 50 μm in main image, 20 μm for inset. *P<0.05, **P<0.01. Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; C, control side; CPP, 3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid; DCX, doublecortin; LTP, long-term potentiation; S, stimulated side.
In line with in vitro results above, the LFS group (n=6) showed no statistically significant change in the number of BrdU-positive cells (Figure 3a; stimulated hemisphere, 4.2±1.1 cells mm−1 of dentate gyrus; control hemisphere, 3.5±1.1 cells mm−1; P>0.05, Student's paired t-test). Similarly, the CPP group (n=5) showed no statistically significant change in the number of BrdU-positive cells between the stimulated hemisphere (5.4±0.8 cells mm−1 of dentate gyrus) and the control hemisphere (5.1±0.5 cells mm−1; P>0.05, Student's paired t-test). Thus, it was only following LTP induction that any change in the number of BrdU-positive cells was observed.
To determine whether the increase in proliferation resulted in an increased number of neurons, we stained for doublecortin (DCX) expression, an early marker of cells committed to the neuronal lineage. The LTP(+) hippocampus contained a significantly higher number of BrdU/DCX double-positive cells than the control (Figure 3b; stimulated hemisphere 3.3±0.3 cells mm−1 of dentate gyrus, control hemisphere 2.0±0.2 cellsmm−1; P=0.0053, n=6, Student's paired t-test), whereas in the LFS group no significant change was observed (Figure 3b; stimulated hemisphere 1.9±0.3 cells mm−1 of dentate gyrus, control hemisphere 1.6±0.2 cells mm−1; P>0.05, n=6, Student's paired t-test). Similarly, no statistically significant difference in the number of BrdU/DCX double-positive cells between hemispheres was observed in the CPP group (Figure 3b; stimulated hemisphere 2.1±0.2 cells mm−1, control hemisphere 2.2±0.2 cells mm−1; P>0.05, n=6, Student's paired t-test). Moreover, the stimulated hemisphere of the LTP(+) group revealed a significantly greater number of BrdU/DCX double-positive cells than the CPP group (Figure 3b; P=0.0121, Student's unpaired t-test) and the LFS group (P=0.0084, Student's unpaired t-test). Therefore, only in the LTP(+)-stimulated hippocampus did enhanced proliferation lead to an increase in neurogenesis (Figures 3b and c).
Discussion
Here we have demonstrated that LTP-inducing stimulation of the hippocampus activates a large number of neural precursors capable of forming neurospheres in vitro, with a concomitant increase in the number of proliferative BrdU-positive cells and neuronally committed BrdU/DCX double-positive cells in vivo. Most importantly, it was only with successful and persistent LTP induction that precursor activation occurred, providing the first evidence for how synaptic activity associated with functions encoded by LTP, such as learning and memory, can specifically regulate neuronal production at the precursor level.
The two-fold increase in precursor number relative to unstimulated controls obtained by the induction of LTP indicates that the hippocampus contains a large pool of precursor cells that can be regulated by physiological inputs. We have reported previously that there is a large latent pool of hippocampal precursors that can be activated in vitro using depolarizing levels of K+, and that this population is normally quiescent.12 The precursor population activated in the present study appears to be similar to the population activated by high K+ in vitro, as no significant additive effect of LTP stimulation was observed in the presence of high K+. Furthermore, LTP stimulation revealed a comparable number of latent precursors to that observed with the addition of K+ in vitro, suggesting that LTP and K+ depolarization may share similar mechanisms of precursor cell activation. Nevertheless, very few large, stem cell-derived neurospheres (>250 μm diameter)12 were generated by the induction of LTP (data not shown). A lack of activation of the small number of stem cells believed to be present in the mouse hippocampus is consistent with our previous finding, where we showed activation of the precursor pool in a pilocarpine model of epilepsy.12 As such, we hypothesize that the successful induction of LTP activates latent precursor cells in the dentate gyrus, downstream of the quiescent stem cell population described recently by Lugert et al.13 This suggests that the true hippocampal stem cell may be tightly regulated in situ, as is the case for many tissue-specific stem cells, and only activated under abnormal circumstances such as neurogenic depletion.
Synaptic activation of such a large pool of latent precursors appears to be unique to the hippocampus, as we have previously shown that the subventricular zone precursor pool, which provides interneurons to the olfactory bulb, does not contain a latent precursor population.12 This may in part explain why hippocampal neurogenesis is highly responsive to environmental stimuli.
First, the activation of neurogenesis by LTP appears to be highly specific, as demonstrated by the inhibition of neurogenesis when NMDA receptors were blocked by CPP. It is now widely accepted that triggering LTP at synapses made by perforant pathway fibers onto dentate granule cells requires activation of the NMDA receptor and a postsynaptic rise in Ca2+ concentration.14, 15 Second, animals that received identical HFS stimulation, but failed to demonstrate LTP (LTP(−) group) or persistent LTP (early-LTP group), showed no change in hippocampal neurosphere numbers compared with controls. These results demonstrate that the activation process is highly sensitive and specific to LTP, and indicates that neither the delivery of HFS alone, which can result in modulatory neurotransmitter release capable of activating precursor cells in the subgranular zone,16, 17, 18 nor the mechanisms underlying the induction of early-LTP,19, 20 are sufficient to activate the precursor cell population in the dentate gyrus of the mouse.
Although we have shown here that only the induction of late-phase LTP leads to precursor activation, the exact mechanism by which LTP activates precursor cells requires further investigation. Although it has been reported previously that a large number of factors are able to stimulate neurogenesis, few researchers have been able to identify the site of action. Recent studies (unpublished observations) have revealed that K+ activation in vitro may occur via release of soluble growth factors; thus, it may be that LTP mediates precursor activation in a similar manner. Previous studies suggest that growth factor signaling may be a more likely mechanism than direct stimulation, as neural precursors in the hippocampus and subventricular zone express growth factor receptors,21, 22, 23 but lack the necessary receptors and ion channels to be directly activated.24, 25 Furthermore, new mRNA transcription and protein synthesis, which underlie the mechanisms of late-phase LTP,20 may be required to mediate the activation of precursor cells, as proliferation-related genes are significantly enhanced following the induction of LTP in vivo.26
The results of the BrdU-labeling experiments following LTP were largely consistent with our neurosphere assay results, and with previous rat stimulation experiments,8, 9, 10 showing significantly enhanced proliferation and neurogenesis in the stimulated hippocampus of the LTP(+) group compared with the CPP and LFS groups. Surprisingly, although we saw a significant inhibition of neurosphere formation after the delivery of HFS in the presence of CPP, this reduction of proliferation did not translate to our in vivo BrdU studies (which are consistent with previous results conducted in rats9). Previous reports have shown NMDA receptor antagonism increases neurogenesis,27 which again appears to be in conflict with our neurosphere assay results. This may indicate that there are multiple populations of precursors with varying proliferative capacities. As we only measured precursors capable of giving rise to several hundred progeny, inhibiting the activation of this population may not have a significant effect on late-stage progenitors, which only have limited proliferative capacity. The mechanism underlying this differential effect may relate to the reported ability of NMDA receptor antagonists to arrest the cell cycle in the G1 phase,28, 29 which would have a much more profound effect on precursor pools than on late-stage progenitors. Alternatively, our inhibitory effect of CPP on neurosphere formation following the delivery of HFS may also be dependent on in vivo synaptic activity. We replicated this experiment with the omission of electrophysiological stimulation, and found no effect on neurosphere formation when hippocampi were assayed 60 min, 3 h and 2 days following CPP administration (data not shown).
We also observed a difference in the read out of proliferation 4 days following HFS, between the formation of neurospheres in vitro, and the BrdU-labeled cells in vivo. This could be because of the activation state of the precursor cell. For instance, we saw a maximal effect on neurosphere formation 2 days following LTP-inducing HFS. We hypothesize that following the induction of LTP, the precursor cell has become primed to enable a greater response to the EGF/bFGF mitogens present in the culture media. However, 4 days following HFS, the precursor cell may have either lost this priming effect or matured into a slightly later-stage progenitor, still capable of proliferating (and thus able to incorporate BrdU), but no longer more responsive to EGF/bFGF stimulation in vitro than cells isolated from the untetanized hippocampus. At 11 days following HFS, some of the BrdU-labelled cells already express DCX, indicating the transition into neuronally committed progenitors/neuroblasts.30
The present findings, which reveal a strong correlation between the magnitude of LTP induced and the activation of a large latent neural precursor pool in the adult mouse dentate gyrus, could explain the ability of learning paradigms and environmental stimuli to increase the rate of neurogenesis in the hippocampus.1, 31 The intimate relationship between synaptic activity and neurogenesis is further highlighted by recent findings that show ablation of neurogenesis impairs the induction of LTP.32, 33
Clinical advances are now starting to show that in humans, patterns of electrical stimulation can be utilized to successfully treat psychiatric and neurological conditions such as depression, obsessive–compulsive disorder, Tourette's syndrome, neuropathic pain and movement disorders.34, 35 For instance, transcranial direct current stimulation has been shown to enhance aspects of memory performance in healthy controls and individuals with Parkinson's or Alzheimer's disease.34 Similarly, early evidence suggests that deep brain stimulation may have an impact on the progression of symptoms in patients with Alzheimer's disesase,36 and has been shown to promote subgranular zone proliferation and facilitate spatial memory performance in mice.7 Electroconvulsive seizure is widely used as an animal model of electroconvulsive therapy, the most successful treatment for severe depression in humans.37 Electroconvulsive seizure has been shown to robustly increase the proliferation and neuronal differentiation of hippocampal precursor cells in rodents5, 38, 39 and non-human primates.40 Furthermore, electroconvulsive seizure has been shown to increase hippocampal volume in patients with depression,41 suggesting a role for enhanced neurogenesis.
The future holds intriguing possibilities for the modulation of human memory and cognition. With further research, and an understanding of the mechanisms underlying the association between LTP and the modulation of precursor activation, proliferation, differentiation and survival, neurogenesis may be able to be harnessed to preserve and enhance hippocampal function in situations such as age-related cognitive decline and the development of neuropsychological diseases.
Acknowledgments
This study was funded by a National Health and Medical Research Council program Grant (P.F.B.), and was supported by the Estate of Dr Clem Jones AO. We thank the staff at the University of Queensland School of Biomedical Sciences Animal Facility for maintaining the animals used in this study, and Rowan Tweedale, Dr Peter Cook and Ashley Cooper for editorial assistance. We also thank Betty Mason-Parker (University of Otago) for technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1999;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Hamani C, Fawcett AP, Hutchinson WD, Lozano AM. The regulation of adult rodent hippocampal neurogenesis by deep brain stimulation. J Neurosurg. 2008;108:132–138. doi: 10.3171/JNS/2008/108/01/0132. [DOI] [PubMed] [Google Scholar]
- Stone SSD, Teixeira CM, DeVito LM, Kaslavsky K, Josselyn SA, Lozano AM, et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011;31:13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SK, Sun W, Park JJ, Jung MW. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem. 2006;86:322–329. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Chun SK, Sun W, Jung MW. LTD induction suppresses LTP-induced hippocampal adult neurogenesis. NeuroReport. 2009;20:1279–1283. doi: 10.1097/WNR.0b013e3283303794. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Murayama A, Sugiyama H, Inokuchi K. LTP induction within a narrow critical period of immature stages enhances the survival of newly generated neurons in the adult rat dentate gyrus. Mol Brain. 2010;3:13–20. doi: 10.1186/1756-6606-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker TL, White A, Black DM, Wallace RH, Sah P, Bartlett PF. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J Neurosci. 2008;28:5240–5247. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Götz M, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation - a decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long-term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Bronzino JD, Kehoe P, Mallinson K, Fortin DA. Increased extracellular release of hippocampal NE is associated with tetanization of the medial perforant pathway in the freely moving adult male rat. Hippocampus. 2001;11:423–429. doi: 10.1002/hipo.1057. [DOI] [PubMed] [Google Scholar]
- Jhaveri DJ, Mackay EW, Hamlin AS, Marathe SV, Nandam LS, Vaidya VA, et al. Norepinephrine directly activates adult hippocampal precursors via β3-adrenergic receptors. J Neurosci. 2010;30:2795–2806. doi: 10.1523/JNEUROSCI.3780-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the ‘long' in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Abraham WC. How long will long-term potentiation last. Phil Trans B. 2003;358:735–744. doi: 10.1098/rstb.2002.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Maurer MH, Tripps WKC, Feldmann RE, Kuschinsky W. Expression of vascular endothelial growth factors and its receptors in rat neural stem cells. Neurosci Lett. 2003;344:165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- Lee D-C, Hsu Y-C, Chung Y-F, Hsiao C-Y, Chen S-L, Chen M-S, et al. Isolation of neural stem/progenitor cells using EGF/FGF1 and FGF1B promoter-driven green fluorescence from embryonic and adult mouse brains. Mol Cell Neurosci. 2009;41:348–363. doi: 10.1016/j.mcn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Stafford MR, Bartlett PF, Adams DJ. Purinergic receptor activation inhibits mitogen-stimulated proliferation in primary neurospheres from the adult mouse subventricular zone. Mol Cell Neurosci. 2007;35:535–548. doi: 10.1016/j.mcn.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Walker TL, Yasuda T, Adams DJ, Bartlett PF. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J Neurosci. 2007;27:3734–3742. doi: 10.1523/JNEUROSCI.5060-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MM, Mason-Parker SE, Tate WP, Abraham WC, Williams JM. Rapidly induced gene networks following induction of long-term potentiation at perforant path synapses in vivo. Hippocampus. 2011;21:541–543. doi: 10.1002/hipo.20770. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JY, Kim BW, Lee JS, Park JY, Kim S, Yun YJ, et al. Activation of NMDA receptors increases proliferation and differentiation of hippocampal neural progenitor cells. J Cell Sci. 2007;120:1358–1370. doi: 10.1242/jcs.002154. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kanno T, Oshima T, Miwa H, Tashiro C, Nishizaki T. The NMDA receptor NR2A subunit regulates proliferation of MKN45 human gastric cancer cells. Biochem Biophys Res Commun. 2008;367:487–490. doi: 10.1016/j.bbrc.2007.12.167. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Khun CM, Winkler J, Aigner L, Khun HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neur. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Singer BH, Gamelli AE, Fuller CL, Temme SJ, Parent JM, Murphy GG. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc Natl Acad Sci USA. 2011;108:5437–5442. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Koehl M, Wiesner T, Grosjean N, Revest J-M, Piazza P-V, et al. Conditional reduction of adult neurogenesis impairs bidirectional hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2011;108:6644–6649. doi: 10.1073/pnas.1016928108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreines JL, McClintock SM, Holtzheimer PE. Neuropsychologic effects of neuromodulation techniques for treatment-resistant depression: A review. Brain Stimul. 2011;4:17–27. doi: 10.1016/j.brs.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O'Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, et al. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010;68:521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- Bolwig TG, Madsen TM. Electroconvulsive therapy in melancholia: The role of hippocampal neurogenesis. Acta Psychiatr Scand. 2007;115:130–135. doi: 10.1111/j.1600-0447.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wojtowicz JM, Burnham WM. Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol. 2000;165:231–236. doi: 10.1006/exnr.2000.7458. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: A volumetric magnetic resonance imaging study. J ECT. 2010;26:62–67. doi: 10.1097/YCT.0b013e3181a95da8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.