Abstract
Nylon-3 polymers contain β-amino acid-derived subunits and can be viewed as higher homologues of poly(α-amino acids). This structural relationship raises the possibility that nylon-3 polymers offer a platform for development of new materials with a variety of biological activities, a prospect that has recently begun to receive experimental support. Nylon-3 homo- and copolymers can be prepared via anionic ring-opening polymerization of β-lactams, and use of an N-acyl-β-lactam as co-initiator in the polymerization reaction allows placement of a specific functional group, borne by the N-acyl-β-lactam, at the N-terminus of each polymer chain. Controlling the unit at the C-termini of nylon-3 polymer chains, however, has been problematic. Here we describe a strategy for specifying C-terminal functionality that is based on the polymerization mechanism. After the anionic ring-opening polymerization is complete we introduce a new β-lactam, approximately one equivalent relative to the expected number of polymer chains. Because the polymer chains bear a reactive imide group at their C-termini, this new β-lactam should become attached at this position. If the terminating β-lactam bears a distinctive functional group, that functionality should be affixed to most or all C-termini in the reaction mixture. We use the new technique to compare the impact of N- and C-terminal placement of a critical hydrophobic fragment on the biological activity profile of nylon-3 copolymers. The synthetic advance described here should prove to be generally useful for tailoring the properties of nylon-3 materials.
Introduction
Polymeric materials have drawn considerable attention in recent years because of their biomedical promise for applications that include tissue engineering, gene delivery and therapeutic agent development.1 Recent results from our group have shown that nylon-3 polymers can display promising biological characteristics, as exemplified by antibacterial activities, 2,3 cell-adhesion properties4 or lung-surfactant mimicry.5 The similarity of the nylon-3 and protein backbones, which feature β-amino acid-derived subunits and α-amino acid-derived subunits, respectively, suggests that nylon-3 polymers could be intrinsically biocompatible. Synthetic advances that enhance our ability to control the molecular structure of nylon-3 chains should improve prospects for tailoring the properties of these materials. The work described here focuses on placing specific functional groups at nylon-3 polymer C-termini.
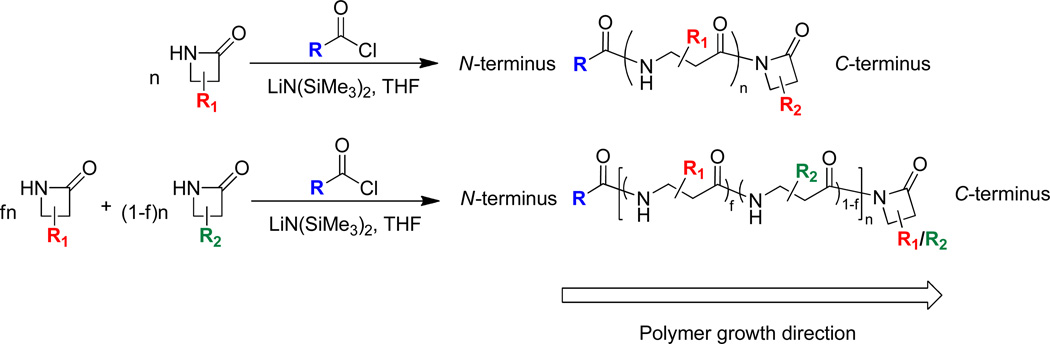
Nylon-3 materials are prepared by anionic ring-opening polymerization (AROP) of β-lactams.6,7 Use of an N-acyl-β-lactam as co-initiator facilitates control of chain length in the AROP process. The N-acyl-β-lactam can be synthesized in a separate operation, or this agent can be generated in situ from an acid chloride or anhydride at the start of the polymerization reaction.7 The latter approach is particularly convenient since many acid chlorides and anhydrides are commercially available. The acyl fragment of the co-initiator defines the unit at the N-termini of the nylon-3 polymer chains (Figure 1). We have shown that a broad variety of functional groups can be placed at nylon-3 N-termini, via appropriate co-initiator choice and, in some cases, via post-polymerization transformations.7 The identity of the N-terminal unit can exert a substantial impact on the biological properties of nylon-3 polymers.2c, 5
Figure 1.
Polymerization of β-lactams and structures of the resulting nylon-3 homopolymer (top) or random copolymer (bottom).
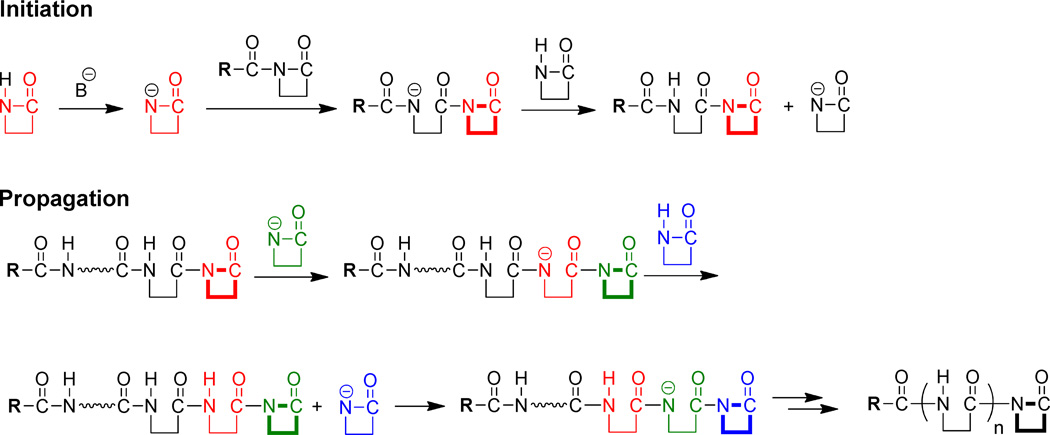
Optimization of nylon-3 behavior and evaluation of new applications for this relatively under-explored class of polymers would be facilitated if it were possible to place specific groups at the C-termini of the polymer chains. The AROP mechanism is expected to produce polymers with a C-terminal N-acyl-β-lactam (Scheme 1), and this imide functionality should provide a site of unique electrophilic reactivity within each polymer chain. The proposed existence of C-terminal imide group is consistent with the generation of diblock nylon-3 copolymers via sequential addition of two distinct β-lactams during AROP reactions.6, 7 We have provided direct evidence for the existence of a C-terminal imide moiety via 13C NMR spectroscopy.7 One might expect that this imide moiety would be broadly susceptible to reaction with nucleophiles, but we found it to be difficult or impossible to achieve efficient reaction of nylon-3 polymer end groups with amines or alcohols (unpublished), despite reports that the nylon-3 polymer shown in Figure 2 undergoes C-terminal reaction with benzylamine,8 methyl α-D-glucoside or a polysaccharide.9 To the best of our knowledge, there are no other reports describing C-terminal functionalization of nylon-3 polymers. Our failure to achieve efficient C-terminal functionalization with simple nucleophiles led us to explore specifically functionalized β-lactams for this purpose. Since the AROP mechanism requires β-lactamate attack on the C-terminal imide for chain elongation, we reasoned that addition of one equivalent (per polymer chain) of a new β-lactam to the reaction vessel, after polymerization was complete, would enable us to generate nylon-3 samples in which most or all polymer chains bear a single, defined functional group at the C-terminus.
Scheme 1.
Proposed mechanism of anionic ROP of β-lactams. The N-terminus (R) and the C-terminus (bold bonds) during and after the polymerization are highlighted.
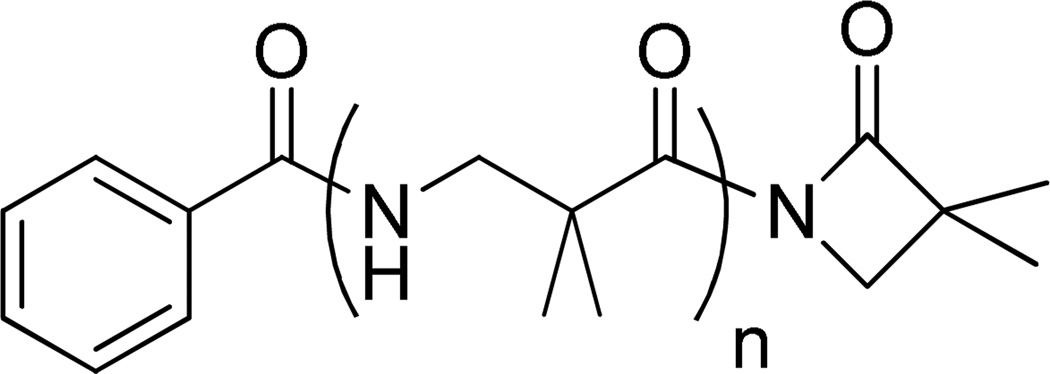
Figure 2.
Structure of the nylon-3 polymer that was reported to undergo C-terminal reaction with benzylamine, methyl α-D-glucoside or a polysaccharide.8, 9
The ability to place diverse and specific groups at the C-terminus of a nylon-3 polymer would represent a very useful tool for function-oriented design. The established methodology for controlling the N-terminal moiety has allowed us to explore the impact of varying this parameter on the activity profile of random nylon-3 copolymers that mimic natural host-defense peptides (HDPs)2c or lung-surfactant proteins.5 It would be valuable to conduct complementary studies involving C-terminal moieties, in order to determine whether the two ends of nylon-3 polymer chains are functionally equivalent. Differences between modifications at N- vs. C-termini are commonly encountered among peptides and proteins, but such differences might not be expected for nylon-3 copolymers if the subunit sequence is truly random.
A robust strategy for placing diverse functional groups at nylon-3 chain C-termini should ultimately allow expansion of the range of applications for these materials. For example, immobilization of antimicrobial copolymers can lead to surfaces that resist colonization by microbes such as bacteria and fungi,10,11 but pursuing this goal with nylon-3 copolymers would require the ability to place specific groups at both ends of the polymer chains. Optimal antibacterial activity in solution requires that a hydrophobic unit be placed at one end of each polymer chain (so far we have been able to explore only the N-terminus for this purpose),2c and it seems likely that comparable tailoring of the polymer chains would be required at surfaces as well. In addition, each polymer chain must bear a single group with unique reactivity, such as a thiol, for covalent attachment to the surface of interest; ideally, this group would be placed at the other chain terminus, relative to the hydrophobic group. Thus, this application requires orthogonal strategies for functionalizing the N- and C-termini of nylon-3 copolymer chains. The resulting dual-functionalized polymers would have other uses as well; for example, the C-terminal thiol could be a site for fluorophore attachment, 12 to generate molecules that enable studies of antibacterial mechanism of action,13 or the C-terminal thiol could enable the preparation of polymer-protein conjugates.14
Results and Discussion
C-terminal functionalization of a nylon-3 homopolymer
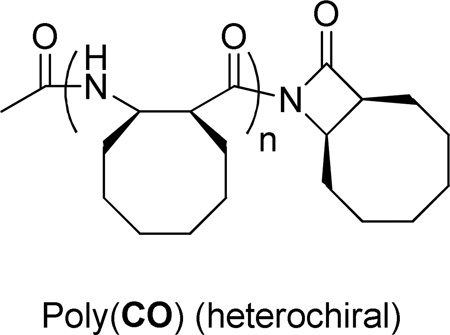
Our initial studies involved poly(cyclooctyl β-lactam), poly(CO), which is more easily characterized than many other nylon-3 homopolymers because of favorable solubility properties.7 We attempted to introduce C-terminal functionality by adding various amine or alcohol nucleophiles (along with a base) to the reaction vessel after the formation of poly(CO) was complete. These efforts included experiments that followed conditions reported for C-terminal functionalization of nylon-3 polymers with amines8 and experiments based on methods for nucleophilic opening of N-Boc β-lactams.15 None of these approaches led to efficient C-terminal modification of poly(CO); in each case, poly(CO) itself was the major product we recovered. Exploratory studies suggested that certain thiol nucleophiles (e.g., benzyl mercaptan) could react with the C-terminal imide of poly(CO) in a highly polar solvent such as dimethylacetamide. Moreover, it appeared that the thiol could facilitate the addition of some amine nucleophiles to the C-terminus of poly(CO), presumably in a two-stage process: ring-opening with the thiol to generate a C-terminal thioester, which reacts with the amine to form a C-terminal amide. (See Supporting Information for details.) However, this thiol-based strategy has so far been too capricious to be used for functionalization of the nylon-3 random copolymers that will be discussed in the next section.
Inspired by results of Gerona-Navarro et al.,16 we examined 1,8-diazabicyclo[5.4.0] undec-7-ene (DBU) as an additive to facilitate the ring-opening of the C-terminal imide of poly(CO) by alcohols. When DBU and a simple alcohol were added to poly(CO) in THF after completion of the AROP reaction, modest to good levels of alcohol incorporation, presumably to generate C-terminal esters, were observed within 24 h. Without DBU, no reaction could be detected between alcohols and poly(CO) even after three days. Unfortunately, attempts to use DBU with alcohols bearing additional functional groups or with amines were largely unsuccessful (See Supporting Information), which led us to seek a more reliable method.
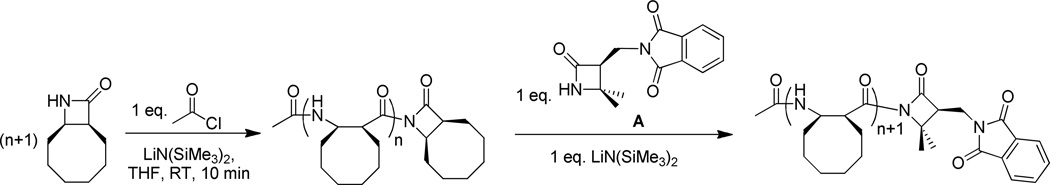
As we were grappling with the challenge of attaching diverse groups to the nylon-3 C-terminus via amine or alcohol nucleophiles, we began to consider the prospect of using a suitably functionalized β-lactam for this purpose. The competence of β-lactamate nucleophiles is evident from the success of the AROP process. Indeed, a simple experiment suggested that this approach could be fruitful: shortly after the AROP to form poly(CO) was complete, without isolation of poly(CO) from the reaction vessel, we added one equivalent of β-lactam A relative to the expected number of polymer chains, plus one equivalent of the base used in the polymerization process [LiN(SiMe3)2] (Figure 3). After 24 hr, the polymer was precipitated by addition of pentane. Mass spectrometric analysis (Figure 4) suggested that most polymer chains had incorporated one equivalent of A, presumably at the C-terminus.
Figure 3.
C-terminal functionalization of poly(CO) using β-lactam A as nucleophile.
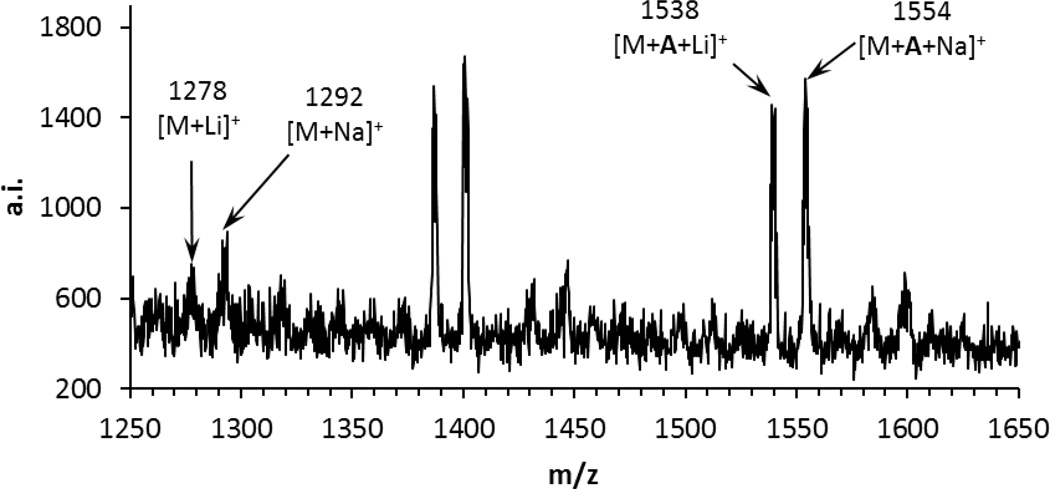
Figure 4.
A portion of the MALDI-TOF spectrum of the final product from the procedure indicated in Figure 3. M refers to poly(CO) containing eight subunits with an acetyl N-terminus and an imide C-terminus. M+A refers to this octamer plus the unit indicated as A in Figure 3; presumably this species has been formed by attack of the β-lactamate formed from A on the C-terminal imide of M. The pair of signals near 1400 MW are unit A plus a poly(CO) heptamer with an acetyl N-terminus and an imide C-terminus. Additional MALDI-TOF data may be found in the Supporting Information.
C-terminal functionalization of antibacterial nylon-3 random copolymers
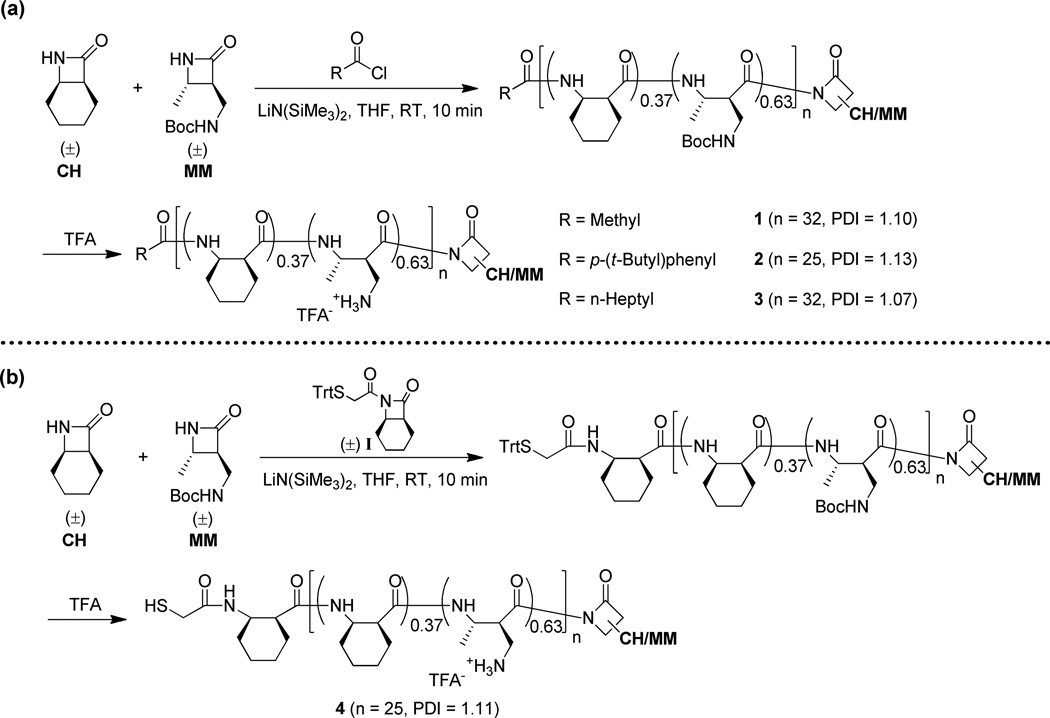
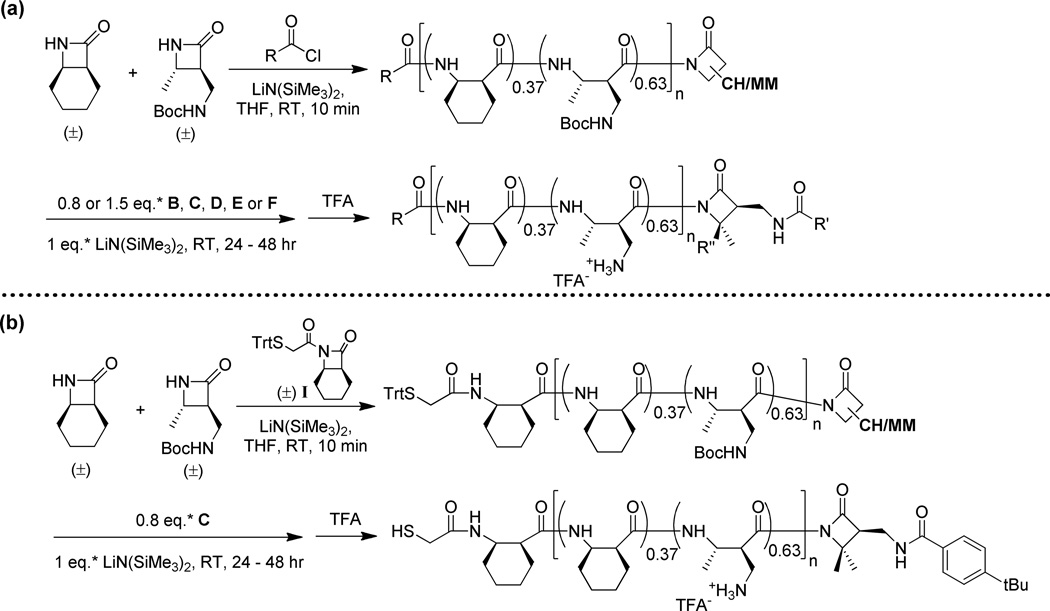
Having identified a strategy that provided an acceptable degree of C-terminal functionalization with poly(CO), we sought to extend this strategy to nylon-3 random copolymers that had previously been shown to display antibacterial activity.2a, c Our target polymers were prepared by copolymerizing β-lactams CH (for "cyclohexyl") and MM (for "monomethyl") in a 37:63 molar ratio (Scheme 2). Earlier results revealed that varying the N-terminal group can cause significant changes in activity among 37:63 CH:MM materials. Polymer 1, bearing the small acetyl group at the N-terminus, displays relatively weak antibacterial activity and weak hemolytic activity. Changing the N-terminal group to p-t-butylbenzoyl, to generate polymer 2, enhances antibacterial activity and provides the highest selectivity for inhibition of bacterial growth relative to hemolysis among all the nylon-3 random polymers we have examined.2a, c Polymer 3, with an N-terminal octanoyl group, displays an activity profile similar to that of 2. Polymer 4 is interesting because the N-terminus bears a thiol group, which can be used to attach the polymer to a surface or to other molecules.2c Exposing any sample among 1 – 4 to amine nucleophiles or simple alcohols in the presence of DBU did not produce C-terminally functionalized polymers, which supports our conclusion that this modification strategy is capricious.
Scheme 2.
Preparation and structures of selected antibacterial 37:63 CH:MM nylon-3 random copolymers. Trt = Trityl, CH/MM = side chain from either CH or MM.
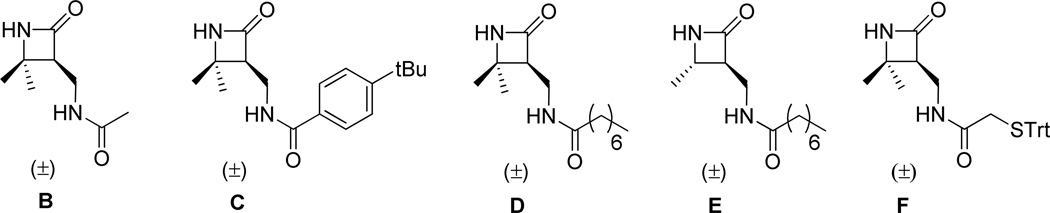
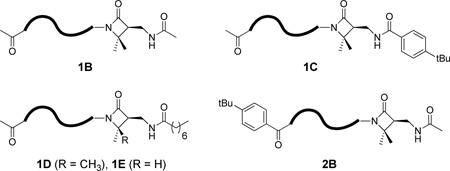
We used functionalized β-lactams B – F (Figure 5) to introduce specific groups at the C-termini of nylon-3 copolymers 1 – 4, as illustrated in Scheme 3. These β-lactams are derivatives of two previously reported β-lactams7 that bear a protected side chain amino group. In B – F, this side chain nitrogen has been acylated to introduce an acetyl, p-t-butylbenzyoyl, octanoyl or thioacetyl group; thus, this β-lactam set allows us to introduce C-terminal groups that mirror the N-terminal groups found among nylon-3 copolymers 1 – 4.
Figure 5.
Functionalized β-lactams employed in the C-terminal functionalization of 37:63 CH:MM nylon-3 random copolymers.
Scheme 3.
Preparation of C-terminal functionalized 37:63 CH:MM nylon-3 random copolymers using β-lactam B – F as nucleophile. R” is hydrogen when E is used, and is methyl when B, C, D or F is used. See Table 1 for all combinations of R and R’ in this study. * Relative to the anticipated number of polymer chains.
We explored the β-lactam-based C-terminal functionalization strategy by using two different stoichiometries, 0.8 or 1.5 equiv. relative to the anticipated number of polymer chains, for the β-lactam that was added after the polymerization was complete. This variation was motivated by our desire to functionalize the largest possible proportion of polymer chains, but to minimize the formation of polymer chains bearing more than one unit derived from this β-lactam. Post-polymerization incorporation of the β-lactam was observed in all cases, but the degree of C-terminal functionalization, estimated by 1H NMR, seemed to vary somewhat with the identity of the β-lactam (Tables 1 and S3). β-Lactam B provided higher levels of functionalization than expected based on the anticipated number of polymer chains; however, quantification in this case was hampered because the NMR signal for the C-terminal acetyl CH3 group partially overlaps with CH resonances. The deprotonated froms of β-lactams bearing an octanoyl group, E and F, appear to be poor nucleophiles, based on the low levels of incorporation observed.
Table 1.
Characterization of C-terminally functionalized polymers generated from selected 37:63 CH:MM nylon-3 random copolymers.a
 | |||||
|---|---|---|---|---|---|
| Polymerb | nc | PDIc | Eqd | Fe | %f |
| 1B-a | 26 | 1.13 | 0.8 | 0.86 | 108 |
| 1B-b | 31 | 1.07 | 1.5 | 1.6 | 107 |
| 1C-a | 29 | 1.09 | 0.8 | 0.53 | 66 |
| 1C-b | 32 | 1.12 | 1.5 | 0.88 | 59 |
| 1D-a | 33 | 1.06 | 0.8 | 0.11 | 14 |
| 1D-b | 29 | 1.09 | 1.5 | 0.14 | 9 |
| 1E | 28 | 1.11 | 0.8 | 0.35 | 44 |
| 2B-a | 19 | 1.13 | 0.8 | 1.1 | 137 |
| 2B-b | 25 | 1.12 | 1.5 | 1.9 | 127 |
R” is hydrogen for 1E and methyl for the other polymers; see Table S3 for additional examples.
In each polymer designation, the numeral defines the N-terminal group (R; see also Scheme 2), and the capital letter defines the C-terminal group (based on the β-lactam employed as nucleophile; see also Figure 5). When small letters (a or b) are present, they indicate distinct batches of the polymer that were prepared with differing amounts of the β-lactam employed as the final nucleophile.
The average degree of polymerization (n) and the polydispersity (PDI) were determined for polymers bearing Boc protecting groups on the side chain amines using a GPC equipped with a MALS-RI detector; polymer samples eluted with THF, dn/dc = 0.1.
The number of equivalents of β-lactam nucleophile added after the polymerization reaction was complete relative to the amount of co-initiator employed in the polymerization; the molar amount of co-initiator is supposed to determine the number of polymer chains.
The average number of C-terminal functional groups per polymer chain, estimated by 1H NMR.
The efficiency of C-terminal functionalization = F/Eq × 100.
GPC analysis of each C-terminally functionalized polymer showed a unimodal and symmetric peak (see Supporting Information), and each sample had molecular weight (MW) and polydispersity comparable to that of an analogous polymer sample that had not be subjected to the C-terminal functionalization step (See Tables 1 and S3). Thus, the relatively good control of polymer length offered by the AROP process is not eroded by this C-terminal functionalization technique. Nevertheless, it must be noted that the NMR-based analysis provides only average values for extent of C-terminal functionalization; the resulting polymer population almost certainly includes chains bearing two or more units derived from the β-lactam added after the AROP step as well as chains that do not contain this unit at all.
Effect of C-terminal groups on biological activities of nylon-3 copolymers
In our previous studies of antibacterial nylon-3 materials we explored several polymer parameters in an effort to maximize growth inhibition for four different bacteria and minimize disruption of human red blood cell mezmbranes ("hemolysis").2a,c The antibacterial effects are believed to reflect disruption of bacterial membranes,2b and comparing bacterial growth inhibition with hemolytic activity provides a measure of the selectivity with which a particular polymer targets prokaryotic cell membranes relative to eukaryotic cell membranes. This study identified 37:63 CH:MM as a particularly favorable composition, and lengths in the 20-mer to 30-mer range as ideal. A number of acyl units at the N-terminus were compared, and moderate hydrophobicity at this position was found to be optimal. In particular, the p-t-butylbenzoyl N-terminal unit (in 2) and the octanoyl N-terminal unit (in 3) led to the most favorable activity profiles. The non-hydrophobic acetyl N-terminal unit (in 1), in contrast, provided only weak antibacterial activity. Highly hydrophobic N-terminal units, such as dodecanoyl, caused a large increase in hemolytic activity, and further increases in N-terminal hydrophobicity (e.g., to hexadecanoyl) led to declining antibacterial activity while hemolytic activity remained strong. The highly hydrophobic end groups were shown to promote polymer chain aggregation under bioassay conditions.2c
Is there an operational difference between the N- and C-termini of CH-MM copolymers in terms of introducing the critical hydrophobic moiety? The technique described above for introducing specific groups at nylon-3 chain C-termini via post-polymerization addition of stoichiometric β-lactams such as B – E allows us to address this question. The answer is important because some future applications will require polymer chains that bear specific and distinct functionality at the two termini, e.g., a thiol at one end for attachment to a macromolecule or surface, and a hydrophobic group at the other end for antibacterial activity. It should be noted that the C-termini of the copolymers analyzed in our previous studies2a–c were presumably heterogeneous, since either a hydrophobic CH unit or a cationic MM unit could occupy this position in any given nylon-3 molecule.
We examined selected C-terminally functionalized nylon-3 copolymers for growth-inhibitory activity toward four bacteria, Escherichia coli, Bacillus subtilis, Enterococcus faecium and Staphylococcus aureus, and for hemolytic activity. These studies involved laboratory strains of E. coli17 and B. subtilis,18 but the E. faecium19 and S. aureus20 strains were originally isolated in the clinic and display resistance to conventional antibiotics. Table 2 shows the minimum inhibitory concentration (MIC) values for the bacteria and the minimum hemolytic concentration (MHC) values for a set of C-terminally functionalized polymers along with comparison data for analogous polymers that were not subject to a C-terminal modification step. (The biological activities of other polymers can be found in Table S4.) For polymers 1 and 2, lacking defined C-termini, the MIC and MHC values in Table 2 are consistent with published results,2d which highlights the reproducibility of the β-lactam AROP process.
Table 2.
Growth-inhibitor activities of selected 37:63 CH:MM nylon-3 random copolymers toward four bacteria and lytic activity toward human red blood cells.
| Polymer | na | Fb | MIC (µg/mL)c | MHC (µg/mL)d |
|||
|---|---|---|---|---|---|---|---|
| E. coli | B. subtilis | S. aureus | E. faecium | ||||
| 1 | 32 | - | 200 | 6.25 | 200 | 50 | 800 |
| 2 | 25 | - | 12.5 | 3.13 | 25 | 12.5 | 800 |
| 1B-a | 26 | 0.86 | 200 | 6.25 | 400 | 100 | 1600 |
| 1B-b | 31 | 1.6 | 200 | 6.25 | 200 | 50 | 1600 |
| 1C-a | 29 | 0.53 | 50 | 6.25 | 100 | 25 | 25 |
| 1C-b | 32 | 0.88 | 50 | 12.5 | 200 | 50 | 3.13 |
| 2B-a | 19 | 1.1 | 25 | 3.13 | 50 | 25 | 800 |
| 2B-b | 25 | 1.9 | 25 | 6.25 | 100 | 25 | 800 |
The average number of the specific C-terminal functional groups per polymer chain.
MIC = minimum inhibitory concentration.
MHC = minimum hemolytic concentration.
The data for 1 and 2 illustrate the previously reported2c importance of a terminal hydrophobic group: nylon-3 copolymer 2 shows significantly higher antibacterial activity for E. coli and E. faecium than does 1. (The MIC values are determined based on serial two-fold dilutions, and the uncertainty of a given MIC value is one two-fold dilution on either side.) Neither polymer shows strong hemolytic activity; in both cases the concentration must approach 1000 µg/mL before hemolysis can be detected. The results obtained for polymers 1B and 2B show that C-terminal functionalization with a β-lactam that bears an acetyl group on the side chain nitrogen, a unit that is neither strongly hydrophobic nor strongly hydrophilic, has little effect on either bacterial growth inhibition or hemolytic activity. Thus, placing a defined side chain at the C-terminus exerts only minimal impact on biological activity; the observation that a small terminal side chain is relatively benign constitutes an important control for interpretation of the data for polymers 1C.
The data in Table 2 for 1C suggest that the N- and C-termini of 37:63 CH:MM random copolymer chains are not functionally equivalent as sites for introducing a hydrophobic end group. If the two chain ends were equivalent, then we would expect the biological activity profile of 2B (p-t-butylbenzoyl at the N-terminus, acetyl at the C-terminus) to match the biological activity profile of 1C (acetyl at the N-terminus, p-t-butylbenzoyl at the C-terminus). In terms of bacterial growth inhibition, 2B and 1C are similar, although perhaps 1C is slightly less effective than 2B. This trend can be seen by comparing 2B-a and 1C-b, which have similar extents of C-terminal functionalization; although the differences in MIC values are within uncertainty for all bacteria, it seems noteworthy that for each species the MIC for 2B-a is lower than that for 1C-b. However, a striking difference is seen in hemolytic activities between 2B, bearing the hydrophobic group at the N-terminus, and 1C, bearing the hydrophobic group at the C-terminus: 1C is much more hemolytic. As a result, the nylon-3 copolymer with the N-terminal hydrophobic group is a selective antibacterial agent, while the version with the C-terminal hydrophobic group is not selective for prokaryotic vs. eukaryotic cells. A significant difference in hemolytic activity can be observed in the 1C set as a function of extent of C-terminal modification, with 1C-b (~88% modification) more hemolytic than 1C-a (~53% modification). Comparable promotion of hemolytic activity was observed for polymer samples bearing C-terminal octanoyl groups: 1E with ~35% C-terminal modification displayed an MHC value of 3.1 µg/mL, while 1D with only ~11 % or ~14% C-terminal modification had an MHC value of 800 µg/mL, which matches the MHC of 3 (N-terminal octanoyl group; undefined C-termini) (see Table S4).
Overall, our data suggest that placing a moderately hydrophobic group at the C-terminus, such as the p-t-butylbenzoyl group in 1C, enhances hemolytic activity of 37:63 CH:MM nylon-3 copolymers, but placing the same hydrophobic group at the N-terminus (e.g., 2B) does not influence hemolytic activity, relative to a polymer with acetyl at both termini (1B). The comparisons in Table 2 may seem somewhat indirect, since the samples of 1C and 2B differ in terms of extent of C-terminal functionalization (%) as well as degree of polymerization (n), but the conclusion is nevertheless valid. Differences in degree of polymerization are small and therefore unlikely to be significant; n = 29 for sample 1C-a, and n = 25 for sample 2B-b, but the former sample is much more hemolytic than the latter. Differences in extent of terminal functionalization are larger, but nature of the variations allows a solid conclusion. For samples 2B, ~100% of the polymer chain bear the hydrophobic unit at the N-terminus, because of the nature of the initiation process. In contrast, only 59–66% of the polymer chains bear the hydrophobic unit at the C-terminus in samples 1C. Despite the smaller extent of hydrophobic modification, polymers 1C are much more hemolytic than polymers 2B. The contrast between the high hemolytic activity of partially C-terminally functionalized 1C and the low hemolytic activity of fully N-terminally functionalized 2B strongly supports the conclusion that the N- and C-termini of nylon-3 copolymers are not equivalent sites for introduction of specific modifications.
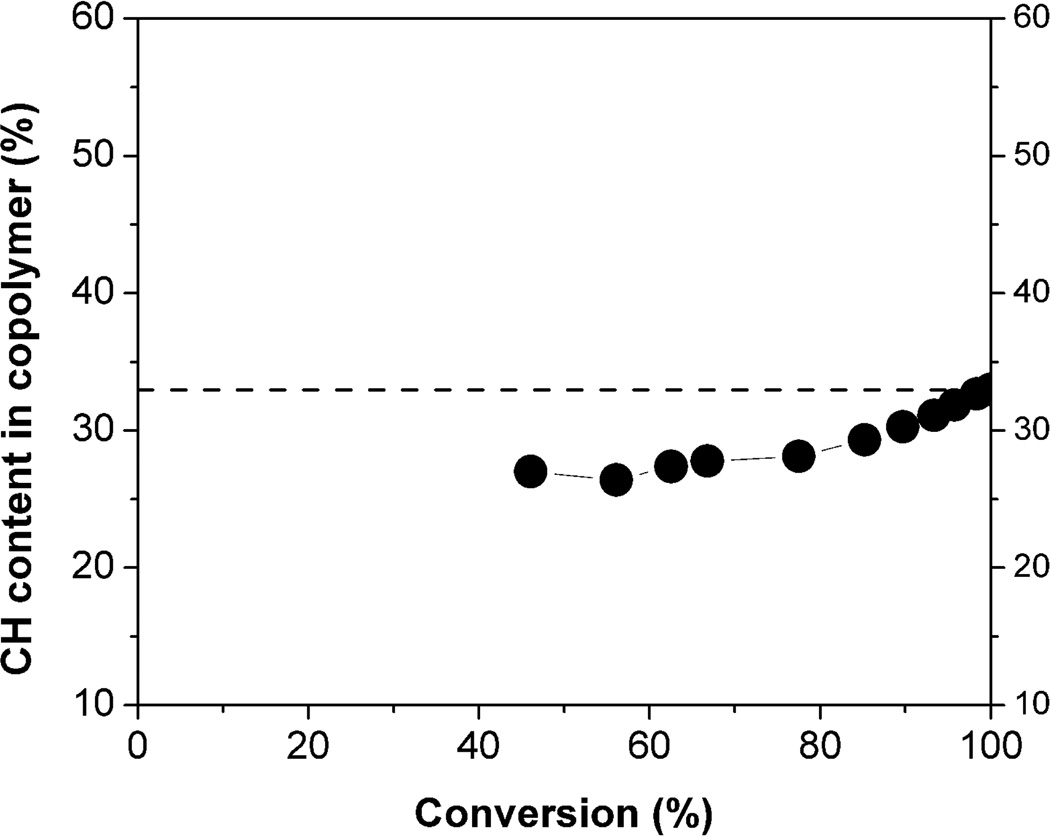
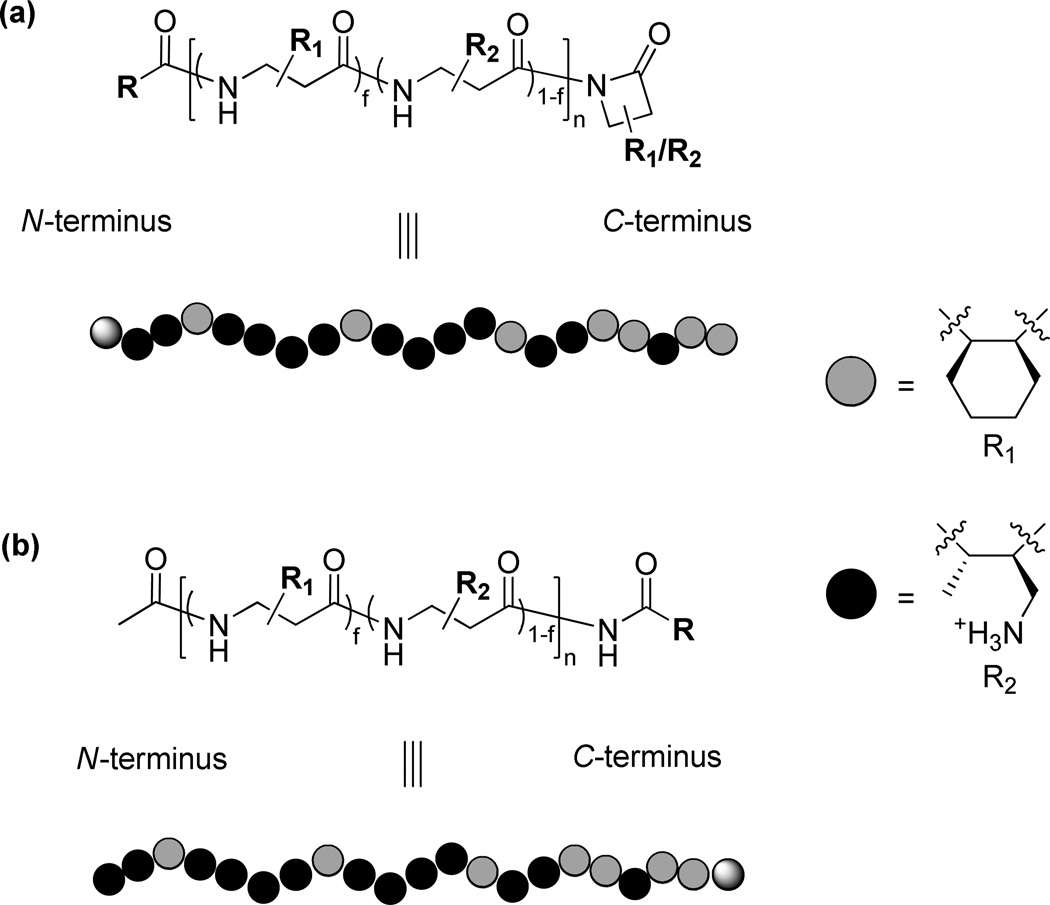
To explain the differential impact of N- vs. C-terminal placement of hydrophobic end groups, we invoke previous studies of CH + MM copolymerization.21 Those studies indicate that although the subunit distribution is fairly random along the polymer chains, there is some degree of compositional drift, which leads to an increase in CH prevalence from N- to C-terminus. Figure 6 illustrates this effect, showing how polymer composition changes as a function of the extent of β-lactam conversion for a polymerization that starts with a 33:67 CH:MM ratio. (This ratio differs slightly from that used to prepare the nylon-3 copolymers discussed above (63:37), but the conclusions drawn from Figure 6 should be general.) Polymeric material isolated after only partial conversion of the starting β-lactam mixture contains somewhat less of the subunit derived from CH than would be expected based on the starting β-lactam ratio (e.g., ~28% rather than 33% CH for material isolated after ~50% conversion). Since the starting ratio is accurately reflected in the polymer obtained after complete conversion, one can deduce that the portions of the polymer chain near the C-terminus must be somewhat enriched in hydrophobic CH units, and the portions near the N-terminus must be somewhat enriched in hydrophilic MM units. Based on this deduction, we can offer an explanation for the differential effects on hemolytic activity of placing the p-t-butylbenzoyl group at the N- vs. the C-terminus of 37:63 CH:MM nylon-3 copolymer samples: C-terminal placement leads to greater local hydrophobicity than does N-terminal placement. This effect is illustrated by the cartoons in Figure 7. Our previous studies involving exclusively N-terminal functionalization of 37:63 CH:MM nylon-3 copolymers showed that very large hydrophobic groups lead to high hemolytic activity;2c we now speculate that the impact of a given hydrophobic unit (such as p-t-butylbenzoyl) is larger at the C-terminus than at the N-terminus because the C-terminus has an intrinsically higher local hydrophobicity for CH:MM copolymers.
Figure 6.
The composition of copolymers (fraction of unit derived from CH) as a function of total conversion for the copolymerization of β-lactams CH and MM at 0 °C. The starting ratio of CH:MM is 33:67. CH content in polymer samples was deduced from the amounts of unreacted CH and MM β-lactams measured by GC after various extents of reaction.
Figure 7.
Proposed structures of 37:63 CH:MM nylon-3 polymers with the C-terminus not defined (a) or modified by post-polymerization addition of a specific β-lactam nucleophile (b). The ball cartoons are meant to illustrate the different effects on local hydrophobicity at the N-terminus vs. the C-terminus that result from placing a given hydrophobic group at that terminus. The faded, filled and shaded balls represent CH subunit (R1), MM subunit (R2) and the terminal group (R), respectively.
We tested this hypothesis by measuring the critical aggregation concentration (CAC) of several C-terminally functionalized nylon-3 copolymers using a dye solubilization method. The measurements were performed in Tris-buffered saline, the medium that was used for hemolysis assays. As shown in Table 3, the polymers that are C-terminally functionalized with the moderately hydrophobic p-t-butylbenzoyl group display CAC ≤ 220 µg/mL (1C-a or 1C-b), while no dye solubilization could be detected up to 3200 µg/mL for the polymers that do not have defined C-termini (1 or 2) or that bear the small acetyl group at the C-terminus (1B-a or 2B-a). These results suggest that placement of a hydrophobic group at the C-terminus leads to a greater self-association propensity than is observed for analogues that bear the hydrophobic appendage at the N-terminus and either lack defined C-termini or have an acetyl group at the C-terminus. The trend in CAC values parallels the trend in hemolytic activities: polymers with a hydrophobic group at the C-terminus are more hemolytic than analogous polymers with the hydrophobic group at the N-terminus and undefined C-termini or acetyl at the C-terminus.
Table 3.
Selected biological activities of C-terminally functionalized and C-terminally undefined 37:63 CH:MM nylon-3 random copolymers compared to their critical aggregation concentration (CAC) values.
| Polymer | MICE. coli (µg/mL)a | MHC (µg/mL)b | CAC (µg/mL) |
|---|---|---|---|
| 1 | 200 | 800 | > 3200 |
| 2 | 12.5 | 800 | > 3200 |
| 1B-a | 200 | 1600 | > 3200 |
| 2B-a | 25 | 800 | > 3200 |
| 1C-a | 50 | 25 | 220 |
| 1C-b | 50 | 3.13 | 70 |
MIC = minimum inhibitory concentration.
MHC = minimum hemolytic concentration.
While conducting bioactivity assays, we noticed that polymers with hydrophobic groups at the C-termini, e.g., 1C, with a C-terminal p-t-butylbenzoyl unit, displayed limited solubility in the medium used for antibacterial assays (brain-heart infusion). This behavior is consistent with the results of CAC measurements described in the preceding paragraph. Attempts to dissolve polymer to >100 µg/mL led to a cloudy solution. This solubility limit did not seem to affect most MIC determinations (most MIC values were < 100 µ/mL). No solubility limit was observed for any of the nylon-3 copolymers in the medium used for hemolysis assays (Tris-buffered saline, pH = 7.2).
Conclusion and outlook
Nylon-3 copolymers have recently been shown to display an interesting variety of biological activities;2, 4, 5 exploration of the full potential of these materials would be facilitated by the ability to place diverse functional units independently at the N- and C-termini of the polymer chains. Such terminal modifications could be crucial for tailoring polymer properties and can enable the incorporation of the polymers into larger entities. We have previously described a versatile strategy for placing specific functional groups at nylon-3 N-termini, but C-terminal functionalization has been a long-standing challenge. The C-termini generated via the AROP process bear an imide group, and β- lactam-derived imides are known to be susceptible to attack by nucleophiles such as amines (ref. 12; we note, however, that in the β-lactam imides of these small-molecule examples the exo-cyclic carbonyl is derived from a carbonic acid rather than a carboxylic acid). Indeed, there are limited reports of nylon-3 C-terminal modification with nucleophilic attack by amines or alcohols,8, 9 but we found this approach to be capricious.
In this work we have introduced an approach for C-terminal modification that is based on the polymerization mechanism in that a β-lactam (presumably as its conjugate base) is employed as the nucleophile. By controlling the stoichiometry of β-lactam added after the polymerization process is complete, we can generate materials in which each polymer chain bears on average approximately one C-terminal unit derived from this β-lactam. This approach allows us to place specific functional groups at the nylon-3 C-terminus, based on the acyl group attached to the side chain nitrogen of the final β-lactam. The examples we report include a polymer that contains a C-terminal thiol group, which in the future could be used for linkage of nylon-3 polymers to other molecules (e.g., a fluorophore) or immobilization of polymers on surfaces.
We have used the new C-terminal functionalization approach to ask whether the two types of polymer terminus are equivalent in terms of introducing a crucial hydrophobic group to a previously identified antibacterial nylon-3 copolymer. Our results reveal a significant functional difference between the N- and C-termini for a specific family of CH:MM copolymers: N-terminal placement generates a material that selectively inhibits bacterial growth without being disruptive to eukaryotic cell membranes (low hemolytic activity), while C-terminal placement leads to bacterial growth inhibition in conjunction with eukaryotic membrane disruption (high hemolytic activity). We have rationalized this difference between copolymer termini based on kinetic analysis of the polymerization, which suggests modest variation in hydrophobic vs. cationic subunit composition across the length of the polymer chains. The demonstration that nylon-3 copolymer termini are not necessarily fully equivalent as locations for particular end groups represents an important consideration for future efforts to optimize nylon-3 materials for specific applications.
Supplementary Material
Acknowledgment
This research was supported by the UW-Madison NSEC (DMR-0832760). S.H.G. acknowledges partial support from NIH grant GM-093265.
Supporting Information Available. Experimental details, MALDI mass spectra of C-terminally functionalized poly(CO), 1H NMR and GPC data of C-terminally functionalized 37:63 CH:MM nylon-3 random copolymers, and CAC measurements. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Shannon S. Stahl, Email: stahl@chem.wisc.edu.
Samuel H. Gellman, Email: gellman@chem.wisc.edu.
References
- 1.(a) Kiick KL. Science. 2007;317:1182–1183. doi: 10.1126/science.1145951. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Langer R, Tirrell DA. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]; (c) Haag R, Kratz F. Angew. Chem. Int. Ed. 2006;45:1198–1215. doi: 10.1002/anie.200502113. [DOI] [PubMed] [Google Scholar]
- 2.(a) Mowery BP, Lee SE, Kissounko DA, Epand RF, Epand RM, Weisblum B, Stahl SS, Gellman SH. J. Am. Chem. Soc. 2007;129:15474–15475. doi: 10.1021/ja077288d. [DOI] [PubMed] [Google Scholar]; (b) Epand RF, Mowery BP, Lee SE, Stahl SS, Lehrer RI, Gellman SH, Epand RM. J. Mol. Biol. 2008;379:38–50. doi: 10.1016/j.jmb.2008.03.047. [DOI] [PubMed] [Google Scholar]; (c) Mowery BP, Lindner AH, Weisblum B, Stahl SS, Gellman SH. J. Am. Chem. Soc. 2009;131:9735–9745. doi: 10.1021/ja901613g. [DOI] [PubMed] [Google Scholar]
- 3.For leading references on other antibacterial polymers, see: Vucetic JJ, Vandjel VH, Janic MD. Glas. Hem. Drus. Beograd. 1977;42:389–391. Ikeda T, Tazuke S, Suzuki Y. Makromol. Chem. 1984;185:869–876. Kawabata N, Nishiguchi M. Appl. Environ. Microbiol. 1988;54:2532–2535. doi: 10.1128/aem.54.10.2532-2535.1988. Senuma M, Tashiro T, Iwakura M, Kaeriyama K, Shimura Y. J. Appl. Polym. Sci. 1989;37:2837–2843. Li G, Shen J, Zhu Y. J. Appl. Polym. Sci. 1998;67:1761–1768. Chen CZ, Beck-Tan NC, Dhurjati P, Dyk TKV, LaRossa RA, Cooper SL. Biomacromolecules. 2000;1:473–480. doi: 10.1021/bm0055495. Ohta S, Misawa Y, Miyamoto H, Makino M, Nagai K, Shiraishi T, Nakagawa Y, Yamato S, Tachikawa E, Zenda H. Biol. Pharm. Bull. 2001;24:1093–1096. doi: 10.1248/bpb.24.1093. Tiller JC, Liao C-J, Lewis K, Klibanov AM. Proc. Nat. Acad. Sci. USA. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. Tew GN, Liu D, Chen B, Doerksen RJ, Kaplan J, Carroll PJ, Klein ML, DeGrado WF. Proc. Nat. Acad. Sci. USA. 2002;99:5110–5114. doi: 10.1073/pnas.082046199. Gelman MA, Weisblum B, Lynn DM, Gellman SH. Org. Lett. 2004;6:557–560. doi: 10.1021/ol036341+. Arnt L, Nüslein K, Tew GN. J. Polym. Sci. A: Polym. Chem. 2004;42:3860–3864. Ilker MF, Nusslein K, Tew GN, Coughlin EB. J. Am. Chem. Soc. 2004;126:15870–15875. doi: 10.1021/ja045664d. Kuroda K, DeGrado WF. J. Am. Chem. Soc. 2005;127:4128–4129. doi: 10.1021/ja044205+. Sellenet PH, Allison B, Applegate BM, Youngblood JP. Biomacromol. 2007;8:19–23. doi: 10.1021/bm0605513. Sambhy V, Peterson BR, Sen A. Angew. Chem. Int. Ed. 2008;47:1250–1254. doi: 10.1002/anie.200702287. Lienkamp K, Madkour AE, Musante A, Nelson CF, Nüslein K, Tew GN. J. Am. Chem. Soc. 2008;130:9836–9843. doi: 10.1021/ja801662y. Gabriel GJ, Madkour AE, Dabkowski JM, Nelson CF, Nüslein K, Tew GN. Biomacromol. 2008;9:2980–2983. doi: 10.1021/bm800855t. Kuroda K, Caputo GA, DeGrado WF. Chem. Eur. J. 2009;15:1123–1133. doi: 10.1002/chem.200801523. Palermo EF, Kuroda K. Biomacromol. 2009;10:1416–1428. doi: 10.1021/bm900044x. Palermo EF, Sovadinova E, Kuroda K. Biomacromol. 2009;10:3098–3107. doi: 10.1021/bm900784x. Tew GN, Scott RW, Klein ML, DeGrado WF. Acc. Chem. Res. 2010;43:30–39. doi: 10.1021/ar900036b. Palermo EF, Lee D-K, Ramamoorthy A, Kuroda K. J. Phys. Chem. B. 2011;115:366–375. doi: 10.1021/jp1083357. Li P, Poon YF, Li W, Zhu H-Y, Yeap SH, Cao Y, Qi X, Zhou C, Lamrani M, Beuerman RW, Kang E-T, Mu Y, Li CM, Chang MW, Leong SSJ, Chan-Park MB. Nat. Materials. 2011;2:149–156. doi: 10.1038/nmat2915.
- 4.Lee MR, Stahl SS, Gellman SH, Masters KS. J. Am. Chem. Soc. 2009;131:16779–16789. doi: 10.1021/ja9050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohm MT, Mowery BP, Czyzewski AM, Stahl SS, Gellman SH, Barron AE. J. Am. Chem. Soc. 2010;132:7957–7967. doi: 10.1021/ja909734n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto K. Prog. Polym. Sci. 2000;25:1411–1462. [Google Scholar]
- 7.Zhang JH, Kissounko DA, Lee SE, Gellman SH, Stahl SS. J. Am. Chem. Soc. 2009;131:1589–1597. doi: 10.1021/ja8069192. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K, Hotta K, Okada M, Nagata S. J. Polym. Sci. Part A: Polym. Chem. 1995;33:1995–1999. [Google Scholar]
- 9.Hashimoto K, Yasuda J, Kobayashi M. J. Polym. Sci. Part A: Polym. Chem. 1999;37:909–915. [Google Scholar]
- 10.Lichter JA, Van Vliet KJ, Rubner MF. Macromolecules. 2009;42:8573–8586. [Google Scholar]
- 11.Rana D, Matsuura T. Chem. Rev. 2010;110:2448–2471. doi: 10.1021/cr800208y. [DOI] [PubMed] [Google Scholar]
- 12.Bosch P, Catalina F, Corrales T, Peinado C. Chem. Eur. J. 2005;11:4314–4325. doi: 10.1002/chem.200401349. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson AJ, Pomerantz WC, Neilsen KJ, Gellman SH, Palecek SP. ACS Chem. Biol. 2009;4:567–579. doi: 10.1021/cb900093r. [DOI] [PubMed] [Google Scholar]
- 14.Klok HA. Macromolecules. 2009;42:7990–8000. [Google Scholar]
- 15.(a) Ojima I, Sun CM, Park YH. J. Org. Chem. 1994;59:1249–1250. [Google Scholar]; (b) Palomo C, Aizpurua JM, Cuevas C. J. Chem. Soc. Chem. Commun. 1994:1957–1958. [Google Scholar]; (c) Durham TB, Miller MJ. J. Org. Chem. 2003;68:35–42. doi: 10.1021/jo016276m. [DOI] [PubMed] [Google Scholar]; (d) Taubinger AA, Fenske D, Podlech J. Tetrahedron. 2008;64:8659–8667. [Google Scholar]
- 16.Gerona-Navarro G, Garcia-Lopez MT, Gonzalez-Muniz R. Tetrahedron Lett. 2003;44:6145–6148. [Google Scholar]
- 17.Yanisch-Perron C, Vieira J, Messing J. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 18.Young FE, Smith C, Reilly BE. J. Bacteriol. 1969;98:1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisblum B, Demohn V. J. Bacteriol. 1969;98:447–452 . doi: 10.1128/jb.98.2.447-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicas TI, Wu CYE, Hobbs JN, Preston DA, Allen NE. Antimicrob. Agents Chemother. 1989;33:1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JH, Gellman SH, Stahl SS. Macromolecules. 2010;43:5618–5626. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.