Abstract
Anti-PEG antibodies have been reported to mediate the accelerated clearance of PEG-conjugated proteins and liposomes, all of which contain methoxyPEG (mPEG). The goal of this research was to assess the role of the methoxy group in the immune responses to mPEG conjugates and the potential advantages of replacing mPEG with hydroxyPEG (HO-PEG). Rabbits were immunized with mPEG, HO-PEG, or t-butoxyPEG (t-BuO-PEG) conjugates of human serum albumin, human interferon-α, or porcine uricase as adjuvant emulsions. Assay plates for enzyme-linked immunosorbent assays (ELISAs) were coated with mPEG, HO-PEG, or t-BuO-PEG conjugates of the non-cross-reacting protein, porcine superoxide dismutase (SOD). In sera from rabbits immunized with HO-PEG conjugates of interferon-α or uricase, the ratio of titers of anti-PEG antibodies detected on mPEG-SOD over HO-PEG-SOD (“relative titer”) had a median of 1.1 (range 0.9–1.5). In contrast, sera from rabbits immunized with mPEG conjugates of three proteins had relative titers with a median of 3.0 (range 1.1–20). Analyses of sera from rabbits immunized with t-BuO-PEG-albumin showed that t-butoxy groups are more immunogenic than methoxy groups. Adding Tween 20 or Tween 80 to buffers used to wash the assay plates, as is often done in ELISAs, greatly reduced the sensitivity of detection of anti-PEG antibodies. Competitive ELISAs revealed that the affinities of antibodies raised against mPEG-uricase were c. 70 times higher for 10 kDa mPEG than for 10 kDa PEG diol and that anti-PEG antibodies raised against mPEG conjugates of three proteins had >1000 times higher affinities for albumin conjugates with c. 20 mPEGs than for analogous HO-PEG-albumin conjugates. Overall, these results are consistent with the hypothesis that antibodies with high affinity for methoxy groups contribute to the loss of efficacy of mPEG conjugates, especially if multiply-PEGylated. Using monofunctionally activated HO-PEG instead of mPEG in preparing conjugates for clinical use might decrease this undesirable effect.
Introduction
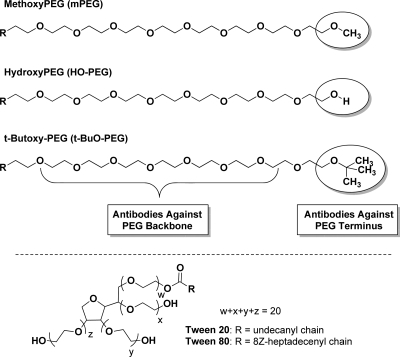
Less than a decade after the first reports of the advantages of coupling proteins to the “non-immunogenic” polymer, poly(ethylene glycol) (PEG),1−3 Richter and Åkerblom provided evidence for the induction of anti-PEG antibodies in rabbits exposed to methoxyPEG (mPEG) conjugates of proteins4 and for the presence of anti-PEG antibodies in the sera of a small percentage of healthy blood donors.5 Nevertheless, during more than three decades of research and the approval for clinical use of numerous PEGylated proteins and other PEGylated therapeutic agents, relatively few research groups have focused on the immunogenicity and antigenicity of the polymer component of PEGylated drugs (reviewed by Armstrong6 and by Su et al.7). Since all of the approved PEGylated drugs, including proteins,8−10 an aptamer,11 and “stealth” liposomes,12 contain mPEG, the role of the methoxy group of mPEG in the immune responses to PEGylated drugs and the potential advantages of using hydroxyPEG (HO-PEG), rather than mPEG, have been of particular interest in our research13,14 (see Figure 1).
Figure 1.
Partial structures of methoxyPEG, hydroxyPEG, and t-butoxyPEG and structures of Tween 20 and Tween 80. In the structures of the PEGs, R designates the rest of the polymer, including the proximal terminal group that reacts with the protein to form PEG conjugates; the domains that may serve as epitopes for the formation of anti-PEG antibodies, namely, the distal terminal groups (in shaded ovals) and the PEG backbone, are indicated. In the structures of Tween 20 and Tween 80, R designates the alkyl groups of the fatty acids that distinguish these two detergents from each other (lauric and oleic acids, respectively); 8Z designates the configuration around the double bond in the heptadecenyl chain.
In a series of research reports and patents that have been published since 1999, Roffler, Cheng, and their colleagues described the development of a series of mouse monoclonal antibodies (mAbs) directed against the backbone of PEG.7,15−21 They demonstrated the utility of such mAbs for the sensitive detection and quantitation of PEGylated drugs7,18 and for the removal of potentially toxic PEGylated drugs from the circulation.7,18−21 Several of these mAbs are available commercially from Abnova (Taipei City, Taiwan). Murine and rabbit anti-PEG mAbs and assay kits incorporating anti-PEG mAbs are now available from several other companies (e.g., ANP Technologies, Newark, DE; Epitomics, Burlingame, CA; Life Diagnostics, Inc., West Chester, PA; Meridian Life Science, Inc., Saco, ME; Silver Lake Research Corp., Monrovia, CA). The majority of these mAbs are directed against the PEG backbone, but at least one of them is directed against the methoxy group of mPEG (Epitomics, Clone PEG-B-47).
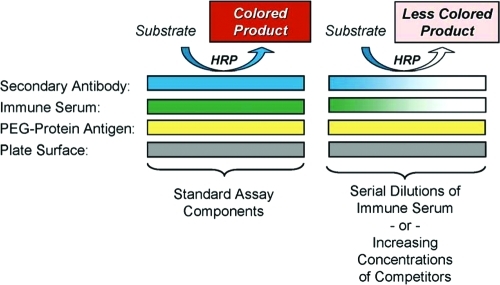
Other research groups have investigated the roles of polyclonal anti-PEG antibodies elicited in experimental animals and patients in altering the pharmacokinetics and pharmacodynamics of PEGylated agents that have been approved for clinical use or are in development. These include PEGylated red blood cells22,23 and PEGylated liposomes,24−29 as well as PEGylated proteins (reviewed by Armstrong6). Various techniques have been used to detect and characterize such antibodies, but the most widely used methods are enzyme-linked immunosorbent assays (ELISAs). These are used in various formats, including direct ELISAs and competitive ELISAs (see Scheme 1). In direct ELISAs, the independent variable is the dilution of immune serum. In competitive ELISAs, a single dilution of serum is tested in the presence of increasing concentrations of competitors, such as unconjugated PEGs or PEG-protein conjugates. In many publications and patents in which anti-PEG antibodies are measured by ELISAs, at least some of the washes of the assay plates contain Tween 20 or Tween 80 (polyoxyethylene sorbitan monolaurate or polyoxyethylene sorbitan monooleate, respectively).7,19−21,30−32 Therefore, we investigated the effects of these PEG-containing detergents on the titers of anti-PEG antibodies detected by direct ELISAs (see Figures 1 and 5).
Scheme 1. Schematic of Enzyme-Linked Immunosorbent Assays (ELISAs) for Determining the Titer of Anti-PEG Antibodies by Direct ELISAs, Using Serial Dilutions of Serum from Immunized Rabbits, or for Determining the Relative Affinities of Binding to Various Competitors, Such as Free PEGs or PEG-Protein Conjugates, Using a Constant Dilution of Serum and Increasing Concentrations of the Competitors.
Linkage of the secondary antibody (goat-anti-rabbit IgG) to horseradish peroxidase (HRP) enables colorimetric quantitation of the binding of the primary antibody to the assay plate.
Figure 5.
Washing the assay plate with Tween 20 or Tween 80, detergents that contain HO-PEG, decreased the titers detected by direct ELISAs of sera from rabbits immunized with HO-PEG-uricase (○, ●) or mPEG-uricase (Δ, ▲) and amplified the differences between the titers of anti-PEG antibodies detected in sera from these two rabbits. Direct ELISAs with mPEG-SOD as the antigen were performed as described in Experimental Procedures, except that the PBS used to wash one of the replicate assay plates before and after the addition of the enzyme-linked secondary antibody contained either 0.05% (v/v) Tween 20 (A) or 0.1% (v/v) Tween 80 (B). Higher titers were detected in the absence of detergent (filled symbols, solid curves) than in the presence of Tween (open symbols, dashed curves). The effects of each type of Tween on the results for the individual rabbits immunized with either HO-PEG-uricase or mPEG-uricase are shown by red arrows and black arrows, respectively. The ratios of titers detected in sera from these two rabbits in the presence or absence of Tween are shown by blue arrows.
Since the terms antigenicity and immunogenicity are sometimes used imprecisely, it seems useful to specify that, in this report, immunogenicity refers to the ability of substances, e.g., protein conjugates of mPEG, HO-PEG, or t-butoxyPEG (t-BuO-PEG), to induce the production of antibodies in experimental animals or in humans. In contrast, antigenicity refers to the abilities of various substances, e.g., unconjugated PEGs or PEG-protein conjugates, to bind to anti-PEG antibodies to which they are exposed in vitro (see Scheme 1). In this research, the immunogens were conjugates of recombinant human interferon-α (IFN-α), porcine uricase, or human serum albumin (albumin), which contained between 1 and 17 molecules of PEG per protein molecule or subunit. The antigens used to coat the assay plates were conjugates of an unrelated protein, porcine Cu–Zn superoxide dismutase (SOD), to which the same small number of molecules of mPEG, HO-PEG, or t-BuO-PEG of the same size (10 kDa) were coupled using the same activation chemistry. In competitive ELISAs, the competitors were free mPEG, HO-PEG, or t-BuO-PEG or conjugates of albumin containing an average of c. 20 molecules of mPEG or HO-PEG per molecule of albumin.
Finally, since the results of the immunologic studies described herein are consistent with the hypothesis that PEG-protein conjugates synthesized with monofunctionally activated HO-PEG might be less antigenic and/or less immunogenic than the analogous mPEG conjugates, we performed experiments to compare the potencies in cell culture of analogous mPEG and HO-PEG conjugates of two recombinant human cytokines.
Experimental Procedures
Reagents and Supplies
Reagents were from Sigma-Aldrich Chemical Co. (St. Louis, MO) unless otherwise noted. PEG molecular weight standards and 10 kDa mono-t-butoxyPEG were from Polymer Laboratories (Shropshire, UK, now part of Agilent Technologies, Santa Clara, CA). Other PEGs were from NOF Corporation (Tokyo, Japan), Shearwater Polymers (Huntsville, AL, now part of Nektar Therapeutics, San Francisco, CA) or SunBio (Anyang City, South Korea), as indicated. Human interferon-α-2b (IFN-α) was from Tianjin Hualida Bioengineering Co. (Tianjin, China). Erythropoietin BRP European Pharmacopoeia Reference Standard (35 280 IU/0.25 mg) and Mircera (100 μg/0.3 mL, Roche) were obtained from Bioassay GmbH (Heidelberg, Germany). Recombinant human erythropoietin-α (EPO, CYT-201) that was used for the synthesis of PEG conjugates was from ProSpec (Rehovot, Israel). Recombinant porcine uricase was from Bio-Technology General Ltd. (Rehovot, Israel, now part of Ferring S.A., Saint-Prex, Switzerland). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF) was obtained from Berlex Biosciences (Richmond, CA). Plasbumin25 from Bayer Biological Products (Elkhart, IN) was used as human serum albumin. Daudi cells and TF-1 cells were from ATCC (Manassas, VA). Goat serum and most reagents used for cell culture were from GIBCO (now part of Invitrogen/Life Technologies, Carlsbad, CA). Alamar Blue, used for cell culture assays, Novex NuPAGE gels and Sypro Ruby were also from Invitrogen. Microplates used for fluorometric assays of cell growth were from Greiner Bio-One North America, Inc. (Monroe, NC). Immulon 2 HB microplates used for ELISAs were from Thermo Scientific (Rochester, NY). Trifluoroacetic acid (TFA) was from Supelco (Bellefonte, PA). Nonfat dry milk solids were from Western Family (Portland, OR). Spectra/Por 1 dialysis tubing was from Spectrum Laboratories (Rancho Dominguez, CA).
PEGylation Reagents and PEG-Protein Conjugates Used as Immunogens, Antigens, and Competitors in Immunologic Assays and in Cell Culture Assays
Table 1 summarizes the reagents used to immunize the rabbits and as antigens or competitors in the ELISAs. Details about the synthesis, purification, and physicochemical analyses of (1) the activated HO-PEGs and t-BuO-PEG; (2) the PEG-protein conjugates listed in Table 1, and (3) the HO-PEG conjugate of erythropoietin used in the cell culture assays are provided in the Supporting Information, which includes seven figures, numbered S1 through S7.
Table 1. Reagents Used as Immunogens and as Antigens and Competitors in ELISAs.
| Immunogens | |||
|---|---|---|---|
| polymer component |
|||
| protein component | end group | PEG M.W. | # of PEGs |
| human interferon-α | methoxy | 20 kDa | 1 |
| 20 kDa | 2 | ||
| hydroxy | 20 kDa | 1 | |
| 20 kDa | 2 | ||
| porcine uricase | methoxy | 10 kDa | 2.3a |
| hydroxy | 10 kDa | 2.3a | |
| human serum albumin | methoxy | 10 kDa | 17 |
| t-butoxy | 10 kDa | 17 | |
| Antigens | |||
|---|---|---|---|
| polymer component |
|||
| protein component | end group | PEG M.W. | # of PEGs |
| porcine superoxide dismutase (SOD) | methoxy | 10 kDa | 2.5b |
| hydroxy | 10 kDa | 2.5b | |
| t-butoxy | 10 kDa | 2.5b | |
| human interferon-α | (No PEG) | ||
| Competitors | ||
|---|---|---|
| free polymers | mPEG | 10 kDa |
| 20 kDa | ||
| PEG Diol | 10 kDa | |
| 20 kDa | ||
| polymer component |
|||
|---|---|---|---|
| PEG-albumin conjugates | end group | PEG M.W. | # of PEGs |
| methoxy | 10 kDa | 17–19 | |
| hydroxy | 10 kDa | 17–22 | |
Number of PEGs per uricase subunit.
Number of PEGs per SOD subunit.
Determination of the Number of PEG Molecules Coupled per Protein Molecule
Size-exclusion chromatography (SEC) was performed on Superdex 200 or TSK G-5000PWXL columns from which the eluates were monitored by both UV absorbance and refractive index (RI). Peaks containing PEG-protein conjugates were analyzed by the method of Kunitani et al.33 This method was used to characterize PEG conjugates of SOD, IFN-α, EPO, recombinant porcine uricase, and human serum albumin (albumin). These conjugates contained between 1 and 22 molecules of PEG per protein molecule or subunit. For conjugates of SOD, IFN-α, EPO, and uricase coupled to fewer than c. 5 molecules of PEG per protein subunit, the results for the PEG-to-protein ratio calculated from the SEC elution profiles were confirmed by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS-PAGE) on Novex NuPAGE gels. Replicate gels were stained for protein with Coomassie blue R-250 or Sypro Ruby and for PEG with a solution of KI and I2 mixed with BaCl2, by an adaptation of the method of Skoog.34
Animals and Immunizations
Eight groups of three rabbits were immunized with a PEG conjugate of one of the following proteins: IFN-α, porcine uricase, or human serum albumin. Specific pathogen-free New Zealand white rabbits were injected with PEGylated immunogens on days 0, 7, 14, 28, 56, and 84. The first immunization was in complete Freund’s adjuvant. Subsequent immunizations were in incomplete Freund’s adjuvant. Blood was collected prior to immunization (prebleed) and 6, 10, and 14 weeks after the first immunization (Bleeds 1, 2, and 3). Serum was prepared from each bleed by centrifugation and was kept frozen at −40 °C until use. All rabbit procedures were performed at Lampire Biological Laboratories (Pipersville, PA) by AAALAC-certified technicians in accordance with IACUC-approved protocols.
Direct Enzyme-Linked Immunosorbent Assays (Direct ELISAs)
The PEGylated antigens used in direct ELISAs consisted of conjugates of SOD with 10 kDa mPEG, 10 kDa HO-PEG, or 10 kDa t-BuO-PEG that were synthesized, purified, and analyzed as described in the Supporting Information. The conjugates contained an average of c. 2–3 molecules of PEG per 16 kDa subunit of SOD. Concentrations of PEGylated antigens are expressed as protein concentrations (μg/mL). For most experiments, 100 μL of a solution of PEGylated SOD (10 μg/mL) prepared in 0.1 M sodium carbonate buffer, pH 10.3, was used to coat each well of the 96-well plates. When IFN-α was used as the antigen, 100 μL of a solution of 10 μg/mL of IFN-α in sodium carbonate buffer was placed in the wells, corresponding to 1 μg/well.
Immulon 2 HB 96-well microplates were coated with IFN-α or PEG-SOD by incubation overnight at 4 °C. Removal of unbound antigen and all washes of the plates were performed with a Denley Wellwash 4 plate washer (Needham Heights, MA). After removal of unbound antigen, the wells were washed three times with phosphate-buffered saline (PBS) and then incubated for 1 h at room temperature with 300 μL/well of Blocking Buffer (5% (w/v) nonfat dry milk solids in PBS). After removal of the Blocking Buffer, the plates were washed three times with PBS. Serial dilutions of rabbit sera in PBS containing 2% (v/v) goat serum (PBS-G) were added to the plates (100 μL/well), which were incubated for 1 h at 37 °C. The plates were then washed three times with PBS. No detergents such as Tween 20 or Tween 80 were included in the wash buffers except as indicated in Figure 5.
Goat anti-rabbit IgG (H & L chain specific) (1 mg/mL) conjugated to horseradish peroxidase (HRP) was used as the secondary antibody (Sigma) (see Scheme 1). For most assays, the secondary antibody was diluted 1/10 000 in PBS-G, so that 100 μL/well contained 0.01 μg of antibody protein. If the peroxidase activity detected under the conditions described below was too high to obtain a linear increase in absorbance for at least 4 min, the secondary antibody was diluted 1/15 000 or 1/20 000 in PBS-G in subsequent experiments. After addition of the secondary antibody, the plates were incubated for 45 min at 37 °C. The plates were washed three times with PBS before the addition of 200 μL/well of peroxidase substrate, which was o-phenylenediamine dihydrochloride35 (OPD), purchased as SIGMAFAST OPD. The substrate solution was prepared as close as possible to the time of use and stored briefly at room temperature, shielded from light. The substrate consists of two tablets (one OPD tablet and one urea hydrogen peroxide/buffer tablet), which were dissolved in 21 mL of water. This solution was filtered through a 0.2 μm syringe filter (Acrodisc 25 mm Syringe Filter, Pall Corporation, Ann Arbor, MI). The absorbance at 440 nm of the colored conjugate formed with peroxide was measured in a SpectraMax 250 microplate reader (Molecular Devices, Sunnyvale, CA) in kinetic mode for 6 min at 12 s intervals. The assay plate was shaken in the plate reader for 15 s before the first reading and for 3 s before each subsequent reading of the plate (see Scheme 1).
Competitive ELISAs
As in the direct ELISAs, Immulon 2 HB 96-well microplates were coated with mPEG-SOD, HO-PEG-SOD, or t-BuO-PEG-SOD by incubation overnight at 4 °C with 100 μL/well of antigens at 10 μg/mL in 0.1 M sodium carbonate buffer, pH 10.3. After removal of unbound antigen and three washes with PBS, the plates were blocked for 1 h at room temperature with 300 μL/well of Blocking Buffer. The plates were washed three times with PBS. Serial dilutions of the competitors in PBS-G (50 μL/well) were added to the wells, followed by 50 μL/well of diluted rabbit serum in PBS-G. Dilutions of sera used for competitive ELISAs were calculated to correspond to the highest dilution factor that provided maximal binding in direct ELISAs. Therefore, for competitive ELISAs, sera with higher titers were diluted more extensively than sera with lower titers. The dilution factors, which are indicated in the legends of Figures 6–8 and Figure 9B, ranged from 1/320 to 1/10 000. Samples were mixed by shaking the plate in the plate reader for 10 s before it was incubated for 1 h at 37 °C. After removal of unbound reactants, the plates were washed three times with PBS. The addition of secondary antibody, washing of plates, addition of HRP substrate, and monitoring of absorbance of the colored product were performed as described above for direct ELISAs.
Figure 6.
Competitive ELISAs were used to compare the inhibition by mPEG and by PEG diol (HO-PEG-OH) of the binding to mPEG-SOD of antibodies raised against mPEG2-IFN-α (A), against mPEG-uricase (B), and against HO-PEG-uricase (C). Concentrations of competitors are expressed as micromolar PEG in the serum-containing assay mixtures. (A) Anti-PEG antibodies in serum from a rabbit immunized with mPEG2-IFN-α bound 10 kDa mPEG (filled circles) 30-fold to 40-fold more tightly than 10 kDa PEG diol (open circles), regardless of the serum dilution in the range of 1/500 to 1/2000. (B) Anti-PEG antibodies in serum from a rabbit immunizedwith mPEG-uricase bound 10 kDa mPEG (●) c. 70 times more tightly than 10 kDa PEG diol (○). The serum was diluted 1/1000. (C) Competitive ELISAs of anti-PEG antibodies in serum from a rabbit immunized with HO-PEG-uricase revealed no preferential binding of 10 kDa mPEG (●) compared to 10 kDa PEG diol (○), although the slopes of the competition curves differed. The serum was diluted 1/500.
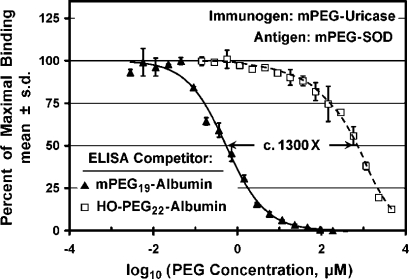
Figure 8.
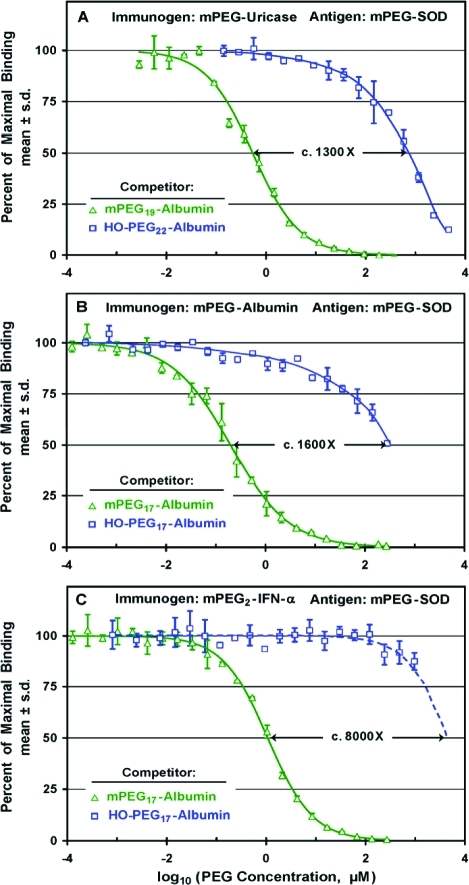
Affinities of antibodies in sera from rabbits immunized with multiply-PEGylated mPEG conjugates of porcine uricase (A), human serum albumin (B), or human interferon-α (C) for multiply-PEGylated conjugates of albumin with mPEG vs HO-PEG were compared in competitive ELISAs with mPEG-SOD as the antigen. Concentrations of all competitors are expressed as micromolar concentrations of PEG in the serum-containing assay mixtures. All of the sera used for these experiments were diluted 1/1000. (A) Increasing concentrations of conjugates of albumin containing c. 20 molecules of either 10 kDa mPEG (△) or 10 kDa HO-PEG (□) were used as competitors for anti-PEG antibodies raised against mPEG-uricase. The c. 1300-fold ratio of the values of IC50 for the HO-PEG and mPEG conjugates of albumin is indicated. (B) Increasing concentrations of conjugates of human serum albumin with 17 molecules of either 10 kDa mPEG (△) or 10 kDa HO-PEG (□) were used as competitors for anti-PEG antibodies raised against mPEG17-albumin. The c. 1600-fold ratio of the values of IC50 for the HO-PEG and mPEG conjugates of albumin is indicated. (C) Increasing concentrations of mPEG17-albumin gave a sigmoid competition curve (△) with an IC50 of c. 1 μM mPEG and complete inhibition at <1 mM mPEG. Since the highest available concentration of HO-PEG17-albumin (corresponding to c. 1 mM HO-PEG) inhibited only c. 13% of the binding (□), the data for HO-PEG17-albumin were extrapolated (dashed curve) to provide an estimate of the relative affinities of the two competitors.
Figure 9.
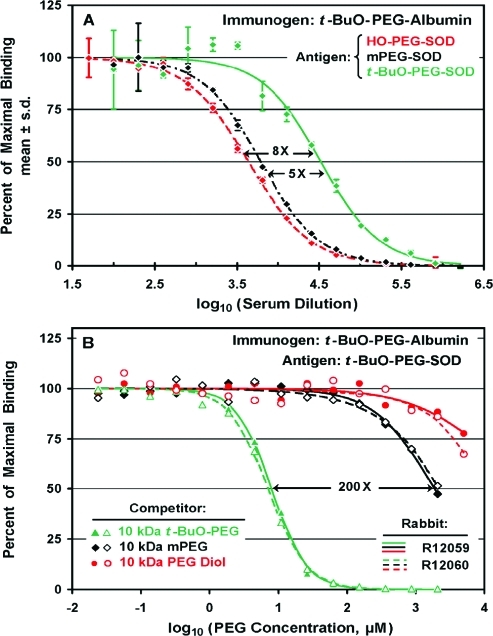
Selectivity of antibodies raised against a t-BuO-PEG conjugate of human serum albumin (t-BuO-PEG17-albumin) was assessed by direct ELISAs (A) and by competitive ELISAs (B). (A) Direct ELISAs were performed on assay plates coated with SOD coupled to 10 kDa HO-PEG (red ◆), to 10 kDa mPEG (black ◆), or to 10 kDa t-BuO-PEG (green ◆) . In this serum, from rabbit R12059, higher titers were detected with t-BuO-PEG-SOD than with mPEG-SOD or with HO-PEG-SOD. (B) Competitive ELISAs were performed on a 1/10 000 dilution of the same serum for which direct ELISA data are shown in (A) (rabbit R12059; filled symbols, solid curves) and on the same dilution of serum from a similarly immunized rabbit (R12060; open symbols, dashed curves). The results revealed a high degree of selectivity of these antisera for 10 kDa t-BuO-PEG (△, ▲) compared to 10 kDa mPEG (◇, ◆) or to 10 kDa PEG diol (○, ●).
Analysis of Data from ELISAs
The rates of increase in absorbance at 440 nm during the period of measurement were exported from the SpectraMax plate reader into Microsoft Excel. Usually, the increase in absorbance was linear during the first 4 min of measurements, with a correlation coefficient (R2) greater than 0.98. For direct ELISAs, the rates (in milli-absorbance units/min) were graphed against the log10 of the serum dilution. For competitive ELISAs, the rates were graphed against the log10 of the final concentration of each competitor, in units of micromolar PEG.
Data in colorimetric assay units (mAU/min) were first analyzed as a function of the log10 of the serum dilution (direct ELISAs) or the log10 of the competitor concentration (competitive ELISAs) using DPlot software (HydeSoft Computing, LLC, Vicksburg, MS) to obtain the best fit to a sigmoid curve, using the equation
The results in colorimetric assay units were converted to percent of the maximal binding by subtracting A (the minimal value), dividing by B (the range of values), and multiplying by 100. The resultant values of the percent of the maximal binding in direct and competitive ELISAs were plotted against the log10 of serum dilution or the log10 of competitor concentration, respectively.
For direct ELISAs, the values of the parameters C and D from DPlot were used to calculate the dilution of serum corresponding to half-maximal binding of the antibodies to the assay plate (D50), using the equation
The ratio of the values of D50 obtained when the same antiserum was tested against mPEG-SOD and against HO-PEG-SOD (at the same antigen concentration) was used as a measure of the relative titer against the two types of PEG (see Figures 2–4 and Table 2).
Figure 2.
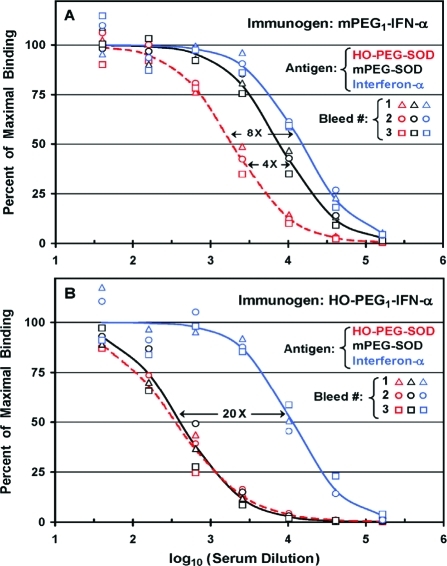
Titers of antibodies to HO-PEG, mPEG, and interferon-α were determined by direct ELISAs of sera prepared from each of three monthly bleeds of rabbits immunized with either mPEG1-IFN-α (A) or HO-PEG1-IFN-α (B). (A) Anti-PEG antibodies were measured on assay plates coated with 2 μg/well of superoxide dismutase (SOD) coupled to either 10 kDa HO-PEG (red symbols) or 10 kDa mPEG (black symbols). Anti-IFN-α antibodies were measured on assay plates coated with 1 μg/well of recombinant human IFN-α (blue symbols). Mean values in sera from Bleeds 1, 2, and 3 were calculated from results obtained with HO-PEG-SOD (red dashed line), mPEG-SOD (black solid line), or IFN-α (blue solid line) as the antigen. Black arrows indicate the ratios of titers detected against the various antigens. (B) Sera from Bleeds 1, 2, and 3 of a rabbit immunized with HO-PEG1-IFN-α were analyzed in the same way as described for (A).
Figure 4.
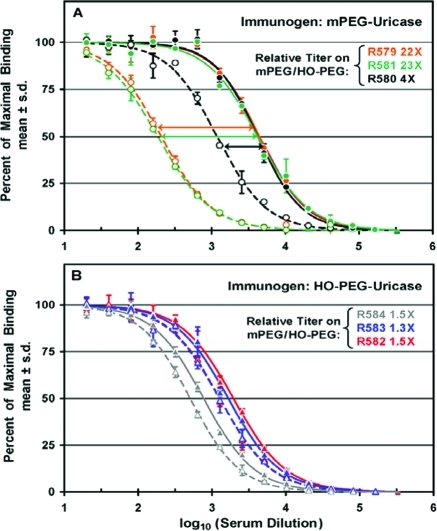
Titers of antibodies against mPEG and HO-PEG in sera from groups of three rabbits immunized with mPEG-uricase (A) or HO-PEG-uricase (B) were determined by direct ELISAs. PEG-uricase conjugates used as immunogens contained an average of 2.3 molecules of 10 kDa mPEG or HO-PEG per uricase subunit. (A) Percentages of maximal binding to mPEG-SOD (filled symbols, solid curves) and to HO-PEG-SOD (open symbols, dashed curves) and the relative titers on mPEG/HO-PEG are indicated for three rabbits immunized with mPEG-uricase. Data for each of rabbits R579, R580, and R581 are shown in orange, black, and green, respectively. (B) Percentages of maximal binding to mPEG-SOD (filled symbols, solid curves) and to HO-PEG-SOD (open symbols, dashed curves) and the relative titers on mPEG/HO-PEG are indicated for three rabbits immunized with HO-PEG-uricase. Data for each of rabbits R582, R583, and R584 are shown in red, blue, and gray, respectively.
Table 2. Relative Titers of Antibodies Detected with mPEG-SOD/HO-PEG-SOD as the Antigen in Sera from Bleeds 2 and/or 3 of 23 Rabbits Immunized with Eight PEGylated Protein Immunogens.
| relative titer |
relative titer |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| protein of immunogen | mPEG/protein (PEG M.W.) | rabbit # | mean | s.d. | n | HO-PEG/protein (PEG M.W.) | rabbit # | mean | s.d. | n |
| Human Interferon-α | 1 (20 kDa) | R8990 | 3.0 | 0.8 | 4 | 1 (20 kDa) | R8991 | 1.1 | 0.1 | 2 |
| R9002 | 3.0 | 0.3 | 4 | R8995 | 1.0 | 0.03 | 2 | |||
| R9003 | 4.8 | 0.9 | 5 | R8997 | 0.9 | 0.2 | 4 | |||
| Human Interferon-α | 2 (20 kDa) | R8993 | 2.7 | 0.5 | 4 | 2 (20 kDa) | R8992 | 0.9 | 0.2 | 2 |
| R9001 | 5.5 | 1.0 | 4 | R8994 | 1.1 | 0.2 | 2 | |||
| R9004a | R9000 | 0.9 | 0.1 | 2 | ||||||
| Porcine Uricase | 2.3/subunit (10 kDa) | R579 | 20 | 4.8 | 4 | 2.3/subunit (10 kDa) | R582 | 1.3 | 0.2 | 3 |
| R580 | 3.1 | 0.6 | 3 | R583 | 1.1 | 0.2 | 3 | |||
| R581 | 18 | 4.8 | 5 | R584 | 1.5 | 0.1 | 3 | |||
| Human Serum Albumin | 17 (10 kDa) | R12056 | 3.0 | 0.5 | 4 | |||||
| R12057 | 1.1 | 0.1 | 3 | |||||||
| R12058 | 2.6 | 0.6 | 3 | |||||||
| Median | 3.0 | Median | 1.1 | |||||||
| Range | 1.1–20 | Range | 0.9–1.5 | |||||||
| t-BuO-PEG/protein (PEG M.W.) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Human Serum Albumin | 17 (10 kDa) | R12059 | 1.8 | 0.5 | 2 | |||||
| R12060 | 1.2 | 0.2 | 2 | |||||||
| R12061 | 1.7 | 0.3 | 2 |
Rabbit R9004 died before Bleed 2.
For competitive ELISAs, the values of the parameters C and D from DPlot were used to calculate the competitor concentration that inhibited 50% of the binding of the antibodies to the antigen on the plate (IC50), using the equation
The ratio of the values of IC50 for two competitors assayed at the same dilution of a particular serum provides a measure of the relative affinities of the antibodies being tested for those competitors (see Figures 6–8 and 9B).
Cell Culture Assay of the Potencies of Interferon-α and PEG Conjugates
Interferon-α and four PEG-IFN-α conjugates were assayed for their abilities to inhibit the proliferation of Daudi cells, measured by a fluorometric assay with Alamar Blue as the indicator.36,37 Daudi cells are human lymphoblastic B cells derived from a male patient with Burkitt’s lymphoma.38 The growth medium was RPMI 1640 with 2 mM l-glutamine containing 10% fetal bovine serum, 10 mM HEPES buffer, 1.5 g/L sodium bicarbonate, 1 mM sodium pyruvate, 1% (v/v) Pen/Strep. and 5.4 g/L d-(+)-glucose. All components of the medium were from GIBCO except for glucose, which was from Sigma-Aldrich. The cells were grown at 37 °C in humidified air containing 5% CO2, with twice-weekly passages.
Cells in the log-phase of growth (150 μL of a suspension in growth medium of 250 000 cells/mL) were incubated for 4 h at 37 °C in humidified air containing 5% CO2 in 96-well, flat, clear-bottom, black microplates. Serial dilutions in growth medium (50 μL) of IFN-α or mPEG-IFN-α or HO-PEG-IFN-α were added to the cells in the assay plates, which were incubated for 72 h under the same conditions as for growth. Alamar Blue (20 μL) was then added, and the incubation was continued for 4 h. The assay plates were shaken for 30 s on a JitterBug orbital shaker (Boekel Scientific, Feasterville, PA) both after the addition of the serial dilutions of IFN-α or the PEG conjugates and after the addition of Alamar Blue. The fluorescent signal was measured at excitation and emission wavelengths of 544 and 589 nm, respectively, in a fluorescence plate reader (Fluoroskan Ascent Model with Ascent software, Thermo Labsystems, Waltham, MA).
As in the analysis of data from ELISAs, data for cell growth in relative fluorescence units as a function of the concentration of IFN-α or PEG-IFN-α were analyzed using DPlot software to fit the equation
Using the minimal value (A) and the range of values (B) from the initial DPlot, the data were converted to Percent of Inhibitable Cell Growth, as shown in Figure 10A.
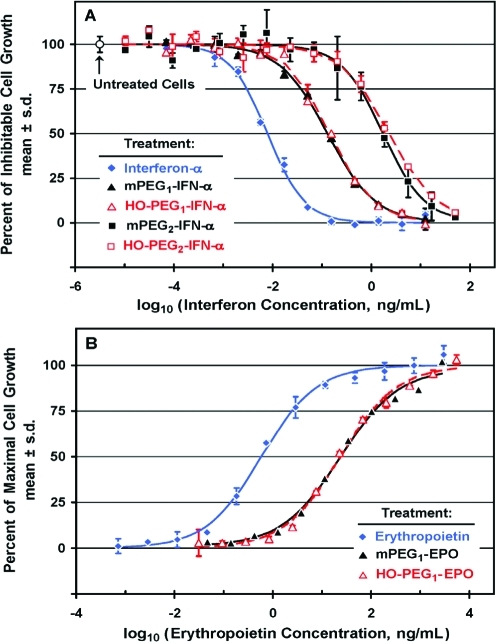
Figure 10.
Cell culture assays responsive to human interferon-α (IFN-α; A) or human erythropoietin (EPO; B) were used to compare the potencies in vitro of analogous conjugates of these cytokines with HO-PEG and mPEG. A: Human lymphoma cells (Daudi cells) were treated for 3 days with serial dilutions of IFN-α (◆), with monoPEGylated conjugates of IFN-α made with 20 kDa mPEG (▲; mPEG1-IFN-α) or 20 kDa HO-PEG (△; HO-PEG1-IFN-α), or with diPEGylated conjugates of IFN-α made with 20 kDa mPEG (■) or with 20 kDa HO-PEG (□). After incubation of the cells with Alamar Blue, the fluorescent signal was measured to quantify cell growth, from which the percent of inhibitable cell growth was calculated. (B) Human erythroleukemic cells (TF-1 cells) were treated for 3 days with serial dilutions of EPO (◆), with a monoPEGylated conjugate with 30 kDa mPEG (▲; mPEG1-EPO; Mircera), or with a monoPEGylated conjugate with 30 kDa HO-PEG (△; HO-PEG1-EPO). After incubation of the cells with Alamar Blue, the fluorescent signal was measured to quantify cell growth, from which the percent of maximal cell growth was calculated.
Cell Culture Assay of the Potencies of Erythropoietin and PEG Conjugates
The effects of EPO and of mPEG and HO-PEG conjugates of EPO on the proliferation of TF-1 cells were assessed using a fluorometric assay with Alamar Blue, as described above for the assay of IFN-α and its PEG conjugates using Daudi cells. TF-1 cells are factor-dependent erythroblastic cells derived from the bone marrow of a male patient with erythroleukemia.39,40 Cells were cultured at 37 °C in humidified air containing 5% CO2 in the same growth medium as used for Daudi cells, except that the growth medium for TF-1 cells was supplemented with 2.4 ng/mL GM-CSF.
TF-1 cells were collected in the log phase of growth and were centrifuged for 10 min at 1000 rpm at 4 °C. The supernatant was removed and the pellet was resuspended twice in 10 mL of assay medium, which is growth medium without added GM-CSF, to minimize the residual concentration of GM-CSF. Cells (100 μL of a suspension in assay medium of 100 000 cells/mL) were mixed with 100 μL of serial dilutions of growth factors (EPO or PEG-EPO conjugates) in assay medium in the wells of a 96-well, flat, clear-bottomed, black microplate. The cells were cultured for 72 h at 37 °C in humidified air containing 5% CO2. Alamar Blue (25 μL) was then added and the incubation was continued for 6 h. The plates were shaken for 30 s on a JitterBug orbital shaker after the addition of the cell suspension to the growth factor dilutions and after the addition of Alamar Blue. The fluorescent signal was measured in a fluorescence plate reader, as described above for the Daudi cell assay. Data in relative fluorescence units were analyzed using DPlot software and the same equation shown above for the analysis of data from the Daudi cell assay. Using the minimal value (A) and range of values (B) from the initial DPlot, the data were converted to Percent of Maximal Cell Growth, as shown in Figure 10B.
Results
Immune Responses to MonoPEG Conjugates of mPEG-Interferon-α and HO-PEG-Interferon-α Differ throughout Immunization
Direct ELISAs were performed on sera from three successive monthly bleeds of two rabbits immunized with either mPEG1-IFN-α (Figure 2A) or HO-PEG1-IFN-α (Figure 2B), using assay plates coated with mPEG-SOD, HO-PEG-SOD, or IFN-α (see Scheme 1). Chromatographic analyses of the PEG conjugates of SOD and IFN-α are provided in Figures S3 and S4 of the Supporting Information, respectively.
The titers of antibodies detected after immunization with IFN-α coupled to one molecule of 20 kDa mPEG (mPEG1-IFN-α) were highest with IFN-α as the antigen, somewhat lower with mPEG-SOD, and lowest for HO-PEG-SOD as the antigen (Figure 2A). There were no significant differences among the results obtained with sera from Bleeds 1, 2, and 3 of this rabbit (represented by triangles, circles, and squares, respectively). Analogous data for sera from three bleeds of a rabbit immunized with IFN-α coupled to one molecule of 20 kDa HO-PEG (HO-PEG1-IFN-α) are shown in Figure 2B. In this case, the titers of anti-PEG antibodies detected with mPEG-SOD and HO-PEG-SOD as the antigens were indistinguishable from each other and were about 20 times lower than the titer of antibodies detected against IFN-α. As in Figure 2A, there were no significant differences among the results obtained with sera prepared from Bleeds 1, 2, or 3 of this rabbit, which was immunized with HO-PEG1-IFN-α.
In contrast with the results in Figure 2, for 1 of the 23 rabbits used in this research (R9003, which was immunized with mPEG1-IFN-α), the titers of anti-PEG antibodies detected in serum from Bleed 1 were lower than those detected in sera from subsequent bleeds (data not shown). Therefore, all subsequent experiments were performed on sera from Bleed 2 and/or Bleed 3 of each rabbit, which gave similar results for all tested parameters.
Rabbits Immunized with mPEG1-Interferon-α, but not with HO-PEG1-Interferon-α, Have Higher Titers of Anti-PEG Antibodies Detected with mPEG-SOD than with HO-PEG-SOD
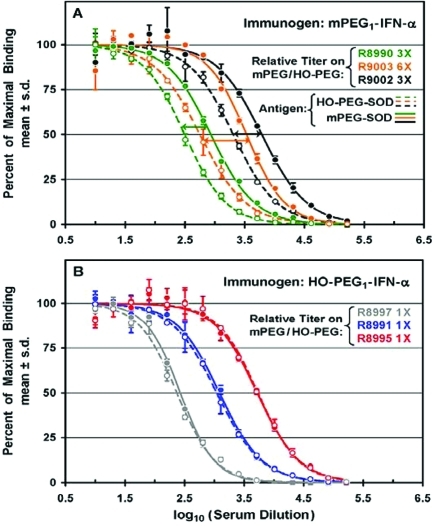
In the sera of three rabbits immunized with mPEG1-IFN-α, a relatively narrow range of titers of antibodies was detected with mPEG-SOD as the antigen (solid curves in Figure 3A) and a slightly wider range of lower titers of antibodies was detected with HO-PEG-SOD (dashed curves in Figure 3A). The highest titer of anti-PEG antibodies detected with mPEG-SOD (in serum from rabbit R9002) was c. 7 times higher than the lowest titer (in serum from rabbit R8990). For each serum sample, the ratio of the titers detected with mPEG-SOD to those detected with HO-PEG-SOD is referred to in this report as the “relative titer”. Among the three rabbits in this experiment, the relative titers varied from 3 to 6 (indicated as 3× and 6×, respectively, in Figure 3A).
Figure 3.
Titers of antibodies against mPEG and against HO-PEG were determined by direct ELISAs of sera from groups of three rabbits immunized with either mPEG1-IFN-α (A) or HO-PEG1-IFN-α (B). (A) Percentages of maximal binding to mPEG-SOD (filled symbols, solid curves) and to HO-PEG-SOD (open symbols, dashed curves) and the ratio of titers on mPEG/HO-PEG (“relative titers”) are indicated for three rabbits immunized with mPEG1-IFN-α. Data for each of rabbits R8990, R9002, and R9003 are shown in green, black, and orange, respectively. (B) Percentages of maximal binding to mPEG-SOD (filled symbols, solid curves) and to HO-PEG-SOD (open symbols, dashed curves) and the ratio of titers on mPEG/HO-PEG are indicated for three rabbits immunized with HO-PEG1-IFN-α. Data for each of rabbits R8991, R8995, and R8997 are shown in blue, red, and gray, respectively.
In sera from each of three rabbits immunized with HO-PEG1-IFN-α, the titers of anti-PEG antibodies detected with mPEG-SOD as the antigen (solid curves in Figure 3B) and with HO-PEG-SOD as the antigen (dashed curves in Figure 3B) were indistinguishable from each other. This was true although the absolute titers of anti-PEG antibodies in the serum with the highest titer (from rabbit R8995) were c. 20 times those in the serum with the lowest titer (from rabbit R8997).
Rabbits Immunized with Multiply-PEGylated mPEG-Uricase, but not HO-PEG-Uricase, Have Higher Titers of Anti-PEG Antibodies Detected with mPEG-SOD than with HO-PEG-SOD
Direct ELISAs similar to those shown in Figure 3 were performed on sera from groups of three rabbits immunized with mPEG or HO-PEG conjugates of porcine uricase in which an average of 2.3 molecules of 10 kDa PEG were coupled per uricase subunit (see Figure S6). This extent of PEGylation corresponds to an average of about nine molecules of PEG per uricase tetramer, which is the active form of the enzyme.14 The titers of anti-PEG antibodies for all three rabbits immunized with this mPEG-uricase conjugate were indistinguishable when tested on assay plates coated with mPEG-SOD (note the superposition of the three solid curves in Figure 4A). However, there were differences among the titers of antibodies detected in the same sera with HO-PEG-SOD as the antigen (dashed curves). The relative titers of antibodies against mPEG/HO-PEG varied from c. 4-fold for rabbit R580 to >20-fold for rabbits R579 and R581.
In sera from three rabbits immunized with HO-PEG-uricase, the titers of antibodies detected against either mPEG or HO-PEG were nearly indistinguishable (Figure 4B). The relative titers of antibodies against mPEG/HO-PEG varied only from about 1.3 to 1.5 in this experiment, in contrast with the much higher relative titers shown in Figure 4A.
Most Rabbits Immunized with mPEG Conjugates of Three Proteins Have Higher Titers of Anti-PEG Antibodies Detected with mPEG-SOD than with HO-PEG-SOD, Unlike Those Immunized with HO-PEG-Proteins
The results obtained in replicate analyses (between 2 and 5), similar to those shown in Figures 2–4, of sera from 23 of the 24 rabbits immunized for this study are summarized in Table 2. Since rabbit R9004 died before Bleed 2, no data for that rabbit are included in the table.
On the basis of the mean values of the relative titers for all 11 rabbits immunized with mPEG conjugates of three proteins, the median value of the relative titer was 3.0, with a range of 1.1–20. In contrast, each of nine rabbits immunized with HO-PEG conjugates of IFN-α or uricase had similar titers of anti-PEG antibodies detected on mPEG and HO-PEG antigens.
Sensitivity of Detection of Anti-PEG Antibodies Is Decreased by Tween 20 or Tween 80
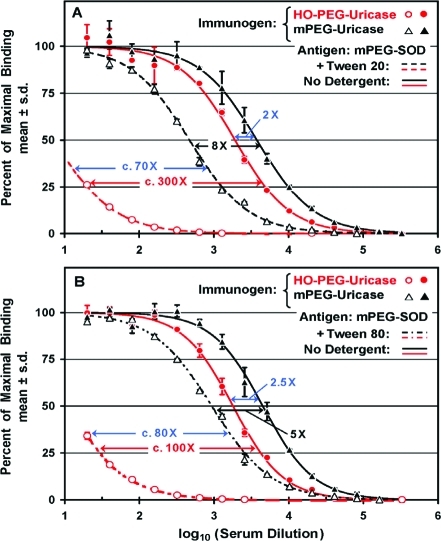
Many procedures commonly used for the performance of direct or competitive ELISAs include the use of the detergents Tween 207,19−21,30,31 or Tween 8032 in the solutions with which the assay plates are washed one or more times. The structures of these detergents are shown in Figure 1. Two of the experiments performed to assess the effects of Tween 20 or Tween 80 on the titers of anti-PEG antibodies detected by direct ELISAs are shown in Figure 5. Sera from two rabbits immunized with either mPEG-uricase or HO-PEG-uricase were tested in direct ELISAs on replicate assay plates coated with mPEG-SOD. In each experiment, one of the assay plates was washed without any detergent, as was done in all of the other ELISAs described in this report. The other assay plate was washed before and after the addition of the secondary antibody with PBS containing either 0.05% (v/v) Tween 20 (Figure 5A) or 0.1% (v/v) Tween 80 (Figure 5B).
When the assays were performed without the use of Tween in the washes (solid curves), the titer of anti-PEG antibodies detected in the serum of the rabbit immunized with mPEG-uricase was only 2–2.5 higher than that of the rabbit immunized with HO-PEG-uricase. In contrast, the titer of anti-PEG antibodies in serum from the same rabbit that was immunized with mPEG-uricase appeared to be higher by a factor of c. 70 or c. 80 when Tween 20 or Tween 80, respectively, was used in the washes before and after the addition of the secondary antibody (dashed curves). Figure 5 also shows that the apparent titers of anti-PEG antibodies detected, on plates coated with mPEG-SOD, in the serum of a rabbit immunized with HO-PEG-uricase were decreased c. 300-fold or c. 100-fold (red arrows) when the plates were washed with buffers containing Tween 20 or Tween 80, respectively. In contrast, washing the assay plates with Tween 20 or Tween 80 decreased the apparent titers in serum from the rabbit immunized with mPEG-uricase only c. 8-fold or c. 5-fold, respectively (black arrows).
MethoxyPEG Binds More Tightly than PEG Diol to Antibodies Raised against mPEG-Interferon-α and mPEG-Uricase, but not to Antibodies Raised against HO-PEG-Uricase
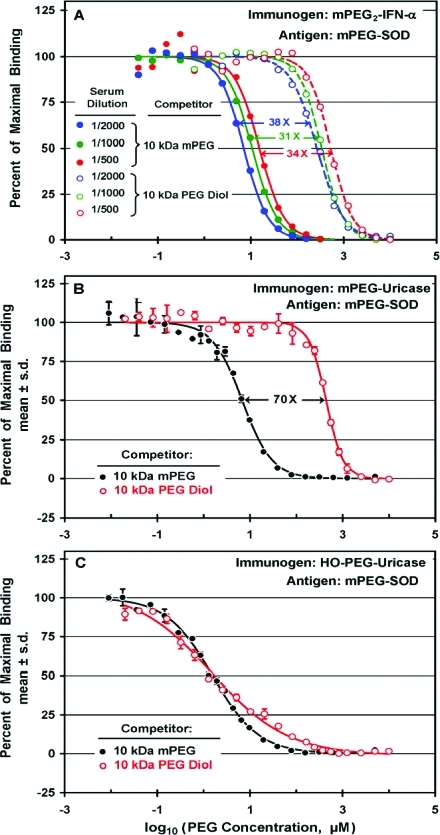
The results of competitive ELISAs with 10 kDa mPEG (filled circles) and 10 kDa PEG diol (open circles) of three dilutions of serum from a rabbit immunized with mPEG2-IFN-α are compared in Figure 6A. For all of the tested dilutions of serum (1/500, 1/1000, and 1/2000), the ratios of affinities for mPEG/PEG diol were between 30 and 40. On the other hand, the values of IC50 for each of the competitors varied in a nonlinear fashion with the dilution of the serum. Thus, a 4-fold decrease in the antibody concentration (from 1/500 to 1/2000) resulted in a 2-fold decrease in the values of IC50.
In Figure 6B, the potency of 10 kDa mPEG (black symbols) is compared to that of 10 kDa PEG diol (red symbols) as competitors for the binding to mPEG-SOD of antibodies elicited by mPEG-uricase. The anti-PEG antibodies in the serum tested in Figure 6B exhibited c. 70-fold higher affinity for mPEG than for PEG diol of the same molecular weight. Similar results were obtained with sera from the other two rabbits immunized with mPEG-uricase (data not shown). In contrast, no significant difference was detected between the affinities of antibodies raised against HO-PEG-uricase for 10 kDa mPEG vs 10 kDa PEG diol, when tested on the same antigen (Figure 6C) or on HO-PEG-SOD as the antigen (data not shown). These results indicate that anti-PEG antibodies raised against mPEG conjugates have much higher affinities for mPEG than for PEG diol, while anti-PEG antibodies elicited by HO-PEG conjugates show no selectivity for the end-group of the polymer.
Although competitive ELISAs on a single dilution of a single serum sample provide information about the relative affinities of those antibodies for various competitors (e.g., 10 kDa mPEG vs 10 kDa PEG diol, as shown in Figure 6B), comparisons of the values of IC50 for different serum samples do not provide a valid comparison of affinities, because the values of IC50 do not vary linearly with the concentration of antibodies in each sample, as shown in Figure 6A.
Anti-PEG Antibodies against mPEG-Interferon-α or mPEG-Uricase Bind Cooperatively to Multiply-PEGylated mPEG-Albumin; Antibodies against HO-PEG-Uricase Bind Similarly to PEG Diol and to HO-PEG-Albumin
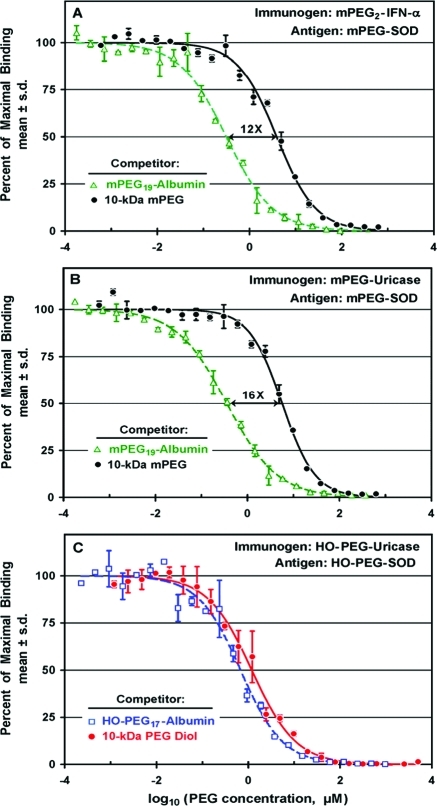
An albumin conjugate containing an average of 19 molecules of 10 kDa mPEG (mPEG19-albumin) and free 10 kDa mPEG were tested as competitors for the binding to mPEG-SOD of antibodies raised against mPEG2-IFN-α (Figure 7A). At the highest tested concentrations (c. 0.1–1 mM PEG), both competitors blocked the binding to the assay plate completely. In a range of lower concentrations, however, the multiply-PEGylated albumin conjugate was much more potent as a competitor than free mPEG. The molar concentration of free mPEG that inhibited 50% of the binding (IC50) was c. 12 times higher than the concentration of mPEG in the albumin conjugate that was similarly inhibitory.
Figure 7.
Competitive ELISAs with mPEG-SOD as the antigen were used to assess the cooperativity of binding by antibodies raised against either mPEG2-IFN-α (A) or mPEG-uricase (B) to mPEG in conjugates with human serum albumin. Competitive ELISAs with HO-PEG-SOD as the antigen showed similar binding of antibodies raised against HO-PEG-uricase to free PEG diol and to HO-PEG in an albumin conjugate (C). Concentrations of all competitors are expressed as micromolar PEG in the serum-containing assay mixtures. (A) For anti-PEG antibodies raised against mPEG2-IFN-α, the concentration of free 10 kDa mPEG (●) that inhibited 50% of the binding to mPEG-SOD (IC50) was 12 times higher than the IC50 for mPEG in an mPEG19-albumin conjugate (△). The serum was diluted 1/1000. (B) For anti-PEG antibodies raised against mPEG-uricase, the IC50 of free 10 kDa mPEG (●) was 16 times higher than the IC50 of mPEG in an mPEG19-albumin conjugate (△). The serum was diluted 1/1000. (C) Binding of antibodies raised against HO-PEG-uricase to HO-PEG-SOD was inhibited to a similar extent by free 10 kDa PEG diol (●) and by a conjugate of albumin with 17 molecules of 10 kDa HO-PEG (□). Because of the low titer of antibodies detected by direct ELISAs (see Figure 4B), this serum was diluted only 1/320.
Experiments analogous to those in Figure 7A were performed on antiserum raised against mPEG-uricase containing an average of nine molecules of mPEG per uricase tetramer (Figure 7B). Again, the multiply-PEGylated albumin conjugate was more potent than free 10 kDa mPEG as an inhibitor of the binding of anti-PEG antibodies to mPEG-SOD. In this antiserum, the IC50 of free mPEG was c. 16 times higher than the IC50 of mPEG in the mPEG19-albumin conjugate.
In contrast with the results for rabbits immunized with mPEG conjugates, the binding to HO-PEG-SOD of antibodies raised against HO-PEG-uricase is inhibited to a similar extent by 10 kDa PEG diol and by a HO-PEG conjugate of albumin (HO-PEG17-albumin) at all tested competitor concentrations (Figure 7C).
Antibodies Raised against mPEG Conjugates of Three Proteins Have Much Higher Affinities for Multiply-PEGylated mPEG-Albumin than for HO-PEG-Albumin of Similar Composition
As shown in Figure 8A, an mPEG conjugate of albumin was much more potent than HO-PEG-albumin as a competitor for the binding to mPEG-SOD of antibodies raised against mPEG-uricase. The potencies of these competitors, each of which contained an average of c. 20 molecules of PEG per albumin molecule, differed by a factor of c. 1300. Indeed, the affinity of the HO-PEG-albumin conjugate was so low that the binding of the anti-PEG antibodies to the assay plate was incompletely inhibited by the highest available concentration of this competitor. Figure 8B illustrates analogous results obtained in a comparison of the potencies ofmPEG17-albumin vs HO-PEG17-albumin as competitors for the binding to mPEG-SOD of anti-PEG antibodies raised against mPEG17-albumin. In this experiment, the highest available concentration of HO-PEG17-albumin inhibited only c. 50% of the binding, and the affinity for mPEG17-albumin was estimated to be c. 1600 times higher than the affinity for HO-PEG17-albumin.
In analogous experiments performed with antiserum raised against mPEG2-IFN-α, the affinity of the mPEG-albumin conjugate was estimated to be c. 8000 times that of the HO-PEG-albumin conjugate (Figure 8C). Since the highest available concentration of the HO-PEG-albumin conjugate inhibited only c. 13% of the binding, the data were extrapolated to obtain a rough estimate of IC50 of the HO-PEG-albumin conjugate (see dashed curve). Thus, anti-PEG antibodies elicited by conjugates of three proteins containing between 2 and 17 molecules of mPEG per protein molecule have affinities for multiply-PEGylated conjugates of albumin with mPEG that are at least 1000-fold higher than their affinities for HO-PEG albumin with a similar PEG-to-protein ratio.
Antibodies Raised against t-BuO-PEG-Albumin Have Higher Titers Detected on t-BuO-PEG than on mPEG or HO-PEG Antigens in Direct ELISAs and Higher Affinities for t-BuO-PEG than for mPEG or PEG Diol in Competitive ELISAs
Although the focus of this research is the role of the methoxy group of mPEG in the immune responses to mPEG-protein conjugates, a few experiments were performed to quantify and characterize the immune responses to a different alkoxy group at the distal terminus of an alkoxyPEG-protein conjugate. Three rabbits were immunized with a human serum albumin conjugate of 10 kDa t-BuO-PEG containing an average of 17 molecules of PEG per molecule of albumin (see Figures 1 and S7). The results of direct ELISAs of serum from one of these rabbits, in which the assay plates were coated with SOD conjugates of t-BuO-PEG, mPEG, or HO-PEG, are shown in Figure 9A. The anti-PEG antibodies generated against t-BuO-PEG17-albumin show significant cross-reactivity with mPEG-SOD and slightly less cross-reactivity with HO-PEG-SOD. The mean titer of antibodies in the sera of these three rabbits detected with t-BuO-PEG-SOD as the antigen (39 000 ± 8000) was higher than the highest titers of anti-PEG antibodies detected with mPEG-SOD as the antigen for the groups of rabbits immunized with mPEG1-IFN-α (see Figure 3A) or mPEG-uricase (see Figure 4A). As shown in Table 2, the antibodies raised against t-BuO-PEG17-albumin have slightly higher titers detected in direct ELISAs with mPEG-SOD than with HO-PEG-SOD as the antigen, but the relative titers (detected on mPEG-SOD/HO-PEG-SOD) for all three rabbits immunized with t-BuO-PEG17-albumin were lower than those of 10 of 11 rabbits immunized with mPEG-protein conjugates from which sera from Bleeds 2 and 3 were available.
Figure 9B shows the results of competitive ELISAs of sera from two rabbits that were immunized with t-BuO-PEG17-albumin, including the rabbit for which the results of direct ELISAs are shown in Figure 9A. About 40-fold higher selectivity of the antibodies raised against t-BuO-PEG17-albumin for t-BuO-PEG compared to mPEG is revealed by the results of the competitive ELISAs for both rabbits (200× in Figure 9B) than by the direct ELISAs (5× in Figure 9A).
MethoxyPEG and HO-PEG Conjugates of Two Cytokines Have Equivalent Potencies in Cell Culture
To complement our studies of the immunologic properties of mPEG and HO-PEG conjugates of various proteins (see Figures 2–9), experiments were performed to determine whether the distal terminal group of the polymer affects the biological activities of PEG conjugates of two cytokines measured in vitro. Human IFN-α and human EPO were selected as examples of cytokines for which reliable cell culture assays of potency were available. MethoxyPEG and HO-PEG conjugates of IFN-α were tested on human Burkitt’s lymphoma cells (Daudi cells), the proliferation of which is profoundly inhibited by IFN-α.37,38 MethoxyPEG and HO-PEG conjugates of EPO were tested on human erythroleukemic cells (TF-1 cells), the proliferation of which is stimulated by EPO and certain other cytokines.39,40
MonoPEGylated conjugates of IFN-α, synthesized by reductive alkylation with 20 kDa mPEG aldehyde or 20 kDa HO-PEG aldehyde (see Figures S1 and S4), were indistinguishable in their potencies as inhibitors of Daudi cell proliferation (Figure 10A). Both conjugates displayed c. 6% of the potency of unPEGylated IFN-α. Similarly, diPEGylated conjugates of IFN-α, synthesized using 20 kDa mPEG aldehyde or 20 kDa HO-PEG aldehyde, were indistinguishable in their potencies as inhibitors of the proliferation of Daudi cells, although both were c. 0.4% as potent as unPEGylated IFN-α and c. 7% as potent as the monoPEGylated conjugates.
The effects on TF-1 cell proliferation of a standard solution of EPO,41 a monoPEGylated conjugate of EPO synthesized by reductive alkylation with 30 kDa HO-PEG aldehyde (see Figure S5), and a monoPEGylated conjugate of EPO containing 30 kDa mPEG, which is commercially available from Roche as Mircera,42,43 were compared (Figure 10B). The potencies of the HO-PEG and mPEG conjugates of EPO were indistinguishable in this assay, although both conjugates were only c. 2% as potent as the EPO standard. Thus, for both IFN-α and EPO, conjugates with HO-PEG and mPEG had equivalent potencies in cell culture.
Discussion
The goal of this research was to assess the role of the methoxy group of mPEG in the immunogenicity and antigenicity of mPEG-protein conjugates and the potential advantages of replacing mPEG with hydroxyPEG (HO-PEG) in the synthesis of PEG conjugates of proteins and other therapeutic agents. The results demonstrate that the methoxy groups of mPEG-protein conjugates contribute significantly to the titers of anti-PEG antibodies detected in the sera of rabbits immunized with mPEG conjugates of human interferon-α, human serum albumin, or porcine uricase (see Figures 2–4 and Table 2). Anti-PEG antibodies raised against mPEG conjugates of these proteins exhibit much higher affinities for multiply-PEGylated conjugates of albumin synthesized with mPEG than with HO-PEG (Figure 8). Additional results include the demonstration that (1) the use of the PEG-containing detergents Tween 20 and Tween 80 decreases the sensitivity of detection of anti-PEG antibodies by enzyme-linked immunosorbent assays (ELISAs) (Figure 5); (2) the titers of anti-PEG antibodies formed against t-BuO-PEG conjugates of albumin were higher than those elicited by mPEG-albumin conjugates, and (3) analogous HO-PEG and mPEG conjugates of two cytokines have indistinguishable biological activities in cell culture assays (Figure 10). While the immunologic data were obtained in experiments on groups of only three rabbits each immunized with an mPEG, a HO-PEG, or a t-BuO-PEG conjugate of one of three proteins, the results have intriguing implications for potential improvements in the pharmacokinetics and pharmacodynamics of a wide range of PEGylated drugs by avoiding the use of activated mPEG or other alkoxy PEGs in synthesizing the next generation of such drugs.
Development of Antibodies against PEGylated Therapeutic Enzymes Can Limit Their Efficacy
In 2000, Müller et al.44 reported the accelerated clearance of an mPEG conjugate of asparaginase (Oncaspar) in a subset of pediatric leukemia patients who had no clinical evidence of an allergic reaction. Although there was no mention of anti-PEG antibodies in that report, subsequent analyses of stored sera from some of the patients in that study provided clear evidence of anti-PEG antibodies that were detected both by serological techniques and by flow cytometry.45 Oncaspar is synthesized by coupling multiple strands of 5 kDa mPEG to l-asparaginase using the succinimidyl succinate derivative of mPEG.9
In 2006, Ganson et al.30 reported that a subset of patients with refractory chronic gout who had received a single subcutaneous injection of an mPEG conjugate of a recombinant mammalian uricase cleared the conjugate exceptionally rapidly and that low titers of anti-PEG antibodies (IgG) were detected in the sera of some of these patients by about one week after the injection. In a subsequent Phase 1 trial in which the same mPEG-uricase was administered intravenously to subjects with refractory chronic gout, antibodies to PEG-uricase, described as “mostly IgG2 and specific for PEG”, developed in 9 of 24 patients, in some of whom the enzyme was cleared rapidly without evidence of allergic reactions.31 The results of a Phase 2 randomized study of this mPEG-uricase (now called pegloticase) were published in 2008,46 and the results of two 6-month, randomized, controlled Phase 3 trials were published in 2011.47 In the Phase 2 and Phase 3 trials, the absence of high titers of anti-pegloticase antibodies was correlated with persistent efficacy of the drug in lowering plasma uric acid concentrations. In the Phase 3 trials, a subset of the patients lost responsiveness within 3–4 months of treatment and this loss was associated with high titers of anti-pegloticase antibodies. However, the specificities of the antibodies for the enzyme vs the polymer component of the drug were not reported.46,47 Pegloticase is synthesized by coupling an average of ten molecules of 10 kDa mPEG per subunit of recombinant mammalian uricase (resembling porcine uricase) using a p-nitrophenylcarbonate derivative of mPEG.9,14
These examples of the loss of responsiveness to mPEG conjugates of two therapeutic enzymes (asparaginase and uricase) that were synthesized using two different coupling chemistries suggest the possible clinical relevance of the present studies of the immunologic responses to mPEG-protein conjugates in experimental animals. The immunogenicity of the polymer component of two mPEG-protein conjugates suggests that patients who have been treated previously with one PEGylated drug may be at increased risk of adverse immunologic responses to a second PEGylated drug. Since the asparaginase sequence in Oncaspar is bacterial44 and the uricase sequence in pegloticase is a mutein of porcine uricase,14 we cannot extrapolate from the cited results with conjugates of foreign proteins in humans and rabbits to predict the relative immunogenicities in humans of mPEG vs HO-PEG conjugates of human proteins.
Anti-PEG Antibodies Formed against HO-PEG-Proteins Have the Same Titers on mPEG and HO-PEG Antigens and the Same Mean Affinities for mPEG and PEG Diol
In contrast with the results obtained for rabbits immunized with mPEG-proteins, direct ELISAs of sera from each of nine rabbits immunized with HO-PEG conjugates of IFN-α or uricase had indistinguishable titers of anti-PEG antibodies detected on mPEG-SOD and HO-PEG-SOD (see Table 2 and Figures 2B, 3B, and 4B). Similarly, competitive ELISAs show that anti-PEG antibodies elicited by HO-PEG-uricase do not bind preferentially to 10 kDa mPEG or 10 kDa PEG diol (see Figure 6C). These results imply that the anti-PEG antibodies elicited by HO-PEG-proteins are directed against the backbone of the polymer (see Figure 1) and that competition for their binding to a PEG-protein antigen is neither enhanced nor inhibited by the presence of a methoxy group in a competitor.
It should be noted that the titers of anti-PEG antibodies elicited by HO-PEG conjugates of uricase tended to be lower than the titers elicited by the corresponding mPEG conjugates (Figure 4). Therefore, sera from the rabbits immunized with HO-PEG-uricase were diluted less extensively than sera from rabbits immunized with mPEG-uricase for competitive ELISAs, e.g., the sera in Figure 6B and C were diluted 1/1000 and 1/500, respectively. Similarly, the sera in Figure 7B and C were diluted 1/1000 and 1/320, respectively. Under these conditions, the values of IC50 are not directly proportional to the affinities of the tested antibodies for the various competitors.
Anti-PEG Antibodies Formed against mPEG-Proteins Vary in Their Selectivities for the PEG Backbone and the Methoxy Group
In sera from 10 of 11 rabbits immunized with mPEG conjugates of three unrelated proteins (human IFN-α, porcine uricase and human serum albumin), the titers of anti-PEG antibodies detected by direct ELISAs on assay plates coated with mPEG conjugates of another unrelated protein, SOD, were higher than the titers detected in the same sera on assay plates coated with HO-PEG-SOD (see Table 2 and Figures 2A, 3A, and 4A]. The unusually high degree of selectivity for mPEG of the anti-PEG antibodies detected in sera from two of three rabbits immunized with mPEG-uricase suggests that the immunogenicity of this porcine enzyme to which the PEG is coupled may influence the impact of the terminal methoxy groups on the specificity of the antigen-binding sites of the anti-PEG antibodies (see Figure 1).
There were noteworthy differences within the two groups of rabbits immunized with mPEG conjugates of porcine uricase and human serum albumin, respectively. Among those immunized with mPEG-uricase, rabbit R580 had a mean relative titer of only 3.1 ± 0.6, while rabbits R579 and R581 had mean relative titers of 20 ± 4.8 and 18 ± 4.8, respectively. Within the group of rabbits immunized with mPEG17-albumin, the mean relative titers of rabbits R12056 and R12058 were 3.0 ± 0.5 and 2.6 ± 0.6, respectively. Surprisingly, rabbit R12057 had indistinguishable titers on mPEG-SOD and HO-PEG-SOD in triplicate assays (mean relative titer = 1.1 ± 0.1). Rabbit R12057 was the only 1 of 11 rabbits immunized with mPEG conjugates of any of three different proteins in which the titer detected on mPEG-SOD was not at least twice that detected on HO-PEG-SOD. Competitive ELISAs of sera from that rabbit also showed no selectivity for mPEG vs PEG diol (data not shown). The results for this anomalous rabbit do not negate the overall conclusion about the importance of the methoxy group in the immune responses of most rabbits to mPEG-protein conjugates, but they signal the potential role of individual variation that might impact population studies in humans.
Antibodies against the Terminal Methoxy Group Dominate the Immune Responses to mPEG-Uricase
Figure 6B illustrates data from one rabbit immunized with mPEG-uricase in which the affinity for 10 kDa mPEG was 70 times higher than for 10 kDa PEG diol. Similar results were obtained when the same serum was tested with 20 kDa mPEG and 20 kDa PEG diol, as well as when sera from the other two rabbits immunized with mPEG-uricase were similarly tested (data not shown). The molecular weight of the methyl group that differentiates mPEG from HO-PEG is only 15 Da, which represents only 0.15% of the molecular weight of the 10 kDa competitors tested in these experiments. Therefore, it may seem surprising that the anti-PEG antibodies display so much higher affinities for the small group at the end of the polymer than for the large number of oxyethylene units (CH2–CH2–O) within the polymer backbone (c. 227 for 10 kDa PEGs). On the other hand, the ends of linear or branched polymers, especially the hydrophobic end containing a methoxy group (as in Figure 6A or B) or a t-butoxy group (as in Figure 9B), are more likely than the polymer backbone to be accessible to the antigen-binding sites of the antibodies (see Figure 1). The lack of absolute specificity of these antibodies for the terminal alkoxy groups is demonstrated by the observation that a sufficiently high concentration of PEG diol can completely inhibit the binding to mPEG-SOD of anti-PEG antibodies raised against mPEG-IFN-α or mPEG-uricase (see Figure 6A and B).
Multiple PEGylation Amplifies the Detectable Selectivity for mPEG vs HO-PEG of Anti-PEG Antibodies Formed against mPEG-Proteins
While several FDA-approved PEGylated therapeutic proteins in clinical use contain a single strand of mPEG (e.g., PegIntron,9,48 Neulasta,9,49 and Mircera9,42,43), several others contain derivatives of diPEG-lysine, which have two methoxy groups (e.g., Pegasys,9,48 Macugen,11 and Cimzia9,50,51), and others contain multiple strands of “linear” mPEG (e.g., Adagen,9,52 Oncaspar,9,45 Somavert,9,53 and KRYSTEXXA9,14,46,47). Our data suggest the potential clinical relevance of the present studies of the impact of multiple PEGylation on the antigenicity of mPEG-protein conjugates.
In contrast with the 12-fold and 16-fold differences between the affinities of anti-PEG antibodies elicited by two mPEG-protein conjugates for free 10 kDa mPEG vs an mPEG-albumin conjugate (see Figure 7A and B), no significant differences were detected between the affinities or the shapes of the competition curves obtained with free 10 kDa PEG diol and a HO-PEG-albumin conjugate as competitors for the binding to HO-PEG-SOD of antibodies raised against HO-PEG-uricase (see Figure 7C). A plausible interpretation is that anti-PEG antibodies directed against the backbone of the polymer can bind cooperatively to at least two sites within the backbone of a polymer as large as 10 kDa PEG diol. In that case, the presence of multiple strands of HO-PEG within the albumin conjugate would not further enhance the cooperativity of the binding. The absence of enhanced cooperativity in binding multiply-PEGylated protein conjugates distinguishes anti-PEG antibodies directed against the polymer backbone from those directed against the methoxy group(s) at the distal terminus or termini.
Anti-PEG Antibodies Raised against mPEG-Protein Conjugates Bind Multiply-PEGylated mPEG-Albumin Conjugates >1000-fold More Tightly than HO-PEG-Albumin Conjugates
In sera from rabbits immunized with mPEG conjugates of uricase, albumin or IFN-α, the affinities of anti-PEG antibodies for mPEG-albumin conjugates were about 3 orders of magnitude higher than for HO-PEG conjugates of albumin (Figure 8). Precise estimates of the relative affinities could not be obtained, since the highest available concentrations of the HO-PEG-albumin conjugates used as competitors inhibited only a fraction of the binding of the antibodies to mPEG-SOD on the assay plate. Su et al.7 previously reported that anti-PEG antibodies bind best when PEG is linked to a protein or liposome or adsorbed on a surface. They also reported that the binding of their monoclonal antibodies to the PEG backbone was not influenced by the nature of the terminal groups of the PEG, which included HO-PEG and mPEG.
The Methoxy Group is Not Unique in Eliciting Anti-PEG Antibodies Directed against the Terminal Group of AlkoxyPEG-Protein Conjugates
In sera from three rabbits immunized with t-BuO-PEG17-albumin (see Figure 9A), the mean titer detected with t-BuO-PEG-SOD as the antigen was about 7–10 times higher than the titers detected in sera of rabbits immunized with mPEG1-IFN-α (see Figure 3A), mPEG-uricase (see Figure 4A), or mPEG-albumin (data not shown).
The immunogenicity of the t-butoxy group shown in Figure 9is relevant to the potential use of this group as a removable blocking group in the synthesis of monofunctionally activated HO-PEG conjugates.13 The use of t-BuO-PEG as an intermediate in the synthesis of the p-nitrophenylcarbonate derivative of HO-PEG (HO-PEG-NPC) is illustrated below:
The failure to remove even a small fraction of the t-butoxy groups in step [2] of the above reaction sequence would result in a preparation of HO-PEG-NPC in which traces of t-BuO-PEG-NPC could form highly immunogenic protein conjugates.
Buffers Containing Tween-Type Non-Ionic Detergents Interfere with Detection of Anti-PEG Antibodies by ELISAs
A few previous investigators have recognized the inhibitory effects of Tween on the detection and quantitation of anti-PEG antibodies6,7 and a few companies that sell anti-PEG mAbs warn their customers to avoid the use of Tween (e.g., Silver Lake Research54). The dramatic effects of Tween 20 and Tween 80, illustrated in Figure 5, have clear implications for clinical studies that include attempts to quantify anti-PEG antibodies.6,30,31,45 The data in Figure 5 and similar results on sera from other rabbits immunized with mPEG- or HO-PEG-uricase (results not shown) illustrate the distortion of the results of direct ELISAs of anti-PEG antibodies resulting from the use of the detergents Tween 20 and Tween 80, which contain short strands of PEG within their structures (see Figure 1).
Therapeutic Proteins Coupled to mPEG and HO-PEG Have Equivalent Potencies in Cell Culture
The in vitro biological activities of two PEGylated cytokines, one of which inhibits proliferation (interferon-α) and the other of which stimulates proliferation (erythropoietin) of the cells on which each was tested were unaffected by the substitution of HO-PEG for mPEG of the same molecular weight in their synthesis (see Figure 10). These results provide support for the proposal that conjugates synthesized with HO-PEG instead of mPEG will retain the desirable attributes of PEGylated therapeutic agents, even if they provoke fewer undesirable immune responses than those observed with mPEG conjugates.
Implications of These Results for Future Research and the Future Design and Development of PEGylated Proteins and Other Therapeutic Agents
Our demonstration that mPEG conjugates of three dissimilar proteins containing as few as 1 or as many as 17 molecules of mPEG elicit anti-PEG antibodies that are directed against the methoxy group has important implications for the clinical use of mPEG conjugates of enzymes, cytokines, and other therapeutic proteins. Armstrong et al.45 recognized that anti-PEG antibodies were involved in the accelerated clearance of mPEG conjugates of asparaginase in a subset of patients treated with Oncaspar, and Ganson, Sundy, and their colleagues recognized that anti-PEG antibodies were correlated with the accelerated clearance of pegloticase in a subset of patients.30,31 However, those reports did not evaluate the role of the methoxy groups of the mPEGs in the conjugates being studied.
The results presented above are consistent with the hypothesis that the accelerated clearance and the consequent loss of efficacy of mPEG conjugates of therapeutic agents might be decreased by synthesizing next-generation versions of these drugs with monofunctionally activated derivatives of hydroxyPEG, instead of mPEG. The potential advantages of HO-PEG conjugates have been suggested in previous publications by several of the present authors.13,14 Despite the convenience inherent in the use of mPEG for the synthesis of monofunctionally activated PEGylation reagents, the extra effort entailed in the use of monofunctionally activated HO-PEG may be justified by the decreased risk of treatment-limiting immune responses to the resultant HO-PEG conjugates (see Supporting Information).
The caveats on extrapolating from the present results to clinical situations include the facts that (1) all immunogens administered to rabbits in this study were emulsified in complete or incomplete Freund’s adjuvant, (2) the clearance rates of the various PEG conjugates could not be measured in these hyperimmune rabbits, and (3) antibody isotyping was not possible in this study because the secondary antibody used for all of the reported ELISAs was specific for the H and L chains of rabbit IgG, which can cross-react with the L chains of rabbit IgM. All of these factors are worthy subjects of future research. Finally, although all of the data in this report were obtained with PEG conjugates of proteins, the potential advantages of replacing activated mPEG with monofunctionally activated HO-PEG for synthesizing polymer conjugates of liposomes, viruses, red blood cells, aptamers, and other types of therapeutic agents also merit investigation.
Acknowledgments
This research was supported in part by a Qualifying Therapeutic Discovery Project grant from the U.S. government under the Patient Protection and Affordable Care Act (P.L. 111-148). We are grateful to John A. French for his contributions throughout this research, particularly with respect to the computer-assisted analysis of the data, and to Alexa L. Martinez for her participation in the early stages of this research. Dr. John A. Katzenellenbogen and Dr. Ralph W. Niven provided many insightful suggestions regarding the manuscript.
Glossary
Abbreviations
- AAALAC
Association for the Assessment and Accreditation of Laboratory Animal Care
- ACN
acetonitrile
- Albumin
human serum albumin
- Blocking Buffer
5% (w/v) nonfat dry milk solids in PBS
- ELISA
enzyme-linked immunosorbent assay
- EPO
recombinant human erythropoietin
- GM-CSF
recombinant human granulocyte-macrophage colony-stimulating factor
- HO-PEG
hydroxyPEG
- HRP
horseradish peroxidase
- IACUC
Institutional Animal Care and Use Committee
- IFN-α
recombinant human interferon-α
- mAU/min
milli-absorbance units/min
- mAbs
monoclonal antibodies
- mPEG
monomethoxypoly(ethylene glycol)
- NPC
p-nitrophenylcarbonate
- OPD
o-phenylenediamine dihydrochloride
- PBS-G
phosphate-buffered saline, pH 7.4, containing 2% (v/v) goat serum
- PEG
poly(ethylene glycol)
- pNP-COCl
p-nitrophenylchloroformate
- RI
refractive index
- RP chromatography
reversed-phase chromatography
- SEC
size-exclusion chromatography
- SOD
porcine Cu–Zn superoxide dismutase
- t-BuO-PEG
t-butoxyPEG
- TFA
trifluoroacetic acid.
Supporting Information Available
Descriptions of the synthesis, purification and physicochemical analyses of (1) the activated HO-PEGs and t-BuO-PEG; (2) the PEG-protein conjugates listed in Table 1, and (3) the HO-PEG conjugate of erythropoietin used in the cell culture assays. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): All of the authors are employees of Mountain View Pharmaceuticals, Inc.
Supplementary Material
References
- Abuchowski A.; van Es T.; Palczuk N. C.; Davis F. F. (1977) Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 252, 3578–3581. [PubMed] [Google Scholar]
- Abuchowski A.; McCoy J. R.; Palczuk N. C.; van Es T.; Davis F. F. (1977) Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 252, 3582–3586. [PubMed] [Google Scholar]
- Lee W. Y.; Sehon A. H. (1978) Suppression of reaginic antibodies with modified allergens. I. Reduction in allergenicity of protein allergens by conjugation to polyethylene glycol. Int. Arch. Allergy Appl. Immunol. 56, 159–170. [DOI] [PubMed] [Google Scholar]
- Richter A. W.; Åkerblom E. (1983) Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 70, 124–131. [DOI] [PubMed] [Google Scholar]
- Richter A. W.; Åkerblom E. (1984) Polyethylene glycol reactive antibodies in man: Titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int. Arch. Allergy Appl. Immunol. 74, 36–39. [DOI] [PubMed] [Google Scholar]
- Armstrong J. K. (2009) The occurrence, induction, specificity and potential effect of antibodies against poly(ethylene glycol). In PEGylated Protein Drugs: Basic Science and Clinical Applications (Veronese F. M., Ed.) pp 147–168, Birkhäuser; Verlag, Basel, Switzerland. [Google Scholar]
- Su Y.-C.; Chen B.-M.; Chuang K.-H.; Cheng T.-L.; Roffler S. R. (2010) Sensitive quantification of PEGylated compounds by second-generation anti-poly(ethylene glycol) monoclonal antibodies. Bioconjugate Chem. 21, 1264–1270. [DOI] [PubMed] [Google Scholar]
- Fishburn C. S. (2008) The pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 97, 4167–4183. [DOI] [PubMed] [Google Scholar]
- Alconcel S. N. S.; Baas A. S.; Maynard H. D. (2011) FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym. Chem. 2, 1442–1448. [Google Scholar]
- Duncan R. (2011) Polymer therapeutics as nanomedicines: New perspectives. Curr. Opin. Biotechnol. 22, 492–501. [DOI] [PubMed] [Google Scholar]
- Ng E. W.; Adamis A. P. (2006) Anti-VEGF aptamer (pegaptanib) therapy for ocular vascular diseases. Ann. N.Y. Acad. Sci. 1082, 151–171. [DOI] [PubMed] [Google Scholar]
- Immordino M. L.; Dosio F.; Cattel L. (2006) Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine 1, 297–315. [PMC free article] [PubMed] [Google Scholar]
- Martinez A. L., Sherman M. R., Saifer M. G. P., and Williams L. D., Mountain View Pharmaceuticals, Inc. Polymer conjugates with decreased antigenicity, methods of preparation and uses thereof. U.S. Patent No. 8,129,330 B1, March 6, 2012.
- Sherman M. R.; Saifer M. G. P.; Perez-Ruiz F. (2008) PEG-uricase in the management of treatment-resistant gout and hyperuricemia. Adv. Drug Delivery Rev. 60, 59–68. [DOI] [PubMed] [Google Scholar]
- Cheng T.-L.; Wu P.-Y.; Wu M.-F.; Chern J.-W.; Roffler S. R. (1999) Accelerated clearance of polyethylene glycol-modified proteins by anti-polyethylene glycol IgM. Bioconjugate Chem. 10, 520–528. [DOI] [PubMed] [Google Scholar]
- Cheng T.-L.; Chen B.-M.; Chern J.-W.; Wu M.-F.; Roffler S. R. (2000) Efficient clearance of poly(ethylene glycol)-modified immunoenzyme with anti-PEG monoclonal antibody for prodrug cancer therapy. Bioconjugate Chem. 11, 258–266. [DOI] [PubMed] [Google Scholar]
- Tsai N.; Cheng T.-L.; Roffler S. R. (2001) Sensitive measurement of polyethylene glycol-modified proteins. BioTechniques 30, 396–402. [DOI] [PubMed] [Google Scholar]
- Cheng T.-L.; Cheng C.-M.; Chen B.-M.; Tsao D.-A.; Chuang K.-H.; Hsiao S.-W.; Lin Y.-H.; Roffler S. R. (2005) Monoclonal antibody-based quantitation of poly(ethylene glycol)-derivatized proteins, liposomes, and nanoparticles. Bioconjugate Chem. 16, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Roffler S., Cheng T.-L., and Wu P.-Y., Academia Sinica, Monoclonal-antibody for analysis and clearance of polyethylene glycol and polyethylene glycol-modified molecules. U.S. Patent No. 6,596,849 B1, Jul. 22, 2003.
- Roffler S., Cheng T.-L., and Wu P.-Y., Academia Sinica, Monoclonal antibody for analysis and clearance of polyethylene glycol and polyethylene glycol-modified molecules. U.S. Patent No. 6,617,118 B2, Sept. 9, 2003.
- Roffler S., Cheng T.-L., and Wu P.-Y., Academia Sinica, Monoclonal antibody for analysis and clearance of polyethylene glycol and polyethylene glycol-modified molecules. U.S. Patent No. 7,320,791 B2, Jan. 22, 2008.
- Armstrong J. K.; Wenby R. B.; Meiselman H. J.; Fisher T. C. (2003) In vivo survival of poly(ethylene glycol)-coated red blood cells in the rabbit. Blood 102, 94a.12623852 [Google Scholar]
- Garratty G. (2008) Modulating the red cell membrane to produce universal/stealth donor red cells suitable for transfusion. Vox Sang. 94, 87–95. [DOI] [PubMed] [Google Scholar]
- Dams E. T. M.; Laverman P.; Oyen W. J. G.; Storm G.; Scherphof G. L.; Van Der Meer J. W. M.; Corstens F. H. M.; Boerman O. C. (2000) Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J. Pharmacol. Exp. Ther. 292, 1071–1079. [PubMed] [Google Scholar]
- Semple S. C.; Harasym T. O.; Clow K. A.; Ansell S. M.; Klimuk S. K.; Hope M. J. (2005) Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic acid. J. Pharmacol. Exp. Ther. 312, 1020–1026. [DOI] [PubMed] [Google Scholar]
- Środa K.; Rydlewski J.; Langner M.; Kozubek A.; Grzybek M.; Sikorski A. F. (2005) Repeated injections of PEG-PE liposomes generate anti-PEG antibodies. Cell. Mol. Biol. Lett. 10, 37–47. [PubMed] [Google Scholar]
- Judge A.; McClintock K.; Phelps J. R.; MacLachlan I. (2006) Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol. Ther. 13, 328–337. [DOI] [PubMed] [Google Scholar]
- Ishida T.; Wang X. Y.; Shimizu T.; Nawata K.; Kiwada H. (2007) PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J. Controlled Release 122, 349–355. [DOI] [PubMed] [Google Scholar]
- Ishida T.; Kashima S.; Kiwada H. (2008) The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J. Controlled Release 126, 162–165. [DOI] [PubMed] [Google Scholar]
- Ganson N. J.; Kelly S. J.; Scarlett E.; Sundy J. S.; Hershfield M. S. (2006) Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res. Ther. 8, R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundy J. S.; Ganson N. J.; Kelly S. J.; Scarlett E. L.; Rehrig C. D.; Huang W.; Hershfield M. S. (2007) Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum. 56, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Hatfield R. M.; Morris B. A.; Henry R. R. (1987) Development of an enzyme linked immunosorbent assay for the detection of humoral antibody to Pasteurella anatipestifer. Avian Pathology 16, 123–140. [DOI] [PubMed] [Google Scholar]
- Kunitani M.; Dollinger G.; Johnson D.; Kresin L. (1991) On-line characterization of polyethylene glycol-modified proteins. J. Chromatogr. 588, 125–137. [Google Scholar]
- Skoog B. (1979) Determination of polyethylene glycols 4000 and 6000 in plasma protein preparations. Vox Sang. 37, 345–349. [DOI] [PubMed] [Google Scholar]
- Porstmann B.; Porstmann T.; Nugel E. (1981) Comparison of chromogens for the determination of horseradish peroxidase as a marker in enzyme immunoassay. J. Clin. Chem. Clin. Biochem. 19, 435–439. [DOI] [PubMed] [Google Scholar]
- Ahmed S. A.; Gogal R. M. Jr.; Walsh J. E. (1994) A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170, 211–224. [DOI] [PubMed] [Google Scholar]
- Evinger M.; Maeda S.; Pestka S. (1981) Recombinant human leukocyte interferon produced in bacteria has antiproliferative activity. J. Biol. Chem. 256, 2113–2114. [PubMed] [Google Scholar]
- Bekisz J.; Baron S.; Balinsky C.; Morrow A.; Zoon K. C. (2010) Antiproliferative properties of Type I and Type II interferon. Pharmaceuticals (Basel) 3, 994–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T.; Tange T.; Terasawa T.; Chiba S.; Kuwaki T.; Miyagawa K.; Piao Y.-F.; Miyazono K.; Urabe A.; Takaku F. (1989) Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J. Cell. Physiol. 140, 323–334. [DOI] [PubMed] [Google Scholar]
- Kitamura T.; Takaku F.; Miyajima A. (1991) IL-1 up-regulates the expression of cytokine receptors on a factor-dependent human hemopoietic cell line, TF-1. Int. Immunol. 3, 571–577. [DOI] [PubMed] [Google Scholar]
- Behr-Gross M.-E.; Daas A.; Burns C.; Bristow A. F. (2007) Collaborative study for the establishment of erythropoietin BRP batch 3. Pharmeuropa Bio. 2007–1, 49–66. [PubMed] [Google Scholar]
- Curran M. P.; McCormack P. L. (2008) Methoxy polyethylene glycol-epoetin beta: A review of its use in the management of anaemia associated with chronic kidney disease. Drugs 68, 1139–1156. [DOI] [PubMed] [Google Scholar]
- Wedekin M.; Ehrich J. H. H.; Pape L. (2011) Effective treatment of anemia in pediatric kidney transplant recipients with methoxy polyethylene glycol-epoetin beta. Pediatr. Transplant. 15, 329–333. [DOI] [PubMed] [Google Scholar]
- Müller H.-J.; Löning L.; Horn A.; Schwabe D.; Gunkel M.; Schrappe M.; von Schütz V.; Henze G.; Casimiro da Palma J.; Ritter J.; Pinheiro J. P. V; Winkelhorst M.; Boos J. (2000) Pegylated asparaginase (OncasparTM) in children with ALL: Drug monitoring in reinduction according to the ALL/NHL-BFM 95 protocols. Br. J. Haematol. 110, 379–384. [DOI] [PubMed] [Google Scholar]
- Armstrong J. K.; Hempel G.; Koling S.; Chan L. S.; Fisher T.; Meiselman H. J.; Garratty G. (2007) Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 110, 103–111. [DOI] [PubMed] [Google Scholar]
- Sundy J. S.; Becker M. A.; Baraf H. S. B.; Barkhuizen A.; Moreland L. W.; Huang W.; Waltrip R. W. II; Maroli A. N.; Horowitz Z. (2008) Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout. Results of a Phase II randomized study. Arthritis Rheum. 58, 2882–2891for the Pegloticase Phase 2 Study Investigators.. [DOI] [PubMed] [Google Scholar]
- Sundy J. S.; Baraf H. S. B.; Yood R. A.; Edwards N. L.; Gutierrez-Urena S. R.; Treadwell E. L.; Vázquez-Mellado J.; White W. B.; Lipsky P. E.; Horowitz Z.; Huang W.; Maroli A. N.; Waltrip R. W. II; Hamburger S. A.; Becker M. A. (2011) Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: Two randomized controlled trials. JAMA 306, 711–720. [DOI] [PubMed] [Google Scholar]
- Foster G. R. (2010) Pegylated interferons for the treatment of chronic hepatitis C: Pharmacological and clinical differences between peginterferon-α-2a and peginterferon-α-2b. Drugs 70, 147–165. [DOI] [PubMed] [Google Scholar]
- Yang B. B.; Kido A. (2011) Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin. Pharmacokinet. 50, 295–306. [DOI] [PubMed] [Google Scholar]
- Sandborn W. J.; Feagan B. G.; Stoinov S.; Honiball P. J.; Rutgeerts P.; Mason D.; Bloomfield R.; Schreiber S.; (2007) Certolizumab pegol for the treatment of Crohn’s disease. N. Engl. J. Med. 357, 228–238. [DOI] [PubMed] [Google Scholar]
- Schreiber S.; Khaliq-Kareemi M.; Lawrance I. C.; Thomsen O. Ø.; Hanauer S. B.; McColm J.; Bloomfield R.; Sandborn W. J.; (2007) Maintenance therapy with certolizumab pegol for Crohn’s disease. N. Engl. J. Med. 357, 239–250. [DOI] [PubMed] [Google Scholar]
- Booth C.; Gaspar H. B. (2009) Pegademase bovine (PEG-ADA) for the treatment of infants and children with severe combined immunodeficiency (SCID). Biologics 3, 349–358. [PMC free article] [PubMed] [Google Scholar]
- Trainer P. J.; Ezzat S.; D’Souza G. A.; Layton G.; Strasburger C. J. (2009) A randomized, controlled multicentre trial comparing pegvisomant alone with combination therapy of pegvisomant and long-acting octreotide in patients with acromegaly. Clin. Endocrinol. 71, 549–557. [DOI] [PubMed] [Google Scholar]
- Silver Lake Research. Reagent Specification Sheet Catalog #: CH2074, Anti-Polyethylene Glycol (PEG) Monoclonal Antibody.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.