Abstract
Background:
The AFCo1 cochleate is a potential novel adjuvant derived from Neisseria meningitidis B proteoliposome.
Aim:
The aim was to assessing the safety of AFCo1 by single and repeated doses in Sprague Dawley rats.
Materials and Methods:
Rats were grouped for treatment with AFCo1, placebo formulation or control. The first study was a single intranasal dose of 100 μl and monitoring body weight, water, and food intakes as well as clinical symptoms. Fourteen days later the rats were killed and anatomopathological studies were conducted. In a second study, four similar doses of the test substance were instilled every 5 days. Clinical observations were carried out as for the single dose study and a number of rats from each group were killed 3 and 14 days after the last dose in order to conduct hematological, hemochemical, and anatomopathological studies.
Results:
No variable showed differences of toxicological relevance; the histological changes found were mild and similarly frequently in the three groups. According to the irritability index calculated form histology of the nasal region, AFCo1 was also classified as nonirritating.
Conclusion:
AFCo1 is potentially safe for human use by nasal route as evidenced by the absence of local and systemic signs of toxicity in Sprague Dawley rats.
Keywords: AFCo1, Cochleate, Rat, Toxicity
Introduction
The mucosal immune system has been recognized as the first defense of the host against pathogens entering the gastrointestinal or the upper respiratory tracts.[1] Mucosal immunization has been well documented and is a highly effective way to stimulate local and systemic immune responses. Local-specific IgA response, systemic–specific IgG response, and cell-mediated immunity have been induced by mucosal immunization.[2,3] However, a close relationship between the adjuvants used and the immune response elicited regarding different antigens has been demonstrated.[4] Thus, the development of new vaccines has increased the need for new and more powerful adjuvants. Finlay Institute has developed a group of adjuvants, of which the AFCo1 cochleate has resulted particularly effective by both, mucosal and parenteral routes.[5,6]
The intranasal administration of adjuvants has been poorly explored from the toxicological point of view. The fact that only one layer of cells separates the lumen of the nasal cavity from a rich vascular net in the lamina propria raises safety concerns. That is why the objective of the present paper was to carry out single and repeated doses safety studies of AFCo1 by intranasal administration in Sprague Dawley rats.
Materials and Methods
Test substance
The active ingredient of AFCo1 is a cochleate structure derived from the proteoliposome of Neisseria meningitidis serogroup B with a protein a content of 1 mg/ml. Every 100 μl also contains sodium chloride (292 μg), Tris (360 μg), calcium chloride (73.5 μg), thiomersal (10 μg), and water for injection as solvent . The placebo formulation contained only the auxiliary components detailed above.
Animal model
Male and female, 5- to 6 week old, 150-200 g body weight, Sprague Dawley rats supplied by the National Center for the Production of Laboratory Animals (CENPALAB, Cuba) were used. Five rats were housed per each T4 (floor area: 1800 cm2) polycarbonate cage (Tecniplast, Italy). Sugar cane bagasse sterilized in autoclave (15 minutes, 121°C) and changed twice a week was used as bedding. Pelleted food produced by CENPALAB and fresh drinking water was administered for ad libitum consumption. Room temperature (22-25°C), relative humidity (60-65%) and light cycle (10 h light-14 h dark) were controlled and recorded twice a day. Animals were killed by intraperitoneal overdose of pentobarbital (100 mg/kg).The protocol of the study was approved by the Ethics Committee for the Care and Use of Laboratory Animals at Finlay Institute and is in accordance with international standards on the topic.[7]
Single-dose toxicity test
Ten rats per sex were inoculated by nasal route with either AFCo1, the placebo formulation or were not treated at all and functioned as controls. A volume of 50 μl (same dose as that proposed for human use) was instilled in each nostril. Animals were clinically examined on a daily basis paying special attention to the appearance of the following signs: Nose irritation, nasal secretion, sneezing, dyspnoea, head shaking, salivation, tearing, and cutaneous reactions. Animals were weighed at the end of quarantine as well as 3, 7, and 14 days after the inoculation. Water and food intakes were measured every other day in order to estimate the average consumption. The rats were killed 2 weeks after the inoculation and anatomopathological studies were conducted with special emphasis on the nasal region and the encephalon. Based on the degree of edema and congestion, the state of the epithelium and the inflammatory cells infiltrating the nasal mucosa, an irritability index was calculated as described elsewhere.[8]
Repeated dose toxicity test
Four doses of 100 μl each were nasally instilled every 5 days. The schedule proposed for human use conceives only there doses, but a further dose was administered to the rats in order to maximize their exposition to the test substance. Animal housing and maintenance as well as the experimental groups were as described for the single-dose toxicity study. Similarly, clinical symptoms, food and water intakes, and body weight were studied. Additionally, hematology, blood biochemistry, and anatomopathological studies were conducted on groups of rats killed 3, 14, and 28 days after the last inoculation.
Hematology included quantification of hemoglobin, hematocrit, leukocytes, differential count of leukocytes, count of erythrocytes, and platelets. Blood serum chemistry measured the levels of glucose, urea, creatinine, alkaline phosphatase, total proteins, triglycerides, cholesterol, direct, and total bilirubin (BIL-D BIL-T), creatine phosphokinase, transaminases, and urates. All the hemochemical determinations were carried out using commercial kits (CENTIS, Havana, Cuba) following the procedures recommended by the producer.
The anatomopathological studies included the necropsy of all the rats and tissue sampling for histological studies. The relative weight of the heart, thymus, lungs, kidneys, liver, and spleen was calculated. The irritation index caused by the product on the nasal mucosa was also calculated.
Statistical analysis
For all the analysis, the statistical package STATISTICA 6 (StatSoft, Inc. (2003); STATISTICA – data analysis software system - version 6, www.statsoft.com) was used. P values under 0.05 were considered significant. Body weights were compared by repeated measures analysis of covariance, using values at the beginning of the assay as covariate. The normality and homogeneity of variance assumptions were tested by means of Shapiro-Wilk's W test and Levene's test, respectively, in order to decide whether to conduct parametric (analysis of variance and least significant difference tests) or nonparametric procedures (Kruskal-Wallis and distribution-free multiple comparisons tests). Finally, the proportion of histological changes was compared by log-lineal analysis.
Results
In the single dose test as well as in the repeated dose toxicity study all the animals increased their body weight (P<0.001) after the inoculation of the test substance. Water and food intakes did not show differences (P>0.1) among the experimental groups. In general, male rats consumed more water and food than female ones (P<0.001). The average daily food consumption was 19.3 g for females and 25.6 g for males. Similarly, the average water intake was 30.0 ml for females and 41.9 for males. Neither local nor general clinical symptoms were observed in any of the two toxicity test carried out.
Single-dose toxicity test
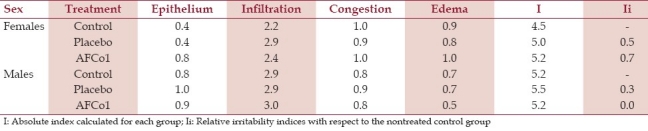
Congestion and small hemorrhages of parotid, submaxillary, retropharyngeal, and cervical lymph nodes were sporadically observed at necropsy. These changes were also found in the histopathological analysis and were found in all groups [Table 1], thus they were associated to the sacrifice method. In a general way, the histopathological changes were mild and similarly frequent in all the groups (P>0.1). The relative irritability index calculated on the basis of histological changes found in the nasal mucosa of rats treated with AFCo1 or the placebo formulation at single dose were all under 1 [Table 2]; therefore, the test product was classified as nonirritant.
Table 1.
Frequency of histological changes in the nasal region and regional lymph nodes in the single-dose toxicity test
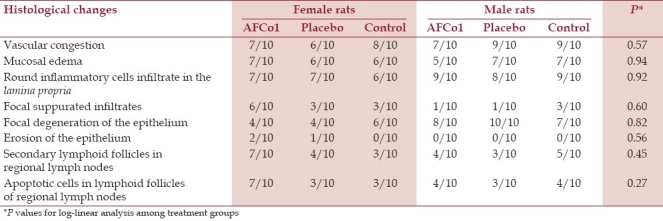
Table 2.
Calculation of nasal irritability indices in rats treated with a single dose
Repeated dose toxicity test
Anatomopathological, hematological, and hemochemical analysis carried out 72 hours after the last inoculation intended to reveal early side effects while those at 14 days were planed to assess recovery of damages or long-term effects.[9]
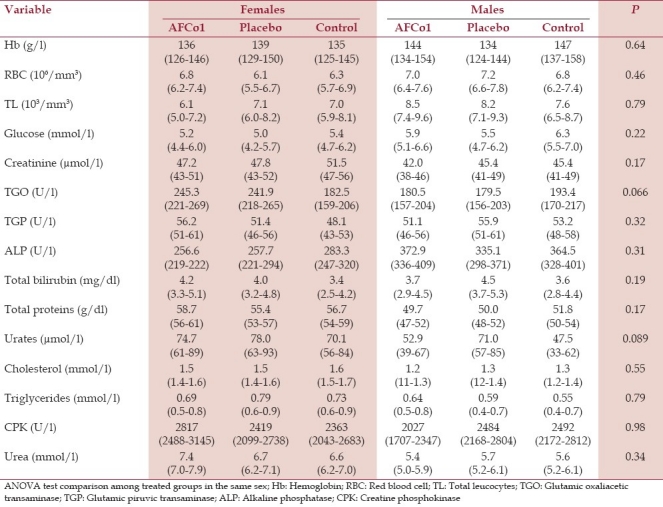
Hematology and blood chemistry analysis did not show statistical differences among experimental groups [Table 3]. Relative organ weights were also statistically similar (data not shown), with the exception of the right kidney weight in females that received AFCo1 and placebo that were higher (P=0.025) to that of control rats. However, no histological changes were found in the kidneys of such animals that could support an association with toxic effects.
Table 3.
Hematology and serum blood chemistry of rats under repeated dosing. Means and 95% confidence intervals (in brackets). P values refer to ANOVA comparisons among treatment groups
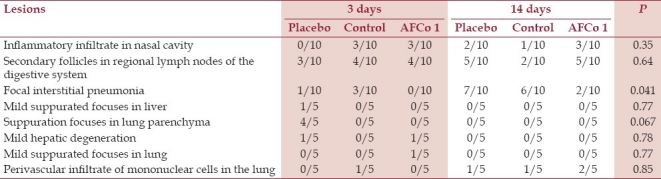
Petechial hemorrhages in cervical lymph nodes in rats regardless of the sex, treatment group, or sacrifice time were found at necropsy again. Due to their unspecific presentation, they were associated to restraint during sedation and bleeding. Histology [Table 4] revealed inflammatory cells infiltrating the nasal cavity. Mononuclear cells and sporadic suppurated focuses were also found at mucosal and submucosal tissues of the nasal septum as well as dorsal and ventral nasal cornets. Besides, nasal congestion, edema, and degeneration of the superficial epithelium were found. However, statistical analysis did not evidence differences (P>0.05) among the histological changes in the three experimental groups. The irritability index calculated after repeated dosing (data not shown) also classified the test substance as nonirritant.
Table 4.
Repeated dose toxicity test: Frequency of lesions in female rats 3 and 14 days after the last dose
Discussion
A relevant animal model for the toxicological assessment of a vaccine or adjuvant must mount an immune response as expected to happen in the target species. Thus, intrinsic toxicity due to the constituents, contaminants, the interaction among them and that related to the immune response elicited by the test product could be evaluated.[9,10] Sprague Dawley is an outbred rat strain preferred for preclinical safety studies due to their heterogeneous response;[7] furthermore, a pilot study demonstrated that they respond immunologically to AFCo1 after nasal instillation. Therefore, that strain was considered a relevant biomodel for the safety assessment of AFCo1.
In order to increase rat exposure to AFCo1 and to test the potential risk of accidental overdosing, a four-dose toxicity study was carried out. This design is in correspondence with current trends in the preclinical evaluation of preventive vaccines that suggest the administration of a further dose to that proposed for the clinical use.[11]
The growth curve, water and food intakes, blood chemistry values, and the relative organ weights of the rats in both tests conducted agreed with those observed for Sprague Dawley rats during the preclinical assessment of other candidate vaccines in our facilities[12–15] and reference values reported elsewhere.[16]
Histological changes were either part of physiologic reactions or incidental, but a connection with toxicity could not be established. Mucosal vascular congestion found reflects the normal physiologic hyperemia of the nasal epithelium.[17] Focally aggregated or diffusely distributed lymphoid cells found at the lamina propria are part of the functional anatomy of mucosal tissues and are mainly represented by T CD4+ and B lymphocytes.[18] The apoptotic lymphocytes and secondary follicles in the cortical and paracortical area of lymph nodes have also been observed by other authors and are associated to the response of secondary lymphoid tissues to the antigenic stimulus.[18,19] Finally, focal interstitial pneumonia is often observed as a consequence of viral infections in rats mainly found in animals like these which are maintained in a conventional sanitary environment.[20–22] However, it is interesting that rats treated with AFCo1 developed interstitial pneumonia in a lower proportion (P<0.05) compared to placebo and control rats. That could probably be related to the immunomodulating effect of AFCo1.[5,6]
Detectable titers of specific IgG have been found in saliva and blood serum 7 and 21 days after AFCo1 nasal instillation in rats, respectively. However, it has not been found in cerebro-spinal fluid, suggesting that AFCo1 does not reach the encephalic cavity, but instead is limited to the nasal region.[14] These results support the idea that AFCo1 is capable of eliciting and modulating immune response after nasal instillation without significantly affecting the histology at the inoculation site as has been previously shown by pharmacology and local toxicity studies.[5,6,14,23,24]
Conclusion
Nasal instillation of AFCo1 in Sprague Dawley rats at a high dose and frequency compared to the schedule proposed for human use was neither local nor systemically toxic. Taking evidence presented here and previous pharmacology and toxicity tests conducted into account, AFCo1, the cochleate derived from Neisseria meningitidis proteoliposome is considered potentially safe for human use.
Acknowledgments
The authors thank technical assistance by personnel of the animal facility of Finlay Institute, standing out the colleagues: Yulieé López, Daiyana Díaz, Jorge L. Prieto, Laura Bencomo, Niurka Rodríguez, Yolanda Valdés and Adriana Ponce. Likewise we wanted to thank our colleagues of the immunology department for their technical support: Caridad Zayas, Osmir Cabrera, Maribel Cuello, Julio Balboa, Belkis Romeu, Elizabeth González, Viviana Pérez and Miriam Lastre.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.McGhee JR, Kiyono H. Mucosal immunology. In: Paul WE, editor. Fundamental Immunology. Vol. 909. San Diego: Academic Press; 1998. pp. 167–79. [Google Scholar]
- 2.Acevedo R, Gil D, del Campo J, Bracho G, Valdés Y, Pérez O. The adjuvant potential of synthetic alkylglycerols. Vaccine. 2006;24(Suppl 2):S32–3. doi: 10.1016/j.vaccine.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto N, St Claire DA, Jr, Homma S, Ngwenya BZ. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 1988;48:6044–9. [PubMed] [Google Scholar]
- 4.Lycke N. From toxin to adjuvant: The rational design of a vaccine adjuvant vector.CTA1-DD/ISCOM. Cell Microbiol. 2004;6:23–32. doi: 10.1046/j.1462-5822.2003.00338.x. [DOI] [PubMed] [Google Scholar]
- 5.Pérez O, Bracho G, Lastre M, Mora N, del Campo J, Gil D, et al. Novel adjuvant based on a proteoliposome-derived cochleate structure containing native lipopolysaccharide as a pathogen-associated molecular pattern. Immunol Cell Biol. 2004;82:603–10. doi: 10.1111/j.1440-1711.2004.01293.x. [DOI] [PubMed] [Google Scholar]
- 6.Del Campo J, Lastre M, Bracho G, Rodríguez T, Gil D, Zayas C, et al. Immunological evaluation of bacterial derived Cochleate and proteoliposome as mucosal adjuvants. Vaccine. 2006;24:50–1. doi: 10.1016/j.vaccine.2005.01.119. [DOI] [PubMed] [Google Scholar]
- 7.Guide to the care and use of experimental animals. Vol. 2. Ottawa, Ontario: 1993. Canadian Council on Animal Care. Laboratory rats; pp. 175–87. [Google Scholar]
- 8.ISO. Norm Part 10: Test for irritation and delayed hypersensitivity. Biological Evaluation of Medical Devises. 2002:26–33. ISO 10993/10. [Google Scholar]
- 9.Sutkowski EM. Proceeding of the Workshop on Non Clinical Safety Evaluation of Preventive Vaccines: Recent Advances and Regulatory Considerations. Vol. 1. Washington, DC: 2002. Non Clinical Safety Assessment of Preventive Vaccines: The FDA Perspective; pp. 203–82. [Google Scholar]
- 10.Ledwith B. Proceeding of the Workshop on Non Clinical Safety Assessment of Preventive Vaccines: Recent Advances and Regulatory Considerations. Vol. 1. Washington, D.C: 2002. The Relevance of Animal Studies for Non-Clinical Safety Assessment of Vaccines; pp. 546–666. [Google Scholar]
- 11.World Health Organization. Guidelines on Nonclinical Assessment of Vaccines. WHO Technical Report Series. Adopted by the 54th meeting of the WHO Expert Committee on Biological Standardization. 2003;54:34–9. [Google Scholar]
- 12.Sosa E, Sifontes S, Infante JF, Díaz D, López Y, Pérez V, et al. Local tolerance of vax-Tyvi® vaccine in Sprague Dawley Rats. Vaccimonitor. 2005;14:21–7. [Google Scholar]
- 13.Infante JF, Sifontes S, Alvarez E, González M, Pérez V, Sosa E, et al. Single dose toxicity and local tolerance evaluation of vax-SPIRAL® vaccine in Sprague Dawley rats. Vaccimonitor. 2004;13:11–6. [Google Scholar]
- 14.Infante JF, Sifontes S, Pérez V, Bracho G, Hernández T, Zayas C, et al. Study of immunogenicity and local toxicity of Neisseria meningitides cochleates in Sprague Dawley rats. Vaccimonitor. 2009;18:1–7. [Google Scholar]
- 15.Sifontes S, Infante JF, Díaz D, López Y, Pérez M, Sosa E, et al. Repeated dose toxicity study of life attenuated oral Cholera vaccine in Sprague Dawley rats. Arch Med Res. 2009;40:527–35. doi: 10.1016/j.arcmed.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Pritchett KR, Coming BF. Biology and Medicine of rats. In: Rueter JD, Suckow MA, editors. Laboratory Medicine and Management. Ithaca NY: International Veterinary Information Service; [Accessed September 29, 2004]. at http://www.ivis.org. No B2503.0904 . [Google Scholar]
- 17.Martín E, Nicolas GM. Nasal mucilliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29:865–85. doi: 10.1016/s0169-409x(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 18.Johansen FE, Baekkevold ES, Carlsen HS, Farstad IN, Soler D, Brandtzaeg P. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: Dispersion from tonsils. Blood. 2005;106:593–600. doi: 10.1182/blood-2004-12-4630. [DOI] [PubMed] [Google Scholar]
- 19.Wlaf JL. The Membranous epithelial (M) cell and the mucosal immune system. Annu Rev Med. 1984;35:95–112. doi: 10.1146/annurev.me.35.020184.000523. [DOI] [PubMed] [Google Scholar]
- 20.López Y, Infante JF, Sifontes S, Díaz D, Pérez V, Año G, et al. Pharmacology and toxicology of an oral tablet whole-cell inactivated cholera vaccine in Sprague Dawley rats. Vaccine. 2011;29:3596–9. doi: 10.1016/j.vaccine.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 21.López Y, Sifontes S, Infante JF, Díaz D, Obaya M, Álvarez E, et al. Evaluation of toxicity by single dose of the Diphtheria-Tetanus Vaccine in Sprague Dawley rats. Vaccimonitor. 2005;14:1–6. [Google Scholar]
- 22.Kuck D, Lau T, Leuchs B, Kern A, Müller M, Gissmann L, et al. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. J Virol. 2006;80:2621–30. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez O, Bracho G, Lastre M, Zayas C, González D, Gil D, et al. Proteoliposome-derived Cochleate as an immunomodulator for nasal vaccine. Vaccine. 2006;24(Suppl 2):S52–3. doi: 10.1016/j.vaccine.2005.01.127. [DOI] [PubMed] [Google Scholar]
- 24.Bracho G, Lastre M, del Campo J, Zayas C, González D, Gil D, et al. Proteoliposome derived cochleate as novel adjuvant. Vaccine. 2006;24(Suppl 2):S30–1. doi: 10.1016/j.vaccine.2005.01.108. [DOI] [PubMed] [Google Scholar]


