Abstract
Hashimoto's thyroiditis (HT), an autoimmune disorder, is the most prevalent cause of subclinical or overt hypothyroidism in areas with sufficient iodine intake. The gland is often diffusely enlarged, and the parenchyma is coarsened, hypoechoic, and often hypervascular on ultrasonograpy. Histopathologic appearance of HT includes lymphocyte aggregates with germinal centers, small thyroid follicles, presence of Hurthle cells, and variable fibrosis. We present a case of a 40-year-old female with suspected follicular neoplasm on fine-needle aspiration cytology of neck swelling. Intraoperatively, thyroid gland was found having four lobes separated from each other. Total thyroidectomy was done and histopathology from all four lobes revealed HT. At present, there is no literature to support the fact that such distorted thyroid anatomy may be due to the underlying disease. If we consider it as thyroid gland anomaly, no such anomaly has been mentioned in the literature till date.
Keywords: Four lobed thyroid gland, Hashimoto's thyroiditis, Thyroid anomaly, Thyroidectomy
Introduction
Hashimoto's thyroiditis (chronic autoimmune thyroiditis) is the most common cause of hypothyroidism in the United States.[1] With a 5–10 times preference over men, the reported prevalence in white women is in the 1–2% range.[2] HT is an autoimmune disorder and is characterized by the presence of antithyroperoxidase (anti-TPO) antibodies. A mixed population of lymphocytes, sometimes represented in the form of crushed lymphocytes and lymphoglandular bodies (cytoplasmic debris) together with the presence of Hurthle cells, is characteristic cytological feature of Hashimoto's thyroiditis (HT). Till date, nowhere in the literature, a case of HT with four lobed thyroid gland has been reported, making our case an interesting and rare one.
Case Report
A 40-year-old married woman came to the out-patient clinic of our institute with chief complaints of a progressively increasing swelling on the anterior aspect of the neck since last 9 months. There was no history of any dysphagia, dyspnea, stridor, hoarseness of voice, fever or loss of weight. There was no history suggestive of hypo- or hyperthyroidism. Systemic examination was grossly normal. On examination of the neck, there was a diffuse swelling on the anterior aspect of neck, nontender, firm, freely mobile horizontally but restricted mobility longitudinally. Ultrasonography of neck demonstrated thyromegaly with hypoechoic thyroid parenchyma and increased vascularity on Color Doppler imaging. fine-needle aspiration cytology (FNAC) of the swelling was advised which revealed features suspicious of follicular neoplasm. Contrast-enhanced computed tomography (CECT) neck revealed diffuse enlargement of thyroid with lobulated appearance of both lobes and isthmus. There was evidence of minimal tracheal compromise at isthmus level. However, there was no CECT evidence of cervical lymphadenopathy. Her hemogram and other basic laboratory parameters were within normal limits. Serum levels of T3, T4, and TSH were 1.16 ng/ml (n=0.8–2.0 ng/ml); 6.5 milligram (mg)/deciliter (n=4.5–11.5 mg/dl), and 4.7 mIU/l (n=0.5–4.70 mIU/l), respectively. The patient was taken for surgery and intraoperatively, we found an unusual anatomy of thyroid gland consisting of four lobes. The additional two lobes were on the antero-medial aspect of enlarged right and left main thyroid lobes and were completely separated from each other. The isthmus was single and prominent [Figure 1]. The veins over the thyroid lobes were also prominent. The gland was firm and was adherent posteriorly to trachea. Due to the nonavailability of frozen section biopsy facility, total thyroidectomy was done and specimen sent for histopathological examination. Histopathology of each of the four lobes showed Hurthle cell changes with lymphocytic background, features suggestive of HT [Figure 2].
Figure 1.
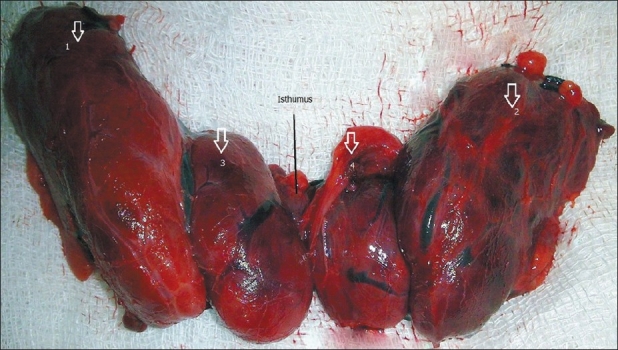
Thyroidectomy specimen showing four lobed thyroid gland with extra lobes (3 and 4) antero-medial to main (1 and 2) thyroid lobes
Figure 2.
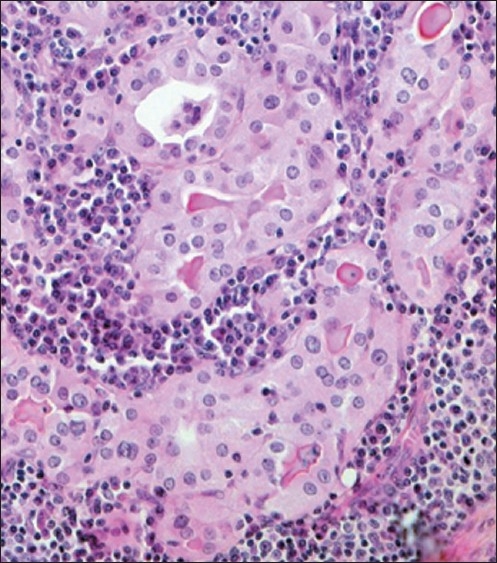
Histopathology of the thyroid specimen showing Hurthle cell changes (pink cells) with lymphocytic background
Discussion
HT is the most prevalent cause of subclinical or overt hypothyroidism in areas with sufficient iodine intake.[3] It has a peak incidence between the ages of 30 and 50, and other autoimmune disorders may coexist with HT.[4] HT can present clinically as painless, firm diffuse goiter often accompanied by hypothyroidism and autoantibodies.[5] The clinical diagnosis is confirmed by low serum T4 levels, high thyroid-stimulating hormone levels, and the presence of autoantibodies to thyroglobulin and thyroid peroxidase.[5,6] The clinical and serological presentation is, however, extremely variable. It is not rare for patients with HT to have neither symptoms nor physical signs.[6,7] In our case also, the patient was asymptomatic and T3/T4 levels were within normal range with a slight increase in TSH levels (subclinical hypothyroidism). If only clinical and serum findings were used to diagnose HT, the diagnosis would be missed in at least half of patients.[6] Since FNAC was suspicious of follicular neoplasm, tests for autoantibodies to thyroglobulin and thyroid peroxidase were not performed in our case.
The sensitivity and specificity improve dramatically by combining ultrasound evaluation with clinical and serological assessments.[6,7] The most accurate method of diagnosing HT, however, remains biopsy and histologic study.[8] On sonography the gland is often diffusely enlarged, and the parenchyma is coarsened, hypoechoic, and often hypervascular. A micronodular pattern on ultrasound is highly diagnostic of HT with a positive predictive value of 95%. These micronodules have been reported to range from 1 to 7 mm in size.[9] They are hypoechoic as a result of lymphocyte infiltration and have an echogenic rim.[9] On Color Doppler, the thyroid parenchyma can vary from slightly hypervascular to markedly hypervascular. In addition to the diffuse form, HT may form discrete nodules within a diffusely altered parenchyma or within a sonographically normal thyroid parenchyma (nodular HT).[10] Ours was a diffuse form of HT with two extra thyroid lobes completely separated from main lobes with increased vascularity. Such distorted anatomy of thyroid gland in a patient of HT has never been reported in the literature till date. If we consider it as an anomaly of thyroid gland, again no literature has mentioned such an anomaly till date. This makes our case an unusual and an interesting one.
Histopathology in HT shows infiltration of the thyroid by T and B cells that are reactive to thyroid antigens. The cytotoxic T cells are largely responsible for the destruction of the thyroid parenchyma, leading to initial thyrotoxicosis followed by hypothyroidism. Typical histopathologic appearance of HT includes lymphocyte aggregates with germinal centers, small thyroid follicles with sparse colloid, oxyphilic changes of epithelial cells, presence of Hurthle cells and variable fibrosis. In our case, histopathology from all the four lobes showed Hurthle cell changes with lymphocytic background, features suggestive of HT.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jaume JC. Endocrine autoimmunity. In: Gardner DG, Shoback DM, editors. Greenspan's Basic and Clinical Endocrinology. New York: McGraw-Hill Medical; 2007. pp. 59–79. [Google Scholar]
- 2.Weetman AP. Thyroid disease. In: Rose NR, Mackay IR, editors. The Autoimmune Disease. Elsevier: 2006. pp. 467–82. [Google Scholar]
- 3.Hayashi N, Tamaki N, Konishi J, Yonekura Y, Senda M, Kasagi K, et al. Sonography of Hashimoto's thyroiditis. J Clin Ultraound. 1986;14:123–6. doi: 10.1002/jcu.1870140208. [DOI] [PubMed] [Google Scholar]
- 4.Sakiyama R. Thyroiditis: A clinical review. Am Fam Physician. 1993;48:615–21. [PubMed] [Google Scholar]
- 5.Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646–55. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- 6.Nordmeyer JP, Shafeh TA, Heckmann C. Thyroid sonography in autoimmune thyroiditis. A prospective study on 123 patients. Acta Endocrinol. 1990;122:391–5. doi: 10.1530/acta.0.1220391. [DOI] [PubMed] [Google Scholar]
- 7.Gutekunst R, Hafermann W, Mansky T, Scriba PC. Ultrasonography related to clinical and laboratory findings in lymphocytic thyroidits. Acta Endocrinol. 1989;121:129–35. doi: 10.1530/acta.0.1210129. [DOI] [PubMed] [Google Scholar]
- 8.Friedman M, Shimaoka K, Rao U, Tsukada Y, Gavigan M, Tamura K. Diagnosis of chronic lymphocytic thyroiditis (nodular presentation) by needle aspiration. Acta Cytol. 1981;25:513–22. [PubMed] [Google Scholar]
- 9.Yeh HC, Futterweit W, Gilbert P. Micronodulation: Ultrasonographic sign of Hashimoto thyroiditis. J Ultrasound Med. 1996;15:813–9. doi: 10.7863/jum.1996.15.12.813. [DOI] [PubMed] [Google Scholar]
- 10.Kerr L. High-resolution thyroid ultrasound: The value of color Doppler. Ultrasound Q. 1994;12:21–44. [Google Scholar]


